336:
29:
555:
in the United States. The suspension should be warmed gently in the hands for 1–2 minutes before administration to prevent dizziness, and shaken before use. It is necessary to remain still for 1 minute, with the affected ear facing up while lying on one's side, after administration to allow
826:
approved medication in the United States that was first developed by a
Singaporean drug company. Finafloxacin was officially approved by the FDA on December 17, 2014. The company, MerLion Pharmaceuticals, partnered with the North American company
746:, meaning that a substantial portion of a dose taken by mouth reaches a person's systemic circulation. Some people have experienced unintentional, quantifiable absorption of finafloxacin into systemic circulation after administering the drug
711:, despite the harsh acidity) thrive. Other acidic conditions found on the human body include the vagina, urinary tract, and skin, though finafloxacin is currently not used to treat infections in these areas either.
1208:"Activity of finafloxacin, a novel fluoroquinolone with increased activity at acid pH, towards extracellular and intracellular Staphylococcus aureus, Listeria monocytogenes and Legionella pneumophila"
597:
The spectrum of adverse effects caused by finafloxacin vary by the method of administration. People that have administered finafloxacin into their ears in the form of drops have experienced ear
812:
640:
in that population. Adverse effects consistent with an allergic reaction to finafloxacin may include swelling of the lips, tongue, or throat, difficulty swallowing, and shortness of breath.
412:
685:
subclass (referring to the CN substituent at the 8th position). Like other fluoroquinolones, its antibiotic activity is derived from its pharmacological mechanism of action as a
63:
864:
780:
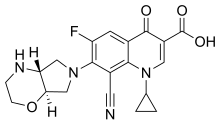
There are some notable differences between the chemistry of finafloxacin and related fluoroquinolones. For example, the 8-cyano-substituent is not found in ciprofloxacin, and
660:
Owing to the local effect of administering finafloxacin into the ears, it is unlikely that it will affect or be affected by other medications that are administered into the
520:
bacteria. In the clinical trial that led to the drug's approval, finafloxacin shortened the time to cessation of ear pain from an average of 6.8 days in patients taking a
1411:
773:-substituent and 7-pyrrolo-oxazinyl moiety." Its low isoelectric point (pH 6.7) is lower than the isoelectric point of another fluoroquinolone class antibiotic called
454:
InChI=1S/C20H19FN4O4/c21-14-5-11-17(25(10-1-2-10)7-13(19(11)26)20(27)28)12(6-22)18(14)24-8-15-16(9-24)29-4-3-23-15/h5,7,10,15-16,23H,1-4,8-9H2,(H,27,28)/t15-,16-/m0/s1
426:
979:
1328:
1080:"Chemical structure and pharmacokinetics of novel quinolone agents represented by avarofloxacin, delafloxacin, finafloxacin, zabofloxacin and nemonoxacin"
2351:
811:
2307:
1404:
1350:
Bartoletti R, Cai T, Perletti G, Wagenlehner FM, Bjerklund
Johansen TE (2015). "Finafloxacin for the treatment of urinary tract infections".
2356:
1518:
1207:
1463:
446:
1397:
847:
in the future. Finafloxacin's manufacturer, MerLion, has invested money in studying the use of finafloxacin for this indication.
149:
84:
1277:
2366:
719:
1251:
1754:
807:
The synthesis of finafloxacin has been described in detail in its patents. An example of its synthesis is provided below:
2243:
896:
481:
235:
581:
The efficacy and safety profile of finafloxacin ear drops are unknown in children younger than the age of 1 years old.
2324:
315:
921:
477:
2361:
731:
1769:
844:
636:
People that are allergic to other quinolones may be allergic to finafloxacin as well, and use may result in an
2312:
1820:
686:
51:
1764:
892:
2196:
1668:
1635:
1607:
1565:
1528:
1513:
1424:
552:
548:
510:
490:
304:
1591:
1587:
1471:
1389:
754:
516:
496:
2085:
2013:
1965:
1899:
1890:
1842:
1814:
661:
128:
788:-substituent instead. The oxygen atom in the ring structure of finafloxacin makes the molecule more
331:
1940:
703:
625:, musculoskeletal problems, and injection site reactions (if IV). Respiratory disorders, including
170:
1714:
2191:
1663:
1375:
1051:
570:
2371:
1447:
1367:
1230:
1188:
1111:
1043:
637:
618:
532:
284:
110:
98:
41:
224:
1936:
1759:
1643:
1615:
1359:
1222:
1178:
1170:
1101:
1091:
1035:
528:
508:
Finafloxacin is used to treat a type of ear infection called acute otitis externa caused by
352:
179:
120:
244:
1832:
1658:
743:
678:
630:
335:
1329:"Singapore's MerLion eyes partner for Phase III of Finafloxacin urinary infection trials"
2318:
2205:
2075:
1875:
1870:
1860:
1724:
1719:
1683:
1541:
1183:
1158:
1106:
1079:
485:
1226:
990:. Contemporary Clinic 2017 Pharmacy & Healthcare Communications, LLC. 31 July 2015
697:. However, unlike other fluoroquinolones, finafloxacin is highly active under acidic (
2345:
2173:
2123:
2113:
2071:
2061:
2051:
1983:
1907:
1865:
1785:
1709:
1678:
1597:
1583:
1571:
1561:
1555:
1551:
1546:
1536:
1491:
1055:
869:
796:
785:
774:
727:
723:
690:
606:
204:
1379:
954:
2128:
2118:
2103:
2093:
2066:
2046:
2041:
2031:
2026:
2021:
2003:
1998:
1993:
1988:
1978:
1973:
1932:
1927:
1729:
1620:
1501:
1432:
1428:
781:
777:(pH 7.4), which accounts for finafloxacin's superior activity at low pH (5.0–6.0).
715:
694:
1363:
718:
activity against a range of bacterial pathogens, especially at acidic pH, with a
2289:
2281:
2261:
2214:
2148:
2108:
2056:
1945:
1795:
1734:
1688:
1648:
1625:
1496:
1476:
1420:
789:
573:
C, meaning that the risk for harming a developing fetus has not been ruled out.
2251:
2177:
2164:
2153:
2098:
1955:
1950:
1922:
1855:
1825:
1673:
1486:
1441:
1096:
1039:
614:
589:
There are no limitations against using finafloxacin ear drops in the elderly.
388:
215:
1174:
20:
2271:
2266:
2256:
2238:
1912:
1880:
1850:
1800:
1653:
1576:
766:
765:
The chemical structure of finafloxacin has been described as a "fluorinated
622:
1371:
1299:
1234:
1192:
1115:
1047:
839:
Owing to its high bactericidal activity in acidic environments, Bartoletti
605:(<1% for both). People that have administered finafloxacin by mouth or
28:
1917:
1704:
1481:
747:
626:
610:
556:
finafloxacin to penetrate the ear canal and reach the site of infection.
1790:
708:
598:
521:
190:
795:
The chemical structure of finafloxacin is nearly identical to that of
602:
295:
843:
have speculated that finafloxacin may be useful in the treatment of
1159:"Who are we? Indigenous microbes and the ecology of human diseases"
1132:
264:
828:
770:
682:
411:
402:
275:
255:
1393:
1026:
McKeage K (April 2015). "Finafloxacin: first global approval".
609:(IV) have experienced gastrointestinal side effects (including
104:
2228:
823:
544:
922:"Finafloxacin: New fluoroquinolone for acute otitis externa"
320:
698:
633:, have also been associated with the use of finafloxacin.
1278:"Biotech sector poised to deliver more health and wealth"
1322:
1320:
1127:
1125:
865:"Health Canada New Drug Authorizations: 2016 Highlights"
652:
is likely to cause severe or life-threatening symptoms.
93:
831:
to produce the drug commercially in the United States.
1073:
1071:
1069:
1067:
1065:
757:
of finafloxacin is approximately 10 hours in humans.
434:
O=C(O)C1=CN(C2CC2)c3c(C1=O)cc(F)c(c3C#N)N4C5(C4)NCCO5
928:. American Pharmacists Association. February 1, 2015
2280:
2237:
2227:
2204:
2190:
2162:
2141:
2084:
2012:
1964:
1898:
1889:
1841:
1813:
1778:
1747:
1697:
1634:
1606:
1527:
1512:
1462:
1440:
400:
387:
351:
346:
314:
294:
274:
254:
234:
214:
189:
169:
140:
127:
119:
83:
78:
62:
50:
40:
35:
1206:Lemaire S, Van Bambeke F, Tulkens PM (July 2011).
1084:Annals of Clinical Microbiology and Antimicrobials
701:5.0–6.0) conditions, where certain bacteria (like
161:)-yl]-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid
980:"Rx Update: Xtoro (Finafloxacin Otic Suspension)"
543:Finafloxacin is commercially available as a 0.3%
1021:
1019:
1017:
1015:
1013:
1011:
1009:
1007:
1005:
707:, a bacterium that is known to infect the human
203:
480:. In the United States, it is approved by the
178:
1246:
1244:
664:(e.g. drugs taken by mouth, or by injection).
1405:
1215:International Journal of Antimicrobial Agents
916:
914:
912:
910:
908:
906:
8:
19:
949:
947:
945:
943:
893:"FDA approves Xtoro to treat swimmer's ear"
157:8-Cyano-1-cyclopropyl-6-fluoro-7-oxazin-6(2
2234:
2201:
1895:
1838:
1524:
1459:
1412:
1398:
1390:
730:bacteria, finafloxacin is classified as a
648:It is not thought that an overdose of the
334:
223:
27:
1182:
1105:
1095:
1078:Kocsis B, Domokos J, Szabo D (May 2016).
243:
1352:Expert Opinion on Investigational Drugs
856:
488:(swimmer's ear) caused by the bacteria
451:
431:
330:
154:
18:
1280:. SPH Digital News. The Straits Times
722:. Owing to its activity against both
303:
283:
7:
984:contemporaryclinic.pharmacytimes.com
263:
194:
14:
1227:10.1016/j.ijantimicag.2011.03.002
527:Finafloxacin cannot be purchased
810:
372:
369:
363:
459:Key:FYMHQCNFKNMJAV-HOTGVXAUSA-N
2352:Drugs not assigned an ATC code
714:Finafloxacin has demonstrated
569:Finafloxacin is classified as
378:
357:
1:
1755:Trimethoprim/sulfamethoxazole
1364:10.1517/13543784.2015.1052401
1331:. Questex LLC. FierceBiotech
1327:Lane EJ (January 27, 2015).
897:Food and Drug Administration
693:and performing other vital,
687:type II topoisomerase poison
482:Food and Drug Administration
2357:Fluoroquinolone antibiotics
1760:Ormetoprim/sulfadimethoxine
955:"finafloxacin (Otic route)"
742:Finafloxacin has good oral
689:, preventing bacteria from
2388:
1157:Blaser MJ (October 2006).
822:Finafloxacin is the first
681:class antibiotic of the 8-
478:fluoroquinolone antibiotic
347:Chemical and physical data
2302:
1770:Pyrimethamine/sulfadoxine
1097:10.1186/s12941-016-0150-4
1040:10.1007/s40265-015-0384-z
732:broad-spectrum antibiotic
442:
422:
145:
26:
1300:"Xtoro Approval History"
1276:Poh LC (July 29, 2017).
1175:10.1038/sj.embor.7400812
845:urinary tract infections
547:(meaning "for the ear")
1669:Sulfamethoxypyridazine
720:post-antibiotic effect
553:topical administration
531:, and is available by
511:Pseudomonas aeruginosa
491:Pseudomonas aeruginosa
58:otic, oral, intavenous
2367:Cyclopropyl compounds
2142:Newer non-fluorinated
1779:Other DHPS inhibitors
1765:Pyrimethamine/dapsone
1592:Succinylsulfathiazole
1588:Phthalylsulfathiazole
1472:2,4-Diaminopyrimidine
755:elimination half-life
517:Staphylococcus aureus
497:Staphylococcus aureus
1566:Acetyl sulfisoxazole
1425:inhibit nucleic acid
899:. December 17, 2014.
792:than ciprofloxacin.
662:systemic circulation
560:Specific populations
1941:Alalevonadifloxacin
1608:Intermediate-acting
704:Helicobacter pylori
673:Mechanism of action
23:
2329:Never to phase III
1830:thereby inhibiting
1818:(inhibit bacterial
1664:Sulfametoxydiazine
1452:thereby inhibiting
1445:(inhibit bacterial
769:derivative with 8-
695:cellular functions
677:Finafloxacin is a
571:pregnancy category
2339:
2338:
2298:
2297:
2223:
2222:
2186:
2185:
2137:
2136:
1809:
1808:
1743:
1742:
1448:purine metabolism
638:allergic reaction
533:prescription only
467:
466:
413:Interactive image
316:CompTox Dashboard
108:
96:
16:Chemical compound
2379:
2362:Carboxylic acids
2235:
2202:
1937:Levonadifloxacin
1896:
1891:Fluoroquinolones
1839:
1644:Sulfadimethoxine
1616:Sulfamethoxazole
1537:Sulfaisodimidine
1525:
1460:
1414:
1407:
1400:
1391:
1384:
1383:
1347:
1341:
1340:
1338:
1336:
1324:
1315:
1314:
1312:
1310:
1296:
1290:
1289:
1287:
1285:
1273:
1267:
1266:
1264:
1262:
1248:
1239:
1238:
1212:
1203:
1197:
1196:
1186:
1154:
1148:
1147:
1145:
1143:
1129:
1120:
1119:
1109:
1099:
1075:
1060:
1059:
1023:
1000:
999:
997:
995:
976:
970:
969:
967:
965:
951:
938:
937:
935:
933:
918:
901:
900:
889:
883:
882:
880:
878:
861:
814:
738:Pharmacokinetics
529:over-the-counter
415:
395:
380:
374:
371:
365:
359:
339:
338:
324:
322:
307:
287:
267:
247:
227:
207:
197:
196:
182:
132:
106:
103:
95:
92:
31:
24:
22:
2387:
2386:
2382:
2381:
2380:
2378:
2377:
2376:
2342:
2341:
2340:
2335:
2334:
2319:Clinical trials
2294:
2276:
2242:
2219:
2195:
2182:
2158:
2133:
2080:
2008:
1960:
1885:
1833:DNA replication
1831:
1829:
1823:
1819:
1817:
1805:
1774:
1739:
1698:Other/ungrouped
1693:
1659:Sulfametomidine
1630:
1602:
1516:
1508:
1455:
1453:
1451:
1446:
1444:
1436:
1418:
1388:
1387:
1349:
1348:
1344:
1334:
1332:
1326:
1325:
1318:
1308:
1306:
1298:
1297:
1293:
1283:
1281:
1275:
1274:
1270:
1260:
1258:
1256:pharmacodia.com
1250:
1249:
1242:
1210:
1205:
1204:
1200:
1156:
1155:
1151:
1141:
1139:
1131:
1130:
1123:
1077:
1076:
1063:
1025:
1024:
1003:
993:
991:
986:. August 2015.
978:
977:
973:
963:
961:
953:
952:
941:
931:
929:
920:
919:
904:
891:
890:
886:
876:
874:
873:. 14 March 2017
863:
862:
858:
853:
837:
820:
805:
763:
744:bioavailability
740:
679:fluoroquinolone
675:
670:
658:
650:otic suspension
646:
631:nasopharyngitis
617:, and nausea),
595:
593:Adverse effects
587:
579:
567:
562:
541:
539:Available forms
506:
484:to treat acute
463:
460:
455:
450:
449:
438:
435:
430:
429:
418:
393:
383:
377:
368:
362:
342:
318:
310:
290:
270:
250:
230:
210:
193:
185:
165:
162:
153:
152:
130:
121:Pharmacokinetic
115:
74:
53:
17:
12:
11:
5:
2385:
2383:
2375:
2374:
2369:
2364:
2359:
2354:
2344:
2343:
2337:
2336:
2333:
2332:
2331:
2330:
2327:
2316:
2310:
2304:
2303:
2300:
2299:
2296:
2295:
2293:
2292:
2286:
2284:
2278:
2277:
2275:
2274:
2269:
2264:
2259:
2254:
2248:
2246:
2244:RNA polymerase
2232:
2225:
2224:
2221:
2220:
2218:
2217:
2211:
2209:
2206:Nitroimidazole
2199:
2188:
2187:
2184:
2183:
2181:
2180:
2174:Aminocoumarins
2170:
2168:
2160:
2159:
2157:
2156:
2151:
2145:
2143:
2139:
2138:
2135:
2134:
2132:
2131:
2126:
2121:
2116:
2111:
2106:
2101:
2096:
2090:
2088:
2082:
2081:
2079:
2078:
2076:Alatrofloxacin
2069:
2064:
2059:
2054:
2049:
2044:
2039:
2034:
2029:
2024:
2018:
2016:
2014:4th generation
2010:
2009:
2007:
2006:
2001:
1996:
1991:
1986:
1981:
1976:
1970:
1968:
1966:3rd generation
1962:
1961:
1959:
1958:
1953:
1948:
1943:
1930:
1925:
1920:
1915:
1910:
1904:
1902:
1900:2nd generation
1893:
1887:
1886:
1884:
1883:
1878:
1876:Piromidic acid
1873:
1871:Pipemidic acid
1868:
1863:
1861:Nalidixic acid
1858:
1853:
1847:
1845:
1843:1st generation
1836:
1811:
1810:
1807:
1806:
1804:
1803:
1798:
1793:
1788:
1782:
1780:
1776:
1775:
1773:
1772:
1767:
1762:
1757:
1751:
1749:
1745:
1744:
1741:
1740:
1738:
1737:
1732:
1727:
1725:Sulfaguanidine
1722:
1720:Sulfadicramide
1717:
1712:
1707:
1701:
1699:
1695:
1694:
1692:
1691:
1686:
1684:Sulfaphenazole
1681:
1676:
1671:
1666:
1661:
1656:
1651:
1646:
1640:
1638:
1632:
1631:
1629:
1628:
1623:
1618:
1612:
1610:
1604:
1603:
1601:
1600:
1595:
1581:
1580:
1579:
1569:
1559:
1549:
1544:
1542:Sulfamethizole
1539:
1533:
1531:
1522:
1519:DHPS inhibitor
1510:
1509:
1507:
1506:
1505:
1504:
1499:
1494:
1489:
1484:
1479:
1468:
1466:
1464:DHFR inhibitor
1457:
1438:
1437:
1421:Antibacterials
1419:
1417:
1416:
1409:
1402:
1394:
1386:
1385:
1342:
1316:
1291:
1268:
1252:"Finafloxacin"
1240:
1198:
1169:(10): 956–60.
1149:
1121:
1061:
1001:
971:
939:
926:pharmacist.com
902:
884:
855:
854:
852:
849:
836:
833:
819:
816:
804:
801:
762:
759:
739:
736:
674:
671:
669:
666:
657:
654:
645:
642:
594:
591:
586:
583:
578:
575:
566:
563:
561:
558:
540:
537:
505:
502:
486:otitis externa
465:
464:
462:
461:
458:
456:
453:
445:
444:
443:
440:
439:
437:
436:
433:
425:
424:
423:
420:
419:
417:
416:
408:
406:
398:
397:
391:
385:
384:
381:
375:
366:
360:
355:
349:
348:
344:
343:
341:
340:
332:DTXSID10175096
327:
325:
312:
311:
309:
308:
300:
298:
292:
291:
289:
288:
280:
278:
272:
271:
269:
268:
260:
258:
252:
251:
249:
248:
240:
238:
232:
231:
229:
228:
220:
218:
212:
211:
209:
208:
200:
198:
187:
186:
184:
183:
175:
173:
167:
166:
164:
163:
156:
148:
147:
146:
143:
142:
138:
137:
134:
125:
124:
117:
116:
114:
113:
101:
89:
87:
81:
80:
76:
75:
73:
72:
68:
66:
60:
59:
56:
54:administration
48:
47:
44:
38:
37:
33:
32:
15:
13:
10:
9:
6:
4:
3:
2:
2384:
2373:
2370:
2368:
2365:
2363:
2360:
2358:
2355:
2353:
2350:
2349:
2347:
2328:
2326:
2323:
2322:
2320:
2317:
2314:
2311:
2309:
2306:
2305:
2301:
2291:
2288:
2287:
2285:
2283:
2279:
2273:
2270:
2268:
2265:
2263:
2260:
2258:
2255:
2253:
2250:
2249:
2247:
2245:
2240:
2236:
2233:
2230:
2226:
2216:
2213:
2212:
2210:
2207:
2203:
2200:
2198:
2193:
2189:
2179:
2175:
2172:
2171:
2169:
2166:
2161:
2155:
2152:
2150:
2147:
2146:
2144:
2140:
2130:
2127:
2125:
2124:Pradofloxacin
2122:
2120:
2117:
2115:
2114:Marbofloxacin
2112:
2110:
2107:
2105:
2102:
2100:
2097:
2095:
2092:
2091:
2089:
2087:
2083:
2077:
2073:
2072:Trovafloxacin
2070:
2068:
2065:
2063:
2062:Prulifloxacin
2060:
2058:
2055:
2053:
2052:Clinafloxacin
2050:
2048:
2045:
2043:
2040:
2038:
2035:
2033:
2030:
2028:
2025:
2023:
2020:
2019:
2017:
2015:
2011:
2005:
2002:
2000:
1997:
1995:
1992:
1990:
1987:
1985:
1984:Grepafloxacin
1982:
1980:
1977:
1975:
1972:
1971:
1969:
1967:
1963:
1957:
1954:
1952:
1949:
1947:
1944:
1942:
1938:
1934:
1931:
1929:
1926:
1924:
1921:
1919:
1916:
1914:
1911:
1909:
1908:Ciprofloxacin
1906:
1905:
1903:
1901:
1897:
1894:
1892:
1888:
1882:
1879:
1877:
1874:
1872:
1869:
1867:
1866:Oxolinic acid
1864:
1862:
1859:
1857:
1854:
1852:
1849:
1848:
1846:
1844:
1840:
1837:
1834:
1827:
1822:
1821:topoisomerase
1816:
1812:
1802:
1799:
1797:
1794:
1792:
1789:
1787:
1786:Acediasulfone
1784:
1783:
1781:
1777:
1771:
1768:
1766:
1763:
1761:
1758:
1756:
1753:
1752:
1750:
1746:
1736:
1733:
1731:
1728:
1726:
1723:
1721:
1718:
1716:
1713:
1711:
1710:Sulfacetamide
1708:
1706:
1703:
1702:
1700:
1696:
1690:
1687:
1685:
1682:
1680:
1679:Sulfamerazine
1677:
1675:
1672:
1670:
1667:
1665:
1662:
1660:
1657:
1655:
1652:
1650:
1647:
1645:
1642:
1641:
1639:
1637:
1633:
1627:
1624:
1622:
1619:
1617:
1614:
1613:
1611:
1609:
1605:
1599:
1598:Sulfathiourea
1596:
1593:
1589:
1585:
1584:Sulfathiazole
1582:
1578:
1575:
1574:
1573:
1572:Sulfanilamide
1570:
1567:
1563:
1562:Sulfafurazole
1560:
1557:
1556:Sulfasalazine
1553:
1552:Sulfapyridine
1550:
1548:
1547:Sulfadimidine
1545:
1543:
1540:
1538:
1535:
1534:
1532:
1530:
1526:
1523:
1520:
1515:
1511:
1503:
1500:
1498:
1495:
1493:
1492:Pyrimethamine
1490:
1488:
1485:
1483:
1480:
1478:
1475:
1474:
1473:
1470:
1469:
1467:
1465:
1461:
1458:
1449:
1443:
1439:
1434:
1430:
1426:
1422:
1415:
1410:
1408:
1403:
1401:
1396:
1395:
1392:
1381:
1377:
1373:
1369:
1365:
1361:
1358:(7): 957–63.
1357:
1353:
1346:
1343:
1330:
1323:
1321:
1317:
1305:
1301:
1295:
1292:
1279:
1272:
1269:
1257:
1253:
1247:
1245:
1241:
1236:
1232:
1228:
1224:
1220:
1216:
1209:
1202:
1199:
1194:
1190:
1185:
1180:
1176:
1172:
1168:
1164:
1160:
1153:
1150:
1138:
1134:
1128:
1126:
1122:
1117:
1113:
1108:
1103:
1098:
1093:
1089:
1085:
1081:
1074:
1072:
1070:
1068:
1066:
1062:
1057:
1053:
1049:
1045:
1041:
1037:
1034:(6): 687–93.
1033:
1029:
1022:
1020:
1018:
1016:
1014:
1012:
1010:
1008:
1006:
1002:
989:
985:
981:
975:
972:
960:
956:
950:
948:
946:
944:
940:
927:
923:
917:
915:
913:
911:
909:
907:
903:
898:
894:
888:
885:
872:
871:
870:Health Canada
866:
860:
857:
850:
848:
846:
842:
834:
832:
830:
825:
817:
815:
813:
808:
802:
800:
798:
797:pradofloxacin
793:
791:
787:
783:
778:
776:
775:ciprofloxacin
772:
768:
760:
758:
756:
751:
749:
745:
737:
735:
733:
729:
728:Gram-negative
725:
724:Gram-positive
721:
717:
712:
710:
706:
705:
700:
696:
692:
688:
684:
680:
672:
667:
665:
663:
655:
653:
651:
643:
641:
639:
634:
632:
628:
624:
620:
616:
612:
608:
607:intravenously
604:
600:
592:
590:
584:
582:
576:
574:
572:
564:
559:
557:
554:
550:
546:
538:
536:
534:
530:
525:
524:to 3.5 days.
523:
519:
518:
513:
512:
503:
501:
499:
498:
493:
492:
487:
483:
479:
475:
471:
457:
452:
448:
441:
432:
428:
421:
414:
410:
409:
407:
404:
399:
392:
390:
386:
356:
354:
350:
345:
337:
333:
329:
328:
326:
317:
313:
306:
305:ChEMBL1908370
302:
301:
299:
297:
293:
286:
282:
281:
279:
277:
273:
266:
262:
261:
259:
257:
253:
246:
242:
241:
239:
237:
233:
226:
222:
221:
219:
217:
213:
206:
202:
201:
199:
192:
188:
181:
177:
176:
174:
172:
168:
160:
155:
151:
144:
139:
135:
133:
126:
122:
118:
112:
102:
100:
91:
90:
88:
86:
82:
77:
70:
69:
67:
65:
61:
57:
55:
49:
45:
43:
39:
36:Clinical data
34:
30:
25:
2282:Lipiarmycins
2129:Sarafloxacin
2119:Orbifloxacin
2104:Enrofloxacin
2094:Danofloxacin
2067:Sitafloxacin
2047:Moxifloxacin
2042:Gemifloxacin
2037:Finafloxacin
2036:
2032:Gatifloxacin
2027:Delafloxacin
2022:Besifloxacin
2004:Tosufloxacin
1999:Temafloxacin
1994:Sparfloxacin
1989:Pazufloxacin
1979:Balofloxacin
1974:Levofloxacin
1933:Nadifloxacin
1928:Lomefloxacin
1748:Combinations
1730:Sulfametrole
1715:Sulfaclozine
1621:Sulfadiazine
1529:Short-acting
1514:Sulfonamides
1502:Trimethoprim
1355:
1351:
1345:
1333:. Retrieved
1307:. Retrieved
1303:
1294:
1282:. Retrieved
1271:
1259:. Retrieved
1255:
1218:
1214:
1201:
1166:
1163:EMBO Reports
1162:
1152:
1140:. Retrieved
1136:
1087:
1083:
1031:
1027:
992:. Retrieved
987:
983:
974:
962:. Retrieved
958:
930:. Retrieved
925:
887:
875:. Retrieved
868:
859:
840:
838:
821:
809:
806:
794:
782:moxifloxacin
779:
764:
752:
741:
716:bactericidal
713:
702:
676:
668:Pharmacology
659:
656:Interactions
649:
647:
635:
596:
588:
580:
568:
542:
526:
515:
509:
507:
504:Medical uses
495:
489:
473:
470:Finafloxacin
469:
468:
158:
129:Elimination
85:Legal status
79:Legal status
21:Finafloxacin
2315:from market
2290:Fidaxomicin
2262:Rifapentine
2215:Secnidazole
2208:derivatives
2149:Nemonoxacin
2109:Ibafloxacin
2057:Garenoxacin
1946:Norfloxacin
1796:Solasulfone
1735:Sulfanitran
1689:Sulfamazone
1649:Sulfadoxine
1636:Long-acting
1626:Sulfamoxole
1497:Tetroxoprim
1477:Brodimoprim
1454:DNA and RNA
1442:Antifolates
1221:(1): 52–9.
790:hydrophilic
748:via the ear
691:replicating
396: g·mol
285:CHEBI:85176
180:209342-40-5
141:Identifiers
42:Trade names
2346:Categories
2252:Rifampicin
2239:Rifamycins
2197:inhibitors
2178:Novobiocin
2154:Ozenoxacin
2099:Difloxacin
2086:Veterinary
1956:Rufloxacin
1951:Pefloxacin
1923:Fleroxacin
1856:Flumequine
1826:DNA gyrase
1815:Quinolones
1674:Sulfaperin
1487:Ormetoprim
1456:synthesis)
851:References
615:flatulence
585:Geriatrics
577:Pediatrics
549:suspension
401:3D model (
389:Molar mass
245:D26OSN9Q4R
216:ChemSpider
171:CAS Number
150:IUPAC name
2325:Phase III
2313:Withdrawn
2272:Rifalazil
2267:Rifaximin
2257:Rifabutin
2231:synthesis
2192:Anaerobic
2163:Related (
1913:Ofloxacin
1881:Rosoxacin
1851:Cinoxacin
1801:Sulfoxone
1654:Sulfalene
1577:Prontosil
1335:15 August
1309:15 August
1304:drugs.com
1284:15 August
1261:14 August
1142:15 August
1137:drugs.com
1090:(1): 34.
1056:207488603
994:14 August
964:15 August
959:drugs.com
932:14 August
803:Synthesis
784:has an 8-
767:quinolone
761:Chemistry
623:headaches
565:Pregnancy
131:half-life
52:Routes of
2372:Nitriles
1918:Enoxacin
1705:Mafenide
1482:Iclaprim
1380:27148906
1372:26068714
1235:21596526
1193:17016449
1116:27215369
1048:25808831
835:Research
644:Overdose
627:rhinitis
611:diarrhea
205:11567473
136:10 hours
64:ATC code
1824:and/or
1791:Dapsone
1184:1618379
1133:"Xtoro"
1107:4878067
877:7 April
818:History
786:methoxy
709:stomach
619:fatigue
599:itching
522:placebo
476:) is a
394:398.394
353:Formula
225:9742243
191:PubChem
97::
2308:WHO-EM
1378:
1370:
1233:
1191:
1181:
1114:
1104:
1054:
1046:
603:nausea
427:SMILES
296:ChEMBL
265:D10575
111:℞-only
109:
99:℞-only
1423:that
1376:S2CID
1211:(PDF)
1052:S2CID
1028:Drugs
841:et al
829:Alcon
771:cyano
683:cyano
474:Xtoro
447:InChI
403:JSmol
276:ChEBI
46:Xtoro
1433:J01M
1429:J01E
1368:PMID
1337:2017
1311:2017
1286:2017
1263:2017
1231:PMID
1189:PMID
1144:2017
1112:PMID
1044:PMID
996:2017
966:2017
934:2017
879:2024
753:The
726:and
629:and
601:and
551:for
545:otic
514:and
494:and
256:KEGG
236:UNII
123:data
71:None
2229:RNA
2194:DNA
1360:doi
1223:doi
1179:PMC
1171:doi
1102:PMC
1092:doi
1036:doi
824:FDA
321:EPA
195:CID
2348::
2321::
2176::
2165:DG
1590:,
1431:,
1374:.
1366:.
1356:24
1354:.
1319:^
1302:.
1254:.
1243:^
1229:.
1219:38
1217:.
1213:.
1187:.
1177:.
1165:.
1161:.
1135:.
1124:^
1110:.
1100:.
1088:15
1086:.
1082:.
1064:^
1050:.
1042:.
1032:75
1030:.
1004:^
982:.
957:.
942:^
924:.
905:^
895:.
867:.
799:.
750:.
734:.
699:pH
621:,
613:,
535:.
500:.
367:19
361:20
105:US
94:CA
2241:/
2167:)
2074:/
1939:/
1935:/
1835:)
1828:,
1594:)
1586:(
1568:)
1564:(
1558:)
1554:(
1521:)
1517:(
1450:,
1435:)
1427:(
1413:e
1406:t
1399:v
1382:.
1362::
1339:.
1313:.
1288:.
1265:.
1237:.
1225::
1195:.
1173::
1167:7
1146:.
1118:.
1094::
1058:.
1038::
998:.
988:1
968:.
936:.
881:.
472:(
405:)
382:4
379:O
376:4
373:N
370:F
364:H
358:C
323:)
319:(
159:H
107::
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.