484:
Furthermore, epothilone B has also been shown to induce tubulin polymerization into microtubules without the presence of GTP. This is caused by the formation of microtubule bundles throughout the cytoplasm. Finally, epothilone B also causes cell cycle arrest at the G2-M transition phase, thus leading to cytotoxicity and eventually cell apoptosis. The ability of epothilone to inhibit spindle function is generally attributed to its suppression of microtubule dynamics; but recent studies have demonstrated that suppression of dynamics occurs at concentrations lower than those needed to block mitosis. At higher antimitotic concentrations, paclitaxel appears to act by suppressing microtubule detachment from centrosomes, a process that is normally activated during mitosis. It is quite possible that epothilone can also act through a similar mechanism.
726:
840:
765:
647:
848:
280:
160:
40:
856:
enoyl reductase (ER), and an acyl carrier protein domain (ACP). The EPOS P however, contains a heterocylization, an adenylation, an oxidase, and a thiolation domain. These domains are important because they are involved in the formation of the five-membered heterocyclic ring of thiazole. As seen in
855:
Epothilone B starts with a 2-methyl-4-carboxythiazole starter unit, which was formed through the translational coupling between PKS, EPOS A (epoA) module, and NRPS, EPOS P(epoP) module. The EPOS A contains a modified β-ketoacyl-synthase (malonyl-ACP decarboxylase, KSQ), an acyltransferase (AT), an
1511:
Su, D.-S.; Meng, D.; Bertinato, P.; Balog, D. M.; Sorensen, E. J.; Danishefsky, S. J.; Zheng, Y.-H.; Chou, T.-C.; He, L.; Horwitz, S. B. (1997). "Total
Synthesis of(–)-Epothilone B: An Extension of the Suzuki Coupling Method and Insights into Structure–Activity Relationships of the Epothilones".
483:
and in cultured cells. This is because they share the same binding site, as well as binding affinity to the microtubule. Like paclitaxel, epothilone B binds to the αβ-tubulin heterodimer subunit. Once bound, the rate of αβ-tubulin dissociation decreases, thus stabilizing the microtubules.
867:
Once the 2-methylthiazole ring has been made, it is then transferred to the PKS EPOS B (epoB), EPOS C (epoC), EPOS D (epoD), EPOS E (epoE), and EPOS F (epoF) for subsequent elongation and modification to generate the olefinic bond, the 16-membered ring, and the epoxide, as seen in
454:
which can affect cardiac function and cause severe hypersensitivity) are not needed. Endotoxin-like properties known from paclitaxel, like activation of macrophages synthesizing inflammatory cytokines and nitric oxide, are not observed for epothilone B.
1098:
Höfle, G.; Bedorf, N.; Steinmertz, H.; Schomburg, D.; Gerth, K.; Reichenbach, H. (1996). "Epothilone A and B—Novel 16-Membered
Macrolides with Cytotoxic Activity: Isolation, Crystal Structure, and Conformation in Solution".
1758:
1699:
Molnar, I.; Schupp, T.; Ono, M.; Zirkle, RE.; Milnamow, M.; Nowak-Thompson, B.; Engel, N.; Toupet, C.; Stratmann, A.; Cyr, DD.; Gorlach, J.; Mayo, JM.; Hu, A.; Goff, S.; Schmid, J.; Ligon, JM. (2000).
1667:
Nicolaou, K.C.; Ninkovic, S.; Sarabia, F.; Vourloumis, D.; He, Y.; Vallberg, H.; Finlay, M.R.V.; Yang, Z. (1997). "Total
Syntheses of Epothilones A and B via a Macrolactonization-Based Strategy".
479:
function. Microtubules are essential to cell division, and epothilones, therefore, stop cells from properly dividing. Epothilone B possesses the same biological effects as paclitaxel both
872:. One important thing to note is the synthesis of the gem-dimethyl unit in module 7. These two dimethyls were not synthesized by two successive C-methylations. Instead, one of the
1751:
725:
580:
in 1996. This total synthesis of epothilone A was achieved via an intramolecular ester enolate-aldehyde condensation. Other syntheses of epothilones have been published by
1744:
686:
starting from the hydrazone. Ozonolysis, the last step of the Enders alkylation, was followed by reduction of the aldehyde and silylation of the resulting alcohol.
683:
538:
anticancer activities at tolerated dose levels in several human xenograft models. As a result, patupilone and various analogs underwent various clinical phases.
450:. Their mechanism of action is similar, but their chemical structure is simpler. Due to their better water solubility, cremophors (solubilizing agents used for
714:. Asymmetric allylboration of the α,β-unsaturated aldehyde and protection of the hydroxy group gave the silyl ether, whose terminal olefin was reacted with
616:
was employed to close the bis terminal olefin of the precursor compound. The resulting compounds were cis- and trans-macrocyclic isomers with distinct
1471:"Phase III Ixabepilone Study Demonstrated Significant Improvement In Progression-Free Survival In Patients With Advanced Metastatic Breast Cancer"
1395:"Medical News Today: FDA Approves IXEMPRA(TM) (ixabepilone), A Semi-Synthetic Analog Of Epothilone B, For The Treatment Of Advanced Breast Cancer"
1179:
Balog, D. M.; Meng, D.; Kamanecka, T.; Bertinato, P.; Su, D.-S.; Sorensen, E. J.; Danishefsky, S. J. (1996). "Totalsynthese von (—)-Epothilon A".
1007:
H. Spreitzer (September 15, 2008). "Neue
Wirkstoffe – Sagobepilon – eine synthetische Variation von Epothilon B als Hoffnungsträger gegen Krebs".
1071:
Muhlradt, P.F.; Sasse, F. (1997). "Epothilone B stabilizes microtubuli of macrophages like taxol without showing taxol-like endotoxin activity".
839:
1484:
Luduvico, I.; Hyaric, M. L.; Almeida, M. V.; Da Silva, A. D. (2006). "Synthetic
Methodologies for the Preparation of Epothilones and Analogs".
860:, the EPOS P activates the cysteine and binds the activated cysteine as an aminoacyl-S-PCP. Once the cysteine has been bound, EPOS A loads an
1382:
918:
1324:
Villegas, Cecilia; González-Chavarría, Iván; Burgos, Viviana; Iturra-Beiza, Héctor; Ulrich, Henning; Paz, Cristian (January 2023).
1632:
Bode, J. W.; Carreira, E. M. (2001). "Stereoselective syntheses of epothilones A and B via directed nitrile oxide cycloaddition".
2553:
1778:
1444:
699:
1394:
797:
876:
was derived from the propionate extender unit, while the second methyl group was integrated by a C-methyl-transferase domain.
764:
2235:
1701:"The biosynthetic gene cluster for the microtubule-stabilizing agents epothilones A and B from Sorangium cellulosum So ce90"
1127:
646:
399:
279:
159:
39:
2745:
1306:
516:
1974:
864:
unit onto the EPOS P complex, thus initiating the formation of the thiazoline ring by intramolecular cyclodehydration.
2767:
2280:
1767:
707:
2703:
2055:
2050:
2538:
2085:
1924:
758:
524:
2782:
2663:
2578:
754:
722:
to furnish the aldehyde. Reduction, iodination, and treatment with triphenylphosphine led to phosphonium salt.
497:
1570:
Schinzer, D.; Limberg, A.; Bauer, A.; Böhm, O. M.; Cordes, M. (1997). "Total
Synthesis of(−)-Epothilone A".
1540:
659:
581:
1794:
812:
thioester to catalyze the reaction and modify the substrates by selectively reducing the β carbonyl to the
2225:
1854:
572:
Due to the high potency and clinical need for cancer treatments, epothilones have been the target of many
788:
group connected to the macrocycle by an olefinic bond. The polyketide backbone was synthesized by type I
1984:
1979:
1969:
847:
464:
2070:
2060:
2792:
2633:
2408:
2380:
2370:
2065:
1989:
1964:
1188:
431:
627:
One of the total syntheses of epothilone B is outlined below and was described by the laboratory of
2404:
2210:
2110:
2034:
789:
613:
358:
238:
118:
1834:
2777:
2683:
2438:
2239:
2004:
1809:
1770:
1239:
805:
671:
419:, but in early trials, epothilones have better efficacy and milder adverse effects than taxanes.
2653:
546:
531:
2145:
2024:
1904:
1470:
446:
Early studies in cancer cell lines and human cancer patients indicate superior efficacy to the
2772:
2628:
2388:
2325:
2265:
2095:
1722:
1649:
1614:
1365:
1347:
1288:
1231:
1147:
1080:
1053:
965:
914:
695:
675:
601:
589:
2413:
2019:
1934:
1543:; Nicolaou, K. C. (1997). "Total Synthesis of Epothilone A: The Olefin Metathesis Approach".
2787:
2673:
2393:
2275:
2205:
2090:
2080:
2075:
1994:
1844:
1712:
1676:
1641:
1606:
1579:
1552:
1521:
1493:
1355:
1337:
1278:
1270:
1223:
1196:
1139:
1108:
1043:
1035:
983:
955:
947:
719:
715:
294:
174:
54:
2350:
2245:
2125:
801:
746:
711:
691:
573:
1736:
1597:
Mulzer, J.; Mantoulidis, A.; Öhler, E. (2000). "Total syntheses of epothilones B and D".
1360:
1325:
1192:
2698:
2618:
2488:
2345:
2200:
2165:
1824:
1283:
1258:
960:
935:
885:
750:
687:
628:
609:
605:
576:. The first group to publish the total synthesis of epothilones was S. J. Danishefsky
550:
338:
218:
98:
1717:
1700:
1448:
1048:
1023:
951:
2761:
2638:
2513:
2398:
2360:
2255:
2220:
2155:
1954:
1914:
1814:
1398:
907:
833:
585:
523:
that no longer responds to currently available chemotherapies. In
November 2008, the
520:
501:
427:
1243:
1039:
2723:
2693:
2668:
2623:
2568:
2563:
2548:
2543:
2533:
2528:
2453:
2355:
2335:
2320:
2315:
2310:
2295:
2190:
2180:
2009:
1949:
1884:
1879:
1874:
1869:
1849:
1839:
1214:
Jordan MA, Wilson L (April 2004). "Microtubules as a target for anticancer drugs".
873:
781:
617:
561:
505:
1274:
1155:
564:
in metastatic breast cancer have been announced (2007 – leading to FDA approval).
2708:
2678:
2658:
2608:
2483:
2478:
2463:
2433:
2260:
2215:
2175:
2170:
2120:
2115:
2100:
1939:
1899:
1804:
1024:"Heterologous Expression of Epothilone Biosynthetic Genes in Myxococcus xanthus"
825:
663:
621:
557:
512:
476:
423:
1497:
658:
was generated from the keto aldehyde that was converted to the silyl ether via
2688:
2648:
2593:
2583:
2523:
2508:
2493:
2473:
2448:
2443:
2423:
2418:
2375:
2365:
2330:
2305:
2285:
2250:
2229:
2185:
2160:
1944:
1909:
1894:
1889:
1858:
1819:
1799:
829:
809:
778:
745:
were reacted with each other to deliver epothilone B in an approach including
667:
542:
451:
1351:
1200:
2613:
2603:
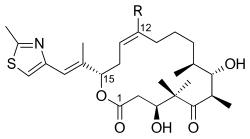
2598:
2588:
2573:
2558:
2503:
2458:
2428:
2290:
2270:
2195:
2135:
2130:
1999:
1929:
1919:
1864:
1143:
493:
17:
1726:
1653:
1618:
1583:
1556:
1525:
1369:
1292:
1235:
1151:
1112:
1057:
969:
1084:
2518:
2498:
2468:
2340:
2300:
2150:
2140:
2014:
1342:
1326:"Epothilones as Natural Compounds for Novel Anticancer Drugs Development"
813:
793:
785:
597:
593:
475:
The principal mechanism of the epothilone class is the inhibition of the
549:
was tested in phase I trials). Patupilone failed a phase III trial for
2728:
2713:
2643:
2105:
1829:
861:
416:
412:
378:
258:
138:
1680:
1645:
1610:
836:, N-methylation, and heterocycle formation occurs in the NRPS enzyme.
519:
for use in the treatment of aggressive metastatic or locally advanced
821:
817:
447:
1227:
1383:
Utidelone Active in
Pretreated, Metastatic Breast Cancer. June 2016
913:(7th ed.). Hagerstwon, MD: Lippincott Williams & Wilkins.
698:
to yield the secondary alcohol. Oxidation of this alcohol with the
710:, and the aldehyde was reacted with the stabilized ylide in the
1740:
905:
Rosenberg, Steven; DeVita, Vincent T.; Hellman, Samuel (2005).
690:
of the benzyl ether gave the alcohol, which was oxidized under
1259:"Paclitaxel-dependent cell lines reveal a novel drug activity"
624:
of cis- and trans-olefins yield epothilone A and its analogs.
415:, they prevent cancer cells from dividing by interfering with
832:
activated on the enzyme as aminoacyl adenylates. Unlike PKS,
828:
the α carbon of the substrate. NRPS, on the other hand, uses
463:
The structure of epothilone A was determined in 1996 using
277:
157:
37:
678:
oxidation of the aldehyde afforded the keto acid. Ketone
1779:
1128:"Epothilones: mechanism of action and biologic activity"
706:
was synthesized from the ester, which was reduced with
496:, was approved in China in 2021 for the treatment of
29:
1432:
800:(NRPS). In this biosynthesis, both PKS and NRPS use
515:, was approved in October 2007 by the United States
2033:
1777:
1418:
London, 20 November 2008 Doc. Ref. EMEA/602569/2008
804:, which have been post-translationally modified by
600:, and ketoacid were constructed and coupled to the
527:refused a marketing authorization for ixabepilone.
906:
1445:"ESMO: Failed Trials Dominate Gyn Cancer Session"
984:"Epothilone - an overview | ScienceDirect Topics"
936:"Epothilones: from discovery to clinical trials"
792:(PKS) and the thiazole ring was derived from a
500:. Utidelone has shown benefits in a phase III
1752:
1694:
1692:
1690:
1257:Ganguly A, Yang H, Cabral F (November 2010).
909:Cancer: Principles & Practice of Oncology
8:
808:groups, to join the growing chain. PKS uses
411:are a class of potential cancer drugs. Like
2035:
1330:International Journal of Molecular Sciences
1174:
1172:
2086:Allopregnanedione (5α-dihydroprogesterone)
1759:
1745:
1737:
1434:American Chemical Society, Washington, DC.
422:Epothilones were originally identified as
1716:
1359:
1341:
1282:
1047:
959:
592:. In this approach, key building blocks
530:Epothilone B, with the generic drug name
492:Epothilone D, with the generic drug name
1784:Tooltip Constitutive androstane receptor
1126:Goodin S, Kane MP, Rubin EH (May 2004).
846:
838:
763:
761:was used to separate the diastereomers.
724:
645:
443:have been identified and characterized.
897:
631:. The retrosynthetic analysis revealed
2539:Pregnanedione (5β-dihydroprogesterone)
1925:Pregnanedione (5β-dihydroprogesterone)
1427:Ojima, I.; Vite, G.D.; Altmann, K.H.;
824:(Enoyl Reductase, ER). PKS-I can also
1307:"New Drug Approvals in China in 2021"
940:Current Topics in Medicinal Chemistry
7:
435:. As of September 2008, epothilones
643:as the building blocks (Figure 1).
545:were tested in phase II trials and
541:Patupilone and the fully synthetic
284:Epothilones E (R = H) and F (R = CH
164:Epothilones C (R = H) and D (R = CH
44:Epothilones A (R = H) and B (R = CH
1539:Yang, Z.; He, Y.; Vourloumis, D.;
1473:. Medical News Today. 4 June 2007.
702:gave the desired ketone. Thiazole
556:Results of a phase III trial with
25:
1486:Mini-Reviews in Organic Chemistry
1447:. 14 October 2010. Archived from
952:10.2174/1568026614666141130095855
560:(BMS-247550) in combination with
1009:Österreichische Apothekerzeitung
718:to a diol that was cleaved with
278:
158:
38:
1040:10.1128/AAC.46.9.2772-2778.2002
798:nonribosomal peptide synthetase
654:As seen in Figure 2, keto acid
534:, was proven to contain potent
2091:Allopregnanolone (brexanolone)
777:Epothilone B is a 16-membered
426:produced by the soil-dwelling
1:
2746:Receptor/signaling modulators
2704:Tropanyl 3,5-dimethulbenzoate
2554:Pregnenolone 16α-carbonitrile
1718:10.1016/S1074-5521(00)00075-2
1275:10.1158/1535-7163.MCT-10-0552
1022:Julien, B.; Shah, S. (2002).
1028:Antimicrob. Agents Chemother
517:Food and Drug Administration
2281:Dodecylbenzenesulfonic acid
2040:Tooltip Pregnane X receptor
1768:Xenobiotic-sensing receptor
1011:(in German) (19/2008): 978.
820:(Dehydratase, DH), and the
708:diisobutylaluminium hydride
27:Class of chemical compounds
2809:
1572:Angew. Chem. Int. Ed. Engl
1545:Angew. Chem. Int. Ed. Engl
1514:Angew. Chem. Int. Ed. Engl
1498:10.2174/157019306775474194
666:of the resulting alcohol.
2737:
816:(Ketoreductase, KR), the
759:thin-layer chromatography
400:Disclaimer and references
397:
32:
2664:Testosterone isocaproate
2579:Reverse triiodothyronine
1201:10.1002/ange.19961082318
757:(Figure 3). Preparative
755:Yamaguchi esterification
660:asymmetric allylboration
498:metastatic breast cancer
488:Medical use and research
2056:17α-Hydroxyprogesterone
2051:17α-Hydroxypregnenolone
1144:10.1200/JCO.2004.12.001
934:Forli, Stefano (2014).
694:and alkylated with the
670:of the silyl ether and
1584:10.1002/anie.199705231
1557:10.1002/anie.199701661
1526:10.1002/anie.199707571
1113:10.1002/anie.199615671
852:
844:
769:
730:
651:
511:One synthetic analog,
1795:6,7-Dimethylesculetin
1705:Chemistry and Biology
1216:Nature Reviews Cancer
988:www.sciencedirect.com
850:
842:
767:
728:
649:
465:x-ray crystallography
2382:Hypericum perforatum
2371:Hetacillin potassium
1343:10.3390/ijms24076063
700:Ley–Griffith reagent
682:was constructed via
504:trial when added to
432:Sorangium cellulosum
2211:Cyproterone acetate
2111:Amlodipine besylate
1193:1996AngCh.108.2976B
790:polyketide synthase
471:Mechanism of action
2768:Mitotic inhibitors
2684:Thonzonium bromide
2439:Methylprednisolone
2240:prasterone sulfate
853:
845:
806:phosphopantetheine
796:incorporated by a
770:
731:
652:
2755:
2754:
2389:Indinavir sulfate
2096:Alpha-Lipoic acid
2071:Δ-Androstenedione
2061:Δ-Androstenedione
1681:10.1021/ja971110h
1646:10.1021/ja0155635
1611:10.1021/jo0007480
946:(20): 2312–2321.
720:lead tetraacetate
684:Enders alkylation
604:precursor via an
602:olefin metathesis
406:
405:
402:
295:Chemical formulae
175:Chemical formulae
55:Chemical formulae
16:(Redirected from
2800:
2674:Thiamylal sodium
2409:γ-linolenic acid
2405:α-Linolenic acid
2403:Linolenic acid:
2394:Lasalocid sodium
2384:(St John's wort)
2276:Docusate calcium
2206:Cyclophosphamide
2081:Allopregnanediol
2066:Δ-Androstenediol
2041:
2037:
1995:Ethinylestradiol
1845:Cyclophosphamide
1785:
1781:
1761:
1754:
1747:
1738:
1731:
1730:
1720:
1696:
1685:
1684:
1669:J. Am. Chem. Soc
1664:
1658:
1657:
1634:J. Am. Chem. Soc
1629:
1623:
1622:
1594:
1588:
1587:
1567:
1561:
1560:
1536:
1530:
1529:
1508:
1502:
1501:
1481:
1475:
1474:
1467:
1461:
1460:
1458:
1456:
1441:
1435:
1425:
1419:
1416:
1410:
1409:
1407:
1406:
1397:. Archived from
1391:
1385:
1380:
1374:
1373:
1363:
1345:
1321:
1315:
1314:
1303:
1297:
1296:
1286:
1263:Mol. Cancer Ther
1254:
1248:
1247:
1211:
1205:
1204:
1176:
1167:
1166:
1164:
1163:
1154:. Archived from
1123:
1117:
1116:
1095:
1089:
1088:
1068:
1062:
1061:
1051:
1019:
1013:
1012:
1004:
998:
997:
995:
994:
980:
974:
973:
963:
931:
925:
924:
912:
902:
802:carrier proteins
716:osmium tetroxide
696:Grignard reagent
614:Grubbs' catalyst
398:
339:Molecular masses
282:
219:Molecular masses
162:
99:Molecular masses
42:
30:
21:
2808:
2807:
2803:
2802:
2801:
2799:
2798:
2797:
2783:Total synthesis
2758:
2757:
2756:
2751:
2733:
2634:Suberoylanilide
2351:Fluorometholone
2246:Dibunate sodium
2126:Aurothioglucose
2039:
2029:
1965:3,17β-Estradiol
1783:
1773:
1765:
1735:
1734:
1698:
1697:
1688:
1666:
1665:
1661:
1631:
1630:
1626:
1605:(22): 7456–67.
1596:
1595:
1591:
1569:
1568:
1564:
1538:
1537:
1533:
1510:
1509:
1505:
1483:
1482:
1478:
1469:
1468:
1464:
1454:
1452:
1451:on 18 June 2010
1443:
1442:
1438:
1426:
1422:
1417:
1413:
1404:
1402:
1393:
1392:
1388:
1381:
1377:
1323:
1322:
1318:
1305:
1304:
1300:
1269:(11): 2914–23.
1256:
1255:
1251:
1228:10.1038/nrc1317
1213:
1212:
1208:
1187:(23–24): 2976.
1178:
1177:
1170:
1161:
1159:
1138:(10): 2015–25.
1125:
1124:
1120:
1097:
1096:
1092:
1073:Cancer Research
1070:
1069:
1065:
1021:
1020:
1016:
1006:
1005:
1001:
992:
990:
982:
981:
977:
933:
932:
928:
921:
904:
903:
899:
894:
882:
775:
747:Wittig reaction
712:Wittig reaction
692:Swern condition
574:total syntheses
570:
568:Total synthesis
490:
473:
461:
388:
368:
348:
331:
327:
323:
316:
314:
310:
306:
287:
268:
248:
228:
211:
207:
203:
196:
194:
190:
186:
167:
148:
128:
108:
91:
87:
83:
76:
74:
70:
66:
47:
28:
23:
22:
15:
12:
11:
5:
2806:
2804:
2796:
2795:
2790:
2785:
2780:
2775:
2770:
2760:
2759:
2753:
2752:
2750:
2749:
2742:
2738:
2735:
2734:
2732:
2731:
2726:
2717:
2716:
2711:
2706:
2701:
2699:Troleandomycin
2696:
2691:
2686:
2681:
2676:
2671:
2666:
2661:
2656:
2651:
2646:
2641:
2636:
2631:
2626:
2621:
2619:Spironolactone
2616:
2611:
2606:
2601:
2596:
2591:
2586:
2581:
2576:
2571:
2566:
2561:
2556:
2551:
2546:
2541:
2536:
2531:
2526:
2521:
2516:
2511:
2506:
2501:
2496:
2491:
2489:Norelgestromin
2486:
2481:
2476:
2471:
2466:
2461:
2456:
2451:
2446:
2441:
2436:
2431:
2426:
2421:
2416:
2411:
2401:
2396:
2391:
2386:
2378:
2373:
2368:
2363:
2358:
2353:
2348:
2346:Flucloxacillin
2343:
2338:
2333:
2328:
2323:
2318:
2313:
2308:
2303:
2298:
2293:
2288:
2283:
2278:
2273:
2268:
2263:
2258:
2253:
2248:
2243:
2233:
2223:
2218:
2213:
2208:
2203:
2201:Corticosterone
2198:
2193:
2188:
2183:
2178:
2173:
2168:
2166:Chlorpromazine
2163:
2158:
2153:
2148:
2143:
2138:
2133:
2128:
2123:
2118:
2113:
2108:
2103:
2098:
2093:
2088:
2083:
2078:
2073:
2068:
2063:
2058:
2053:
2044:
2042:
2031:
2030:
2028:
2027:
2022:
2017:
2012:
2007:
2002:
1997:
1992:
1987:
1985:17-Androstanol
1982:
1980:3β-Androstanol
1977:
1975:3α-Androstenol
1972:
1970:3α-Androstanol
1967:
1958:
1957:
1952:
1947:
1942:
1937:
1932:
1927:
1922:
1917:
1912:
1907:
1902:
1897:
1892:
1887:
1882:
1877:
1872:
1867:
1862:
1852:
1847:
1842:
1837:
1832:
1827:
1825:Chlorpromazine
1822:
1817:
1812:
1807:
1802:
1797:
1788:
1786:
1775:
1774:
1766:
1764:
1763:
1756:
1749:
1741:
1733:
1732:
1686:
1659:
1640:(15): 3611–2.
1624:
1589:
1562:
1531:
1503:
1476:
1462:
1436:
1420:
1411:
1386:
1375:
1316:
1298:
1249:
1206:
1168:
1132:J. Clin. Oncol
1118:
1107:(1314): 1567.
1090:
1079:(16): 3344–6.
1063:
1014:
999:
975:
926:
919:
896:
895:
893:
890:
889:
888:
886:Discodermolide
881:
878:
774:
771:
751:aldol reaction
688:Hydrogenolysis
629:K. C. Nicolaou
610:esterification
606:aldol reaction
569:
566:
551:ovarian cancer
489:
486:
472:
469:
460:
457:
404:
403:
395:
394:
381:
375:
374:
361:
355:
354:
341:
335:
334:
329:
325:
321:
312:
308:
304:
297:
291:
290:
285:
275:
274:
261:
255:
254:
241:
235:
234:
221:
215:
214:
209:
205:
201:
192:
188:
184:
177:
171:
170:
165:
155:
154:
141:
135:
134:
121:
115:
114:
101:
95:
94:
89:
85:
81:
72:
68:
64:
57:
51:
50:
45:
35:
34:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2805:
2794:
2791:
2789:
2786:
2784:
2781:
2779:
2776:
2774:
2771:
2769:
2766:
2765:
2763:
2748:
2747:
2743:
2740:
2739:
2736:
2730:
2727:
2725:
2722:
2719:
2718:
2715:
2712:
2710:
2707:
2705:
2702:
2700:
2697:
2695:
2692:
2690:
2687:
2685:
2682:
2680:
2677:
2675:
2672:
2670:
2667:
2665:
2662:
2660:
2657:
2655:
2652:
2650:
2647:
2645:
2642:
2640:
2639:Sulfisoxazole
2637:
2635:
2632:
2630:
2627:
2625:
2622:
2620:
2617:
2615:
2612:
2610:
2607:
2605:
2602:
2600:
2597:
2595:
2592:
2590:
2587:
2585:
2582:
2580:
2577:
2575:
2572:
2570:
2567:
2565:
2562:
2560:
2557:
2555:
2552:
2550:
2547:
2545:
2542:
2540:
2537:
2535:
2532:
2530:
2527:
2525:
2522:
2520:
2517:
2515:
2514:Phenobarbital
2512:
2510:
2507:
2505:
2502:
2500:
2497:
2495:
2492:
2490:
2487:
2485:
2482:
2480:
2477:
2475:
2472:
2470:
2467:
2465:
2462:
2460:
2457:
2455:
2452:
2450:
2447:
2445:
2442:
2440:
2437:
2435:
2432:
2430:
2427:
2425:
2422:
2420:
2417:
2415:
2412:
2410:
2406:
2402:
2400:
2399:Levothyroxine
2397:
2395:
2392:
2390:
2387:
2385:
2383:
2379:
2377:
2374:
2372:
2369:
2367:
2364:
2362:
2361:Guggulsterone
2359:
2357:
2354:
2352:
2349:
2347:
2344:
2342:
2339:
2337:
2334:
2332:
2329:
2327:
2324:
2322:
2319:
2317:
2314:
2312:
2309:
2307:
2304:
2302:
2299:
2297:
2294:
2292:
2289:
2287:
2284:
2282:
2279:
2277:
2274:
2272:
2269:
2267:
2264:
2262:
2259:
2257:
2256:Dicloxacillin
2254:
2252:
2249:
2247:
2244:
2241:
2237:
2234:
2231:
2227:
2224:
2222:
2221:Dexamethasone
2219:
2217:
2214:
2212:
2209:
2207:
2204:
2202:
2199:
2197:
2194:
2192:
2189:
2187:
2184:
2182:
2179:
2177:
2174:
2172:
2169:
2167:
2164:
2162:
2159:
2157:
2156:Cephaloridine
2154:
2152:
2149:
2147:
2144:
2142:
2139:
2137:
2134:
2132:
2129:
2127:
2124:
2122:
2119:
2117:
2114:
2112:
2109:
2107:
2104:
2102:
2099:
2097:
2094:
2092:
2089:
2087:
2084:
2082:
2079:
2077:
2074:
2072:
2069:
2067:
2064:
2062:
2059:
2057:
2054:
2052:
2049:
2046:
2045:
2043:
2038:
2032:
2026:
2023:
2021:
2018:
2016:
2013:
2011:
2008:
2006:
2003:
2001:
1998:
1996:
1993:
1991:
1988:
1986:
1983:
1981:
1978:
1976:
1973:
1971:
1968:
1966:
1963:
1960:
1959:
1956:
1955:Valproic acid
1953:
1951:
1948:
1946:
1943:
1941:
1938:
1936:
1933:
1931:
1928:
1926:
1923:
1921:
1918:
1916:
1915:Phenobarbital
1913:
1911:
1908:
1906:
1903:
1901:
1898:
1896:
1893:
1891:
1888:
1886:
1883:
1881:
1878:
1876:
1873:
1871:
1868:
1866:
1863:
1860:
1856:
1853:
1851:
1848:
1846:
1843:
1841:
1838:
1836:
1833:
1831:
1828:
1826:
1823:
1821:
1818:
1816:
1815:Carbamazepine
1813:
1811:
1808:
1806:
1803:
1801:
1798:
1796:
1793:
1790:
1789:
1787:
1782:
1776:
1772:
1769:
1762:
1757:
1755:
1750:
1748:
1743:
1742:
1739:
1728:
1724:
1719:
1714:
1711:(2): 97–109.
1710:
1706:
1702:
1695:
1693:
1691:
1687:
1682:
1678:
1674:
1670:
1663:
1660:
1655:
1651:
1647:
1643:
1639:
1635:
1628:
1625:
1620:
1616:
1612:
1608:
1604:
1600:
1593:
1590:
1585:
1581:
1577:
1573:
1566:
1563:
1558:
1554:
1550:
1546:
1542:
1535:
1532:
1527:
1523:
1519:
1515:
1507:
1504:
1499:
1495:
1491:
1487:
1480:
1477:
1472:
1466:
1463:
1450:
1446:
1440:
1437:
1433:
1430:
1424:
1421:
1415:
1412:
1401:on 2011-05-16
1400:
1396:
1390:
1387:
1384:
1379:
1376:
1371:
1367:
1362:
1357:
1353:
1349:
1344:
1339:
1335:
1331:
1327:
1320:
1317:
1313:. 2 May 2022.
1312:
1311:diaglobal.org
1308:
1302:
1299:
1294:
1290:
1285:
1280:
1276:
1272:
1268:
1264:
1260:
1253:
1250:
1245:
1241:
1237:
1233:
1229:
1225:
1222:(4): 253–65.
1221:
1217:
1210:
1207:
1202:
1198:
1194:
1190:
1186:
1182:
1175:
1173:
1169:
1158:on 2006-02-13
1157:
1153:
1149:
1145:
1141:
1137:
1133:
1129:
1122:
1119:
1114:
1110:
1106:
1102:
1094:
1091:
1086:
1082:
1078:
1074:
1067:
1064:
1059:
1055:
1050:
1045:
1041:
1037:
1034:(9): 2772–8.
1033:
1029:
1025:
1018:
1015:
1010:
1003:
1000:
989:
985:
979:
976:
971:
967:
962:
957:
953:
949:
945:
941:
937:
930:
927:
922:
920:0-7817-4450-4
916:
911:
910:
901:
898:
891:
887:
884:
883:
879:
877:
875:
874:methyl groups
871:
865:
863:
859:
849:
841:
837:
835:
834:epimerization
831:
827:
823:
819:
815:
811:
807:
803:
799:
795:
791:
787:
784:with a methyl
783:
780:
772:
766:
762:
760:
756:
752:
748:
744:
740:
736:
727:
723:
721:
717:
713:
709:
705:
701:
697:
693:
689:
685:
681:
677:
673:
669:
665:
661:
657:
648:
644:
642:
638:
634:
630:
625:
623:
619:
618:stereocenters
615:
611:
607:
603:
599:
595:
591:
587:
583:
579:
575:
567:
565:
563:
559:
554:
552:
548:
544:
539:
537:
533:
528:
526:
522:
521:breast cancer
518:
514:
509:
507:
503:
502:breast cancer
499:
495:
487:
485:
482:
478:
470:
468:
466:
458:
456:
453:
449:
444:
442:
438:
434:
433:
429:
428:myxobacterium
425:
420:
418:
414:
410:
401:
396:
393:
391:
386:
382:
380:
377:
376:
373:
371:
366:
362:
360:
357:
356:
353:
352:523.68 g/mol
351:
346:
342:
340:
337:
336:
333:
319:
302:
298:
296:
293:
292:
289:
281:
276:
273:
271:
266:
262:
260:
257:
256:
253:
251:
246:
242:
240:
237:
236:
233:
232:491.68 g/mol
231:
226:
222:
220:
217:
216:
213:
199:
182:
178:
176:
173:
172:
169:
161:
156:
153:
151:
146:
142:
140:
137:
136:
133:
131:
126:
122:
120:
117:
116:
113:
112:507.68 g/mol
111:
106:
102:
100:
97:
96:
93:
79:
62:
58:
56:
53:
52:
49:
41:
36:
31:
19:
2744:
2724:Ketoconazole
2721:Antagonists:
2720:
2694:Troglitazone
2669:Tetracycline
2624:Spiroxatrine
2569:Quingestrone
2564:Progesterone
2549:Pregnenolone
2544:Pregnanolone
2534:Pregnanediol
2529:Prednisolone
2454:Mifepristone
2381:
2356:Griseofulvin
2336:Fenbendazole
2321:Famprofazone
2316:Erythromycin
2311:Epothilone B
2296:Eburnamonine
2191:Clotrimazole
2181:Clofenvinfos
2116:Antimycotics
2047:
2010:Okadaic acid
1962:Antagonists:
1961:
1950:Troglitazone
1885:Mifepristone
1880:Methoxychlor
1875:Griseofulvin
1870:Ellagic acid
1850:Cypermethrin
1840:Clotrimazole
1791:
1708:
1704:
1675:(34): 7974.
1672:
1668:
1662:
1637:
1633:
1627:
1602:
1599:J. Org. Chem
1598:
1592:
1575:
1571:
1565:
1548:
1544:
1541:Vallberg, H.
1534:
1517:
1513:
1506:
1489:
1485:
1479:
1465:
1453:. Retrieved
1449:the original
1439:
1431:
1428:
1423:
1414:
1403:. Retrieved
1399:the original
1389:
1378:
1333:
1329:
1319:
1310:
1301:
1266:
1262:
1252:
1219:
1215:
1209:
1184:
1180:
1160:. Retrieved
1156:the original
1135:
1131:
1121:
1104:
1100:
1093:
1076:
1072:
1066:
1031:
1027:
1017:
1008:
1002:
991:. Retrieved
987:
978:
943:
939:
929:
908:
900:
869:
866:
857:
854:
782:macrolactone
776:
773:Biosynthesis
742:
738:
734:
732:
703:
679:
655:
653:
640:
636:
632:
626:
608:and then an
584:, Schinzer,
577:
571:
562:capecitabine
555:
540:
535:
529:
510:
506:capecitabine
491:
480:
474:
462:
445:
440:
436:
430:
421:
408:
407:
389:
384:
383:
372:208518-52-9
369:
364:
363:
349:
347:509.66 g/mol
344:
343:
317:
300:
299:
283:
269:
264:
263:
252:189453-10-9
249:
244:
243:
229:
227:477.66 g/mol
224:
223:
197:
180:
179:
163:
149:
144:
143:
132:152044-54-7
129:
124:
123:
109:
107:493.66 g/mol
104:
103:
77:
60:
59:
43:
33:Epothilones
18:Epothilone B
2793:Polyketides
2709:Zafirlukast
2679:Thiothixene
2659:Terconazole
2609:Simvastatin
2484:Nisoldipine
2479:Nilvadipine
2464:Nicardipine
2434:Metacycline
2261:Dimercaprol
2216:Demecolcine
2176:Clindamycin
2171:Ciglitazone
2121:Artemisinin
2101:Ambrisentan
2005:Nigramide J
1940:Telmisartan
1900:Nicardipine
1810:Benfuracarb
1805:Artemisinin
1551:(12): 166.
1336:(7): 6063.
1181:Angew. Chem
1101:Angew. Chem
830:amino acids
622:Epoxidation
612:coupling.
558:ixabepilone
513:ixabepilone
477:microtubule
424:metabolites
409:Epothilones
367:201049-37-8
359:CAS numbers
247:186692-73-9
239:CAS numbers
127:152044-53-6
119:CAS numbers
2762:Categories
2689:Tianeptine
2654:Tenylidone
2649:Tacrolimus
2594:Rimexolone
2584:Rifampicin
2524:Plicamycin
2509:Paclitaxel
2494:Omeprazole
2474:Nifedipine
2449:Mevastatin
2444:Metyrapone
2424:Lovastatin
2419:Loratadine
2376:Hyperforin
2366:Haloprogin
2331:Felodipine
2306:Enzacamene
2286:Dronabinol
2251:Diclazuril
2230:prasterone
2186:Chloroxine
2161:Cephradine
2131:Bile acids
1945:Tolnaftate
1910:Permethrin
1895:Nevirapine
1890:Nefazodone
1859:prasterone
1820:Carvedilol
1800:Amiodarone
1771:modulators
1578:(5): 523.
1520:(7): 757.
1488:(Review).
1455:26 October
1405:2009-02-17
1162:2006-05-14
993:2022-06-18
892:References
810:coenzyme-A
779:polyketide
733:Fragments
668:Ozonolysis
664:silylation
547:BMS-310705
543:sagopilone
532:patupilone
452:paclitaxel
2778:Thiazoles
2614:Sirolimus
2604:Ritonavir
2599:Riodipine
2589:Rifaximin
2574:Reserpine
2559:Proadifen
2504:Oxatomide
2459:Nafcillin
2429:Meclizine
2291:Droxidopa
2271:Docetaxel
2196:Colforsin
2146:Bumecaine
2136:Bithionol
2048:Agonists:
2025:T-0901317
2000:Meclizine
1930:Reserpine
1920:Phenytoin
1905:Octicizer
1865:Efavirenz
1792:Agonists:
1492:: 49–75.
1352:1422-0067
826:methylate
598:glycidols
553:in 2010.
494:utidelone
2773:Epoxides
2741:See also
2629:SR-12813
2519:Piperine
2499:Orlistat
2469:Nicotine
2341:Fentanyl
2326:Febantel
2301:Ecopipam
2266:Dinaline
2151:Cafestol
2141:Bosentan
2015:PK-11195
1727:10662695
1654:11472140
1619:11076603
1370:37047035
1361:10093981
1293:20978163
1244:10228718
1236:15057285
1152:15143095
1058:12183227
970:25434353
880:See also
870:Figure 5
858:Figure 4
851:Figure 5
843:Figure 4
814:hydroxyl
794:cysteine
786:thiazole
768:Figure 3
729:Figure 2
672:Lindgren
650:Figure 1
594:aldehyde
590:Carreira
582:Nicolaou
481:in vitro
392:9914741
2788:Lactams
2729:Sesamin
2714:Zeranol
2644:Suramin
2414:LOE-908
2106:AMI-193
2020:S-07662
1935:TCPOBOP
1830:Chrysin
1284:2978777
1189:Bibcode
1085:9269992
961:4629788
862:acetate
676:Pinnick
536:in vivo
459:History
448:taxanes
417:tubulin
413:taxanes
387:9806341
379:PubChem
272:447865
267:9891226
259:PubChem
152:448013
139:PubChem
2236:DHEA-S
2076:AA-861
1725:
1652:
1617:
1368:
1358:
1350:
1291:
1281:
1242:
1234:
1150:
1083:
1056:
1049:127399
1046:
968:
958:
917:
822:alkane
818:alkene
753:, and
741:, and
639:, and
588:, and
586:Mulzer
578:et al.
147:448799
1835:CITCO
1240:S2CID
2407:and
2226:DHEA
1990:AITC
1855:DHEA
1723:PMID
1650:PMID
1615:PMID
1457:2010
1429:2001
1366:PMID
1348:ISSN
1289:PMID
1232:PMID
1148:PMID
1081:PMID
1054:PMID
966:PMID
915:ISBN
662:and
525:EMEA
2036:PXR
1780:CAR
1713:doi
1677:doi
1673:119
1642:doi
1638:123
1607:doi
1580:doi
1553:doi
1522:doi
1494:doi
1356:PMC
1338:doi
1279:PMC
1271:doi
1224:doi
1197:doi
1185:108
1140:doi
1109:doi
1044:PMC
1036:doi
956:PMC
948:doi
620:.
439:to
2764::
1721:.
1707:.
1703:.
1689:^
1671:.
1648:.
1636:.
1613:.
1603:65
1601:.
1576:36
1574:.
1549:36
1547:.
1518:36
1516:.
1364:.
1354:.
1346:.
1334:24
1332:.
1328:.
1309:.
1287:.
1277:.
1265:.
1261:.
1238:.
1230:.
1218:.
1195:.
1183:.
1171:^
1146:.
1136:22
1134:.
1130:.
1105:35
1103:.
1077:57
1075:.
1052:.
1042:.
1032:46
1030:.
1026:.
986:.
964:.
954:.
944:14
942:.
938:.
749:,
737:,
635:,
596:,
508:.
467:.
390:F:
385:E:
370:F:
365:E:
350:F:
345:E:
332:S
328:NO
326:41
322:27
318:F:
311:NO
309:39
305:26
301:E:
288:)
270:D:
265:C:
250:D:
245:C:
230:D:
225:C:
212:S
208:NO
206:41
202:27
198:D:
191:NO
189:39
185:26
181:C:
168:)
150:B:
145:A:
130:B:
125:A:
110:B:
105:A:
92:S
88:NO
86:41
82:27
78:B:
71:NO
69:39
65:26
61:A:
48:)
2242:)
2238:(
2232:)
2228:(
1861:)
1857:(
1760:e
1753:t
1746:v
1729:.
1715::
1709:7
1683:.
1679::
1656:.
1644::
1621:.
1609::
1586:.
1582::
1559:.
1555::
1528:.
1524::
1500:.
1496::
1490:3
1459:.
1408:.
1372:.
1340::
1295:.
1273::
1267:9
1246:.
1226::
1220:4
1203:.
1199::
1191::
1165:.
1142::
1115:.
1111::
1087:.
1060:.
1038::
996:.
972:.
950::
923:.
743:3
739:2
735:1
704:3
680:2
674:–
656:1
641:3
637:2
633:1
441:F
437:A
330:7
324:H
320:C
315:S
313:7
307:H
303:C
286:3
210:5
204:H
200:C
195:S
193:5
187:H
183:C
166:3
90:6
84:H
80:C
75:S
73:6
67:H
63:C
46:3
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.