602:
InChI=1S/C59H89N19O13S/c60-37(14-5-19-67-57(61)62)48(82)72-38(15-6-20-68-58(63)64)52(86)75-22-8-18-43(75)54(88)77-30-35(80)26-44(77)50(84)70-28-47(81)71-40(27-36-13-9-23-92-36)49(83)74-41(31-79)53(87)76-29-34-12-2-1-10-32(34)24-46(76)55(89)78-42-17-4-3-11-33(42)25-45(78)51(85)73-39(56(90)91)16-7-21-69-59(65)66/h1-2,9-10,12-13,23,33,35,37-46,79-80H,3-8,11,14-22,24-31,60H2,(H,70,84)(H,71,81)(H,72,82)(H,73,85)(H,74,83)(H,90,91)(H4,61,62,67)(H4,63,64,68)(H4,65,66,69)/t33-,35+,37+,38-,39-,40-,41-,42-,43-,44-,45-,46+/m0/s1
480:
560:
42:
1252:
736:
to market
Firazyr in the European Union's 27 member states, as well as Switzerland, Liechtenstein and Iceland, making it the first product to be approved in all EU countries for the treatment of hereditary angioedema. In the US, the drug was granted FDA approval in August 2011.
205:
260:)-2-amino]-5-(diaminomethylideneamino)pentanoyl]pyrrolidine-2-carbonyl]-4-hydroxypyrrolidine-2-carbonyl]amino]acetyl]amino]-3-thiophen-2-ylpropanoyl]amino]-3-hydroxypropanoyl]-3,4-dihydro-1
1287:
160:
1044:
618:
872:
72:
574:
582:
C1CC2(C1)C(N2C(=O)3CC4=CC=CC=C4CN3C(=O)(CO)NC(=O)(CC5=CC=CS5)NC(=O)CNC(=O)6C(CN6C(=O)7CCCN7C(=O)(CCCN=C(N)N)NC(=O)(CCCN=C(N)N)N)O)C(=O)N(CCCN=C(N)N)C(=O)O
1037:
939:
684:
Bradykinin is a peptide-based hormone that is formed locally in tissues, very often in response to a trauma. It increases vessel permeability,
796:
1030:
1272:
594:
811:
248:
190:
90:
1277:
378:
264:-isoquinoline-3-carbonyl]-2,3,3a,4,5,6,7,7a-octahydroindole-2-carbonyl]amino]-5-(diaminomethylideneamino)pentanoic acid
459:
1092:
109:
704:
receptors. Icatibant acts as a bradykinin inhibitor by blocking the binding of native bradykinin to the bradykinin B
1242:
1203:
845:
1198:
1188:
327:
146:
900:"Randomized Trial of Icatibant for Angiotensin-Converting Enzyme Inhibitor-Induced Upper Airway Angioedema"
669:
448:
153:
797:
https://www.tga.gov.au/resources/prescription-medicines-registrations/icatibant-wkt-wockhardt-bio-pty-ltd
1282:
1119:
933:
898:
Sinert R, Levy P, Bernstein JA, Body R, Sivilotti ML, Moellman J, et al. (September–October 2017).
643:
318:
729:
status in
Australia, the EU, Switzerland, and the US for the treatment of hereditary angioedema (HAE).
1005:"FDA Approves Shire's Firazyr (icatibant injection) for Acute Attacks of Hereditary Angioedema (HAE)"
475:
1170:
273:
1083:
779:
689:
120:
986:
921:
873:"Jerini Receives European Commission Approval for Firazyr (Icatibant) in the Treatment of HAE"
771:
428:
367:
218:
54:
1175:
976:
966:
911:
840:
763:
496:
282:
387:
1256:
1004:
82:
479:
981:
954:
658:
1266:
1193:
1144:
1103:
1098:
767:
651:
783:
307:
1179:
1139:
1134:
1125:
1088:
1078:
1064:
1054:
1022:
955:"Management of acute attacks of hereditary angioedema: potential role of icatibant"
685:
647:
173:
168:
700:, overheating and pain. These symptoms are mediated by activation of bradykinin B
692:
to contract. Bradykinin plays an important role as the mediator of pain. Surplus
1159:
1149:
726:
916:
899:
693:
666:
662:
532:
358:
1213:
1208:
1073:
733:
708:
receptor. Little is known about the effects of icatibant on the bradykinin B
650:
deficiency. It is not effective in angioedema caused by medication from the
76:
990:
925:
775:
696:
is responsible for the typical symptoms of inflammation, such as swelling,
33:
697:
338:
104:
347:
1108:
971:
293:
17:
732:
In the EU, the approval by the
European Commission (July 2008) allows
41:
876:
439:
642:, is a medication for the symptomatic treatment of acute attacks of
408:
1229:
559:
550:
419:
398:
224:
1026:
212:
99:
464:
904:
The
Journal of Allergy and Clinical Immunology. In Practice
199:
131:
625:
1240:
1222:
1168:
1117:
1062:
806:
804:
548:
531:
495:
490:
458:
438:
418:
397:
377:
357:
337:
326:
317:
292:
272:
239:
189:
184:
159:
145:
119:
89:
71:
63:
53:
48:
665:, which is a selective and specific antagonist of
1288:Drugs developed by Takeda Pharmaceutical Company
812:"Firazyr- icatibant acetate injection, solution"
306:
281:
835:
833:
1038:
8:
867:
865:
108:
32:
232:In general: ℞ (Prescription only)
1045:
1031:
1023:
478:
366:
980:
970:
915:
386:
1247:
746:
599:
579:
474:
346:
253:
931:
31:
754:"Icatibant: HOE 140, JE 049, JE049".
447:
427:
81:
7:
172:
959:Vascular Health and Risk Management
407:
297:
938:: CS1 maint: overridden setting (
25:
1250:
768:10.2165/00126839-200405060-00006
513:
507:
40:
953:Longhurst HJ (September 2010).
607:Key:QURWXBZNHXJZBE-SKXRKSCCSA-N
525:
519:
501:
1:
1053:Other hematological agents (
638:, sold under the brand name
1304:
1204:Lovotibeglogene autotemcel
917:10.1016/j.jaip.2017.03.003
491:Chemical and physical data
846:European Medicines Agency
615:
590:
570:
244:
39:
1273:Anti-inflammatory agents
1199:Exagamglogene autotemcel
1189:Betibeglogene autotemcel
1007:(Press release). Shire
686:dilates blood vessels
648:C1-esterase-inhibitor
646:(HAE) in adults with
644:hereditary angioedema
1278:Peptide therapeutics
1171:sickle cell disease
1093:+desoxyribonuclease
910:(5): 1402–1409.e3.
850:. 17 September 2018
725:Icatibant received
716:Society and culture
690:smooth muscle cells
680:Mechanism of action
208:(Prescription only)
36:
27:Pharmaceutical drug
1084:Desoxyribonuclease
972:10.2147/vhrm.s4332
818:. 16 December 2019
762:(6): 343–8. 2004.
661:consisting of ten
1238:
1237:
875:(Press release).
633:
632:
561:Interactive image
460:CompTox Dashboard
228:
216:
203:
135:
102:
16:(Redirected from
1295:
1255:
1254:
1253:
1246:
1176:beta thalassemia
1047:
1040:
1033:
1024:
1017:
1016:
1014:
1012:
1001:
995:
994:
984:
974:
950:
944:
943:
937:
929:
919:
895:
889:
888:
886:
884:
869:
860:
859:
857:
855:
837:
828:
827:
825:
823:
808:
799:
794:
788:
787:
756:Drugs in R&D
751:
629:
628:
621:
563:
543:
541:
527:
521:
515:
509:
503:
483:
482:
468:
466:
451:
431:
411:
390:
370:
350:
330:
310:
300:
299:
285:
226:
223:
214:
211:
201:
198:
176:
133:
130:
112:
101:
98:
85:
44:
37:
35:
21:
1303:
1302:
1298:
1297:
1296:
1294:
1293:
1292:
1263:
1262:
1261:
1251:
1249:
1241:
1239:
1234:
1218:
1173:
1164:
1121:
1113:
1058:
1051:
1021:
1020:
1010:
1008:
1003:
1002:
998:
952:
951:
947:
930:
897:
896:
892:
882:
880:
871:
870:
863:
853:
851:
839:
838:
831:
821:
819:
810:
809:
802:
795:
791:
753:
752:
748:
743:
723:
718:
711:
707:
703:
682:
673:
624:
622:
619:(what is this?)
616:
611:
608:
603:
598:
597:
586:
583:
578:
577:
566:
539:
537:
524:
518:
512:
506:
486:
462:
454:
434:
414:
393:
373:
353:
333:
313:
296:
288:
268:
265:
252:
251:
235:
180:
148:
141:
122:
115:
67:Hoe 140, JE 049
28:
23:
22:
15:
12:
11:
5:
1301:
1299:
1291:
1290:
1285:
1280:
1275:
1265:
1264:
1260:
1259:
1236:
1235:
1233:
1232:
1226:
1224:
1220:
1219:
1217:
1216:
1211:
1206:
1201:
1196:
1191:
1185:
1183:
1169:Drugs used in
1166:
1165:
1163:
1162:
1157:
1152:
1147:
1142:
1137:
1131:
1129:
1118:Drugs used in
1115:
1114:
1112:
1111:
1106:
1101:
1096:
1086:
1081:
1076:
1070:
1068:
1060:
1059:
1052:
1050:
1049:
1042:
1035:
1027:
1019:
1018:
996:
945:
890:
879:. 15 July 2008
861:
841:"Firazyr EPAR"
829:
800:
789:
745:
744:
742:
739:
722:
719:
717:
714:
709:
705:
701:
681:
678:
671:
659:peptidomimetic
631:
630:
613:
612:
610:
609:
606:
604:
601:
593:
592:
591:
588:
587:
585:
584:
581:
573:
572:
571:
568:
567:
565:
564:
556:
554:
546:
545:
535:
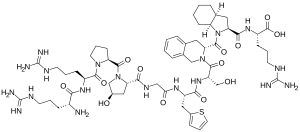
529:
528:
522:
516:
510:
504:
499:
493:
492:
488:
487:
485:
484:
476:DTXSID20903963
471:
469:
456:
455:
453:
452:
444:
442:
436:
435:
433:
432:
424:
422:
416:
415:
413:
412:
406:as salt:
403:
401:
395:
394:
392:
391:
383:
381:
375:
374:
372:
371:
363:
361:
355:
354:
352:
351:
343:
341:
335:
334:
332:
331:
323:
321:
315:
314:
312:
311:
303:
301:
290:
289:
287:
286:
278:
276:
270:
269:
267:
266:
255:
247:
246:
245:
242:
241:
237:
236:
234:
233:
230:
221:
209:
195:
193:
187:
186:
182:
181:
179:
178:
165:
163:
157:
156:
151:
149:administration
143:
142:
140:
139:
137:
127:
125:
117:
116:
114:
113:
95:
93:
87:
86:
79:
69:
68:
65:
61:
60:
57:
51:
50:
46:
45:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1300:
1289:
1286:
1284:
1281:
1279:
1276:
1274:
1271:
1270:
1268:
1258:
1248:
1244:
1231:
1228:
1227:
1225:
1221:
1215:
1212:
1210:
1207:
1205:
1202:
1200:
1197:
1195:
1194:Crizanlizumab
1192:
1190:
1187:
1186:
1184:
1181:
1177:
1172:
1167:
1161:
1158:
1156:
1153:
1151:
1148:
1146:
1145:Conestat alfa
1143:
1141:
1138:
1136:
1133:
1132:
1130:
1127:
1123:
1116:
1110:
1107:
1105:
1104:Streptokinase
1102:
1100:
1099:Hyaluronidase
1097:
1094:
1090:
1087:
1085:
1082:
1080:
1077:
1075:
1072:
1071:
1069:
1066:
1061:
1056:
1048:
1043:
1041:
1036:
1034:
1029:
1028:
1025:
1006:
1000:
997:
992:
988:
983:
978:
973:
968:
964:
960:
956:
949:
946:
941:
935:
927:
923:
918:
913:
909:
905:
901:
894:
891:
878:
874:
868:
866:
862:
849:
847:
842:
836:
834:
830:
817:
813:
807:
805:
801:
798:
793:
790:
785:
781:
777:
773:
769:
765:
761:
757:
750:
747:
740:
738:
735:
730:
728:
720:
715:
713:
699:
695:
691:
687:
679:
677:
675:
668:
664:
660:
655:
653:
652:ACE inhibitor
649:
645:
641:
637:
627:
620:
614:
605:
600:
596:
589:
580:
576:
569:
562:
558:
557:
555:
552:
547:
536:
534:
530:
500:
498:
494:
489:
481:
477:
473:
472:
470:
461:
457:
450:
449:ChEMBL1743581
446:
445:
443:
441:
437:
430:
426:
425:
423:
421:
417:
410:
405:
404:
402:
400:
396:
389:
385:
384:
382:
380:
376:
369:
365:
364:
362:
360:
356:
349:
345:
344:
342:
340:
336:
329:
325:
324:
322:
320:
316:
309:
305:
304:
302:
295:
291:
284:
280:
279:
277:
275:
271:
263:
259:
254:
250:
243:
238:
231:
229: Rx-only
222:
220:
210:
207:
197:
196:
194:
192:
188:
183:
175:
170:
167:
166:
164:
162:
158:
155:
152:
150:
144:
138:
129:
128:
126:
124:
118:
111:
106:
97:
96:
94:
92:
88:
84:
80:
78:
74:
70:
66:
62:
58:
56:
52:
49:Clinical data
47:
43:
38:
30:
19:
1283:Orphan drugs
1154:
1140:C1-inhibitor
1135:Berotralstat
1089:Fibrinolysin
1079:Chymotrypsin
1009:. Retrieved
999:
962:
958:
948:
934:cite journal
907:
903:
893:
881:. Retrieved
852:. Retrieved
844:
820:. Retrieved
815:
792:
759:
755:
749:
731:
724:
721:Legal status
683:
656:
639:
635:
634:
623:
617:
261:
257:
191:Legal status
185:Legal status
154:Subcutaneous
91:License data
29:
1160:Lanadelumab
1150:Ecallantide
965:: 795–802.
727:orphan drug
688:and causes
663:amino acids
544: g·mol
429:CHEBI:68556
283:130308-48-4
240:Identifiers
64:Other names
55:Trade names
1267:Categories
1122:angioedema
1120:hereditary
741:References
712:receptor.
694:bradykinin
667:bradykinin
549:3D model (
533:Molar mass
388:7PG89G35Q7
359:ChemSpider
319:IUPHAR/BPS
274:CAS Number
249:IUPAC name
1214:Voxelotor
1209:Mitapivat
1155:Icatibant
1074:Bromelain
1063:Enzymes (
1011:28 August
877:Jerini AG
674:receptors
636:Icatibant
147:Routes of
121:Pregnancy
110:Icatibant
83:Monograph
77:Drugs.com
34:Icatibant
1257:Medicine
991:20859548
926:28552382
854:17 April
822:17 April
816:DailyMed
784:25491021
776:15563238
657:It is a
626:(verify)
368:16736634
339:DrugBank
161:ATC code
123:category
105:DailyMed
1109:Trypsin
982:2941790
883:22 July
698:redness
654:class.
640:Firazyr
497:Formula
348:DB06196
308:6918173
294:PubChem
177:)
171: (
169:B06AC02
136: C
107::
59:Firazyr
18:Firazyr
1243:Portal
1223:Others
989:
979:
924:
782:
774:
734:Jerini
575:SMILES
440:ChEMBL
409:D04492
219:℞-only
217:
204:
103:
1230:Hemin
1180:B06AX
1126:B06AC
1065:B06AA
848:(EMA)
780:S2CID
595:InChI
551:JSmol
420:ChEBI
1174:and
1013:2011
987:PMID
940:link
922:PMID
885:2008
856:2020
824:2020
772:PMID
399:KEGG
379:UNII
73:AHFS
1055:B06
977:PMC
967:doi
912:doi
764:doi
542:.54
540:304
465:EPA
328:667
298:CID
174:WHO
1269::
985:.
975:.
961:.
957:.
936:}}
932:{{
920:.
906:.
902:.
864:^
843:.
832:^
814:.
803:^
778:.
770:.
758:.
676:.
523:13
517:19
511:89
505:59
256:(2
225:EU
213:US
206:S4
200:AU
132:AU
100:US
1245::
1182:)
1178:(
1128:)
1124:(
1095:)
1091:(
1067:)
1057:)
1046:e
1039:t
1032:v
1015:.
993:.
969::
963:6
942:)
928:.
914::
908:5
887:.
858:.
826:.
786:.
766::
760:5
710:1
706:2
702:2
672:2
670:B
553:)
538:1
526:S
520:O
514:N
508:H
502:C
467:)
463:(
262:H
258:S
227::
215::
202::
134::
75:/
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.