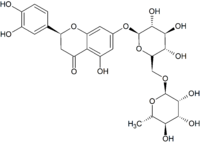566:
Eriomin in reducing hyperglycemia and improving diabetes-related biomarkers in individuals with hyperglycemia above 110 mg/dL (mean 123 ± 18 mg/dL). Subjects (n = 30), divided into two groups (Eriomin or
Placebo), who received a dose of 200 mg/d of the designated supplement for 12 weeks and, after a washout period of 2 weeks, switched to the other supplement in the following 12 weeks. Assessments of biochemical, metabolic, inflammatory, blood pressure, anthropometry, and dietary parameters were performed at the beginning and end of each intervention. Treatment with 200 mg/d of Eriomin significantly decreased blood glucose (−5%), homeostasis model assessment of insulin resistance (−11%), glucagon (−13%), interleukin-6 (−14%), tumor necrosis factor alpha (−20%), and alkaline phosphatase (−13%); but increased glucagon-like peptide 1 (GLP-1) by (17%) (P ≤ .05). At the end of the placebo period, there was a 13% increase in triglycerides (P ≤ .05). Other parameters evaluated did not change with Eriomin or placebo. In conclusion, intervention with Eriomin benefited the glycemic control of prediabetic and diabetic patients, with higher blood glucose levels, by increasing GLP-1 and decreasing systemic inflammation.
562:
metabolic biomarkers in prediabetic individuals. Prediabetes patients (n = 103, 49 ± 10 years) were randomly divided into four parallel groups: (a) Placebo; (b) Eriomin 200 mg; (c) Eriomin 400 mg; and (d) Eriomin 800 mg. Assessment of biochemical, metabolic, inflammatory, hepatic, renal, anthropometric markers, blood pressure, and dietary parameters were performed during 12 weeks of intervention. Treatment with all doses of
Eriomin (200, 400, and 800 mg) had similar effects and altered significantly the following variables: blood glucose (−5%), insulin resistance (−7%), glucose intolerance (−7%), glycated hemoglobin (−2%), glucagon (−6.5%), C-peptide (−5%), hsCRP (−12%), interleukin-6 (−13%), TNFα (−11%), lipid peroxidation (−17%), systolic blood pressure (−8%), GLP-1 (+15%), adiponectin (+19%), and antioxidant capacity (+6%). Eriomin or placebo did not influence the anthropometric and dietary variables. Short-term intervention with Eriomin, at doses of 200, 400, or 800 mg/day, benefited glycemic control, reduced systemic inflammation and oxidative stress, and reversed the prediabetic condition in 24% of the evaluated patients.
207:
924:
860:
460:
552:
996:
24:
164:
561:
The effectiveness of
Eriocitrin in managing hyperglycemia and reversal of prediabetes condition was demonstrated in a double-blind, randomized controlled study. This study evaluated the potential effectiveness of different doses of Eriomin on hyperglycemia and insulin resistance associated with other
565:
A published crossover-randomized clinical trial researched the nutraceutical
Eriocitrin (Eriomin) in reducing hyperglycemia by increasing glucagon-like peptide 1 and downregulates systemic inflammation. This double-blind, randomized, placebo/controlled, crossover study evaluated the efficacy of
445:
The compound has lipid-lowering properties in liver cells. It is marketed as a dietary supplement, usually in conjunction with B and C vitamins and other substances, but there is no established medical use or FDA approved application of the compound.
291:
InChI=1S/C27H32O15/c1-9-20(32)22(34)24(36)26(39-9)38-8-18-21(33)23(35)25(37)27(42-18)40-11-5-14(30)19-15(31)7-16(41-17(19)6-11)10-2-3-12(28)13(29)4-10/h2-6,9,16,18,20-30,32-37H,7-8H2,1H3/t9-,16-,18+,20-,21+,22+,23-,24+,25+,26+,27+/m0/s1
301:
InChI=1/C27H32O15/c1-9-20(32)22(34)24(36)26(39-9)38-8-18-21(33)23(35)25(37)27(42-18)40-11-5-14(30)19-15(31)7-16(41-17(19)6-11)10-2-3-12(28)13(29)4-10/h2-6,9,16,18,20-30,32-37H,7-8H2,1H3/t9-,16-,18+,20-,21+,22+,23-,24+,25+,26+,27+/m0/s1
599:
Cao X, He Y, Kong Y, Mei X, Huo Y, He Y, Liu J (September 2019). "Elucidating the interaction mechanism of eriocitrin with β-casein by multi-spectroscopic and molecular simulation methods".
883:"Nutraceutical Eriocitrin (Eriomin) Reduces Hyperglycemia by Increasing Glucagon-Like Peptide 1 and Downregulates Systemic Inflammation: A Crossover-Randomized Clinical Trial"
681:
Miyake Y, Yamamoto K, Morimitsu Y, Osawa T (1997-12-01). "Isolation of C -Glucosylflavone from Lemon Peel and
Antioxidative Activity of Flavonoid Compounds in Lemon Fruit".
470:
317:
1270:
1028:
819:"Effectiveness of Eriomin® in managing hyperglycemia and reversal of prediabetes condition: A double-blind, randomized, controlled study"
282:
718:"Isolation of Eriocitrin (Eriodictyol 7-rutinoside) from Lemon Fruit (Citrus limon Burm. f.) and Its Antioxidative Activity"
1489:
644:"Lipid-Lowering Effect of Eriocitrin, the Main Flavonoid in Lemon Fruit, in Rats on a High-Fat and High-Cholesterol Diet"
531:
259:
503:
389:
510:
947:
478:
1263:
1021:
434:
or a citrus flavonoid, one of the plant pigments that bring color to fruit and flowers. This antioxidant also
517:
489:
474:
202:
1079:
1000:
717:
50:
36:
643:
499:
948:"Chiral separation of diastereomeric flavanone-7-O-glycosides in citrus by capillary electrophoresis"
770:
1256:
1014:
759:"Eriocitrin ameliorates diet-induced hepatic steatosis with activation of mitochondrial biogenesis"
130:
642:
Miyake Y, Suzuki E, Ohya S, Fukumoto S, Hiramitsu M, Sakaida K, Osawa T, Furuichi Y (2006-11-13).
1221:
1211:
1107:
975:
624:
757:
Hiramitsu M, Shimada Y, Kuroyanagi J, Inoue T, Katagiri T, Zang L, et al. (January 2014).
1463:
1409:
967:
912:
848:
796:
739:
698:
663:
616:
1237:
1054:
959:
902:
894:
838:
830:
786:
778:
729:
690:
655:
608:
340:
430:. It is commonly found in lemons and other citrus fruits. It is colloquially called lemon
268:
184:
140:
1343:
1338:
1333:
1328:
1310:
1305:
1300:
1295:
1287:
1097:
928:
923:
864:
859:
206:
927: This article incorporates text from this source, which is available under the
863: This article incorporates text from this source, which is available under the
774:
524:
1216:
1102:
907:
882:
843:
818:
791:
758:
383:
1483:
1431:
1414:
1157:
659:
628:
195:
979:
424:
1006:
612:
248:
1419:
1376:
1366:
1113:
1069:
1059:
420:
103:-3,6-dioxa-2(2,7)-benzopyrana-4(2,6),7(2)-bis(oxana)-1(1)-benzenaheptaphan-2-one
734:
435:
1381:
1361:
1167:
1162:
1092:
1064:
439:
368:
175:
743:
702:
667:
620:
1468:
1451:
1446:
1441:
1436:
1426:
1404:
1371:
1353:
1279:
1195:
1178:
1144:
1087:
1046:
1038:
580:
431:
417:
413:
971:
963:
916:
852:
800:
995:
898:
1396:
1386:
1184:
1172:
1118:
427:
1458:
488:
if you can. Unsourced or poorly sourced material may be challenged and
235:
23:
782:
694:
1320:
1189:
1134:
834:
382:
Except where otherwise noted, data are given for materials in their
325:
C1((((O1)OC2((((O2)Oc3cc(c4c(c3)O(CC4=O)c5ccc(c(c5)O)O)O)O)O)O)O)O)O
223:
575:
163:
153:
214:
1252:
1010:
453:
1248:
817:
Ribeiro CB, Ramos FM, Manthey JA, Cesar TB (July 2019).
485:
99:)-1,1,2,4,4,4,7,7,7-Nonahydroxy-7-methyl-2,2-dihydro-2
1395:
1352:
1319:
1286:
1230:
1204:
1143:
1127:
1078:
1045:
946:Gel-Moreto N, Streich R, Galensa R (August 2003).
881:Cesar TB, Ramos FM, Ribeiro CB (November 2022).
722:Food Science and Technology International, Tokyo
247:
812:
810:
139:
484:Please review the contents of the section and
1264:
1022:
876:
874:
8:
1271:
1257:
1249:
1029:
1015:
1007:
683:Journal of Agricultural and Food Chemistry
205:
183:
15:
906:
842:
790:
733:
267:
591:
322:
287:
716:Miyake Y, Yamamoto K, Osawa T (1997).
196:
294:Key: OMQADRGFMLGFJF-MNPJBKLOSA-N
7:
304:Key: OMQADRGFMLGFJF-MNPJBKLOBI
238:
222:
44:)-3′,4′,5-Trihydroxy-7-flavan-4-one
14:
1175:(Narigenin 7-O-neohesperidoside)
994:
922:
858:
660:10.1111/j.1750-3841.2006.00192.x
550:
458:
352:
22:
386:(at 25 °C , 100 kPa).
486:add the appropriate references
358:
346:
1:
613:10.1016/j.foodhyd.2019.03.006
436:predominates (38% in 1 study)
1181:(Naringenin 7-O-rutinoside)
1110:(Pinocembrin-7-methylether)
471:reliable medical references
1506:
1192:(Naringenin-7-O-glucoside)
735:10.3136/fsti9596t9798.3.84
887:Journal of Medicinal Food
477:or relies too heavily on
380:
333:
313:
278:
123:
109:
49:
35:
30:
21:
1442:Anthraquinone glycoside
1128:C-methylated flavanones
1080:O-methylated flavanones
648:Journal of Food Science
964:10.1002/elps.200305486
1231:Acetylated glycosides
1037:Flavanones and their
899:10.1089/jmf.2021.0181
823:Phytotherapy Research
450:Clinical Significance
406:eriodictyol glycoside
112:Eriodictyol glycoside
51:Systematic IUPAC name
1490:Flavanone glycosides
1427:Cyanogenic glycoside
1003:at Wikimedia Commons
1452:Flavonoid glycoside
1405:Alcoholic glycoside
775:2014NatSR...4E3708H
408:) is a flavanone-7-
376: g·mol
18:
1447:Coumarin glycoside
1437:Phenolic glycoside
1222:Sophoraflavanone G
1212:8-Prenylnaringenin
763:Scientific Reports
601:Food Hydrocolloids
390:Infobox references
16:
1477:
1476:
1464:Steviol glycoside
1410:Cardiac glycoside
1311:C-glycosidic bond
1306:S-glycosidic bond
1301:N-glycosidic bond
1296:O-glycosidic bond
1246:
1245:
999:Media related to
958:(15): 2716–2722.
893:(11): 1050–1058.
783:10.1038/srep03708
695:10.1021/jf970498x
689:(12): 4619–4623.
559:
558:
535:
398:Chemical compound
396:
395:
165:Interactive image
1497:
1273:
1266:
1259:
1250:
1031:
1024:
1017:
1008:
998:
983:
933:
926:
920:
910:
878:
869:
862:
856:
846:
835:10.1002/ptr.6386
829:(7): 1921–1933.
814:
805:
804:
794:
754:
748:
747:
737:
713:
707:
706:
678:
672:
671:
654:(9): S633–S637.
639:
633:
632:
596:
554:
553:
545:
542:
536:
534:
493:
462:
461:
454:
375:
360:
354:
348:
341:Chemical formula
271:
251:
240:
226:
209:
198:
187:
167:
143:
26:
19:
1505:
1504:
1500:
1499:
1498:
1496:
1495:
1494:
1480:
1479:
1478:
1473:
1391:
1348:
1315:
1282:
1277:
1247:
1242:
1226:
1200:
1139:
1123:
1098:Homoeriodictyol
1074:
1041:
1035:
991:
986:
952:Electrophoresis
945:
941:
939:Further reading
936:
880:
879:
872:
816:
815:
808:
756:
755:
751:
715:
714:
710:
680:
679:
675:
641:
640:
636:
598:
597:
593:
589:
572:
555:
551:
546:
540:
537:
494:
483:
479:primary sources
463:
459:
452:
399:
392:
387:
373:
363:
357:
351:
343:
329:
326:
321:
320:
309:
306:
305:
302:
296:
295:
292:
286:
285:
274:
254:
241:
229:
190:
170:
157:
146:
133:
119:
113:
105:
104:
45:
12:
11:
5:
1503:
1501:
1493:
1492:
1482:
1481:
1475:
1474:
1472:
1471:
1466:
1461:
1456:
1455:
1454:
1449:
1444:
1434:
1429:
1424:
1423:
1422:
1417:
1407:
1401:
1399:
1393:
1392:
1390:
1389:
1384:
1379:
1374:
1369:
1364:
1358:
1356:
1350:
1349:
1347:
1346:
1341:
1336:
1331:
1325:
1323:
1317:
1316:
1314:
1313:
1308:
1303:
1298:
1292:
1290:
1284:
1283:
1278:
1276:
1275:
1268:
1261:
1253:
1244:
1243:
1241:
1240:
1234:
1232:
1228:
1227:
1225:
1224:
1219:
1217:Isoxanthohumol
1214:
1208:
1206:
1202:
1201:
1199:
1198:
1193:
1187:
1182:
1176:
1170:
1165:
1160:
1155:
1149:
1147:
1141:
1140:
1138:
1137:
1131:
1129:
1125:
1124:
1122:
1121:
1116:
1111:
1105:
1103:Isosakuranetin
1100:
1095:
1090:
1084:
1082:
1076:
1075:
1073:
1072:
1067:
1062:
1057:
1051:
1049:
1043:
1042:
1036:
1034:
1033:
1026:
1019:
1011:
1005:
1004:
990:
989:External links
987:
985:
984:
942:
940:
937:
935:
934:
870:
806:
749:
708:
673:
634:
590:
588:
585:
584:
583:
578:
571:
568:
557:
556:
549:
547:
466:
464:
457:
451:
448:
397:
394:
393:
388:
384:standard state
381:
378:
377:
371:
365:
364:
361:
355:
349:
344:
339:
336:
335:
331:
330:
328:
327:
324:
316:
315:
314:
311:
310:
308:
307:
303:
300:
299:
297:
293:
290:
289:
281:
280:
279:
276:
275:
273:
272:
264:
262:
256:
255:
253:
252:
244:
242:
234:
231:
230:
228:
227:
219:
217:
211:
210:
200:
192:
191:
189:
188:
180:
178:
172:
171:
169:
168:
160:
158:
151:
148:
147:
145:
144:
136:
134:
129:
126:
125:
121:
120:
114:Eriodictyol-7-
111:
107:
106:
54:
53:
47:
46:
39:
33:
32:
28:
27:
13:
10:
9:
6:
4:
3:
2:
1502:
1491:
1488:
1487:
1485:
1470:
1469:Thioglycoside
1467:
1465:
1462:
1460:
1457:
1453:
1450:
1448:
1445:
1443:
1440:
1439:
1438:
1435:
1433:
1432:Glycosylamine
1430:
1428:
1425:
1421:
1418:
1416:
1415:Bufadienolide
1413:
1412:
1411:
1408:
1406:
1403:
1402:
1400:
1398:
1394:
1388:
1385:
1383:
1380:
1378:
1375:
1373:
1370:
1368:
1365:
1363:
1360:
1359:
1357:
1355:
1351:
1345:
1344:1,6-Glycoside
1342:
1340:
1339:1,4-Glycoside
1337:
1335:
1332:
1330:
1327:
1326:
1324:
1322:
1318:
1312:
1309:
1307:
1304:
1302:
1299:
1297:
1294:
1293:
1291:
1289:
1285:
1281:
1274:
1269:
1267:
1262:
1260:
1255:
1254:
1251:
1239:
1236:
1235:
1233:
1229:
1223:
1220:
1218:
1215:
1213:
1210:
1209:
1207:
1203:
1197:
1194:
1191:
1188:
1186:
1183:
1180:
1177:
1174:
1171:
1169:
1166:
1164:
1161:
1159:
1158:Neoeriocitrin
1156:
1154:
1151:
1150:
1148:
1146:
1142:
1136:
1133:
1132:
1130:
1126:
1120:
1117:
1115:
1112:
1109:
1106:
1104:
1101:
1099:
1096:
1094:
1091:
1089:
1086:
1085:
1083:
1081:
1077:
1071:
1068:
1066:
1063:
1061:
1058:
1056:
1053:
1052:
1050:
1048:
1044:
1040:
1032:
1027:
1025:
1020:
1018:
1013:
1012:
1009:
1002:
997:
993:
992:
988:
981:
977:
973:
969:
965:
961:
957:
953:
949:
944:
943:
938:
932:
930:
925:
918:
914:
909:
904:
900:
896:
892:
888:
884:
877:
875:
871:
868:
866:
861:
854:
850:
845:
840:
836:
832:
828:
824:
820:
813:
811:
807:
802:
798:
793:
788:
784:
780:
776:
772:
768:
764:
760:
753:
750:
745:
741:
736:
731:
727:
723:
719:
712:
709:
704:
700:
696:
692:
688:
684:
677:
674:
669:
665:
661:
657:
653:
649:
645:
638:
635:
630:
626:
622:
618:
614:
610:
606:
602:
595:
592:
586:
582:
579:
577:
574:
573:
569:
567:
563:
548:
544:
533:
530:
526:
523:
519:
516:
512:
509:
505:
502: –
501:
497:
496:Find sources:
491:
487:
481:
480:
476:
472:
467:This section
465:
456:
455:
449:
447:
443:
441:
437:
433:
429:
426:
422:
419:
415:
411:
407:
403:
391:
385:
379:
372:
370:
367:
366:
345:
342:
338:
337:
332:
323:
319:
312:
298:
288:
284:
277:
270:
266:
265:
263:
261:
258:
257:
250:
246:
245:
243:
237:
233:
232:
225:
221:
220:
218:
216:
213:
212:
208:
204:
201:
199:
197:ECHA InfoCard
194:
193:
186:
182:
181:
179:
177:
174:
173:
166:
162:
161:
159:
155:
150:
149:
142:
138:
137:
135:
132:
128:
127:
122:
117:
108:
102:
98:
94:
90:
86:
82:
78:
74:
70:
66:
62:
58:
52:
48:
43:
38:
34:
29:
25:
20:
1152:
955:
951:
921:
890:
886:
857:
826:
822:
766:
762:
752:
728:(1): 84–89.
725:
721:
711:
686:
682:
676:
651:
647:
637:
604:
600:
594:
564:
560:
538:
528:
521:
514:
507:
500:"Eriocitrin"
495:
475:verification
468:
444:
425:disaccharide
416:between the
409:
405:
401:
400:
124:Identifiers
115:
110:Other names
100:
96:
92:
88:
84:
80:
76:
72:
68:
64:
60:
56:
41:
1420:Cardenolide
1377:Glucuronide
1367:Galactoside
1334:β-Glycoside
1329:α-Glycoside
1114:Sakuranetin
1108:Pinostrobin
1070:Pinocembrin
1060:Eriodictyol
469:needs more
442:infusions.
421:eriodictyol
334:Properties
203:100.033.321
118:-rutinoside
17:Eriocitrin
1382:Rhamnoside
1362:Fructoside
1280:Glycosides
1205:Acetylated
1168:Liquiritin
1163:Hesperidin
1153:Eriocitrin
1145:Glycosides
1093:Hesperetin
1065:Naringenin
1047:Flavanones
1039:glycosides
1001:Eriocitrin
587:References
541:April 2023
511:newspapers
440:Peppermint
402:Eriocitrin
369:Molar mass
269:AS293HR5XQ
176:ChemSpider
152:3D model (
141:13463-28-0
131:CAS Number
37:IUPAC name
1372:Glucoside
1196:Sakuranin
1179:Narirutin
1088:Alpinetin
929:CC BY 4.0
865:CC BY 4.0
744:1881-3976
703:0021-8561
668:0022-1147
629:107490400
621:0268-005X
607:: 63–70.
581:Quercetin
432:flavonoid
418:flavanone
414:glycoside
1484:Category
1397:Aglycone
1387:Riboside
1321:Geometry
1185:Poncirin
1173:Naringin
1119:Sterubin
980:40261445
972:12900888
931:license.
917:35796695
867:license.
853:31183921
801:24424211
769:: 3708.
570:See also
428:rutinose
423:and the
1459:Saponin
1354:Glycone
1238:Nirurin
908:9700344
844:6618084
792:3892443
771:Bibcode
525:scholar
490:removed
374:596.538
236:PubChem
1190:Prunin
1135:Poriol
978:
970:
915:
905:
851:
841:
799:
789:
742:
701:
666:
627:
619:
527:
520:
513:
506:
498:
318:SMILES
224:C09732
31:Names
1055:Butin
976:S2CID
625:S2CID
576:Rutin
532:JSTOR
518:books
283:InChI
249:83489
185:75327
154:JSmol
1288:Bond
968:PMID
913:PMID
849:PMID
797:PMID
740:ISSN
699:ISSN
664:ISSN
617:ISSN
504:news
473:for
260:UNII
215:KEGG
960:doi
903:PMC
895:doi
839:PMC
831:doi
787:PMC
779:doi
730:doi
691:doi
656:doi
609:doi
438:in
239:CID
1486::
974:.
966:.
956:24
954:.
950:.
911:.
901:.
891:25
889:.
885:.
873:^
847:.
837:.
827:33
825:.
821:.
809:^
795:.
785:.
777:.
765:.
761:.
738:.
724:.
720:.
697:.
687:45
685:.
662:.
652:71
650:.
646:.
623:.
615:.
605:94
603:.
492:.
362:15
356:32
350:27
95:,7
91:,7
87:,7
83:,7
79:,7
75:,4
71:,4
67:,4
63:,4
59:,4
55:(2
40:(2
1272:e
1265:t
1258:v
1030:e
1023:t
1016:v
982:.
962::
919:.
897::
855:.
833::
803:.
781::
773::
767:4
746:.
732::
726:3
705:.
693::
670:.
658::
631:.
611::
543:)
539:(
529:·
522:·
515:·
508:·
482:.
412:-
410:O
404:(
359:O
353:H
347:C
156:)
116:O
101:H
97:S
93:R
89:R
85:R
81:R
77:R
73:S
69:S
65:R
61:S
57:S
42:S
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.