30:
768:
17:
358:
209:) fumes proved increasingly hazardous to health, and as telegraphs became more complex, the need for constant voltage became critical. The Grove cell was limited in this respect, because as the cell discharged, voltage reduced. Eventually, Grove cells were replaced in use by
59:
809:
287:
833:
558:
445:
802:
81:
598:
608:
536:
828:
795:
618:
280:
583:
42:
450:
623:
593:
578:
546:
52:
46:
38:
638:
385:
247:
653:
63:
551:
658:
603:
588:
521:
480:
455:
435:
415:
541:
273:
747:
648:
227:
509:
613:
430:
222:
475:
775:
491:
460:
296:
107:
643:
628:
563:
531:
526:
342:
633:
504:
357:
315:
198:
707:
425:
395:
779:
573:
568:
380:
320:
202:
440:
367:
767:
676:
330:
16:
822:
310:
181:
system in the period 1840 – 1860 because it offered a high current output and higher
118:
514:
470:
405:
347:
325:
210:
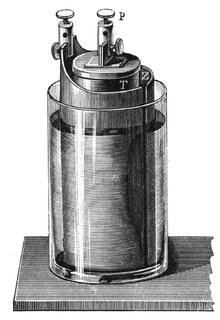
201:, as telegraph traffic increased, the Grove cell's tendency to discharge poisonous
186:
99:
140:
The Grove cell voltage is about 1.9 volts and arises from the following reaction:
742:
727:
465:
390:
232:
129:
686:
400:
732:
722:
712:
681:
337:
178:
410:
122:
265:
717:
182:
125:
702:
177:
The Grove cell was the favored power source of the early
American
114:
103:
15:
111:
269:
737:
23:
783:
695:
667:
489:
365:
303:
51:but its sources remain unclear because it lacks
803:
281:
132:, the two separated by a porous ceramic pot.
8:
189:(at 1.9 volts and 1.1 volts, respectively).
235:, Using cheaper carbon instead of platinum.
810:
796:
288:
274:
266:
82:Learn how and when to remove this message
261:. Cassell and Company. pp. 183–185.
7:
764:
762:
782:. You can help Knowledge (XXG) by
14:
766:
356:
28:
252:. Theodore Bliss. p. 578.
1:
246:Stillman, Benjamin (1861).
850:
834:Analytical chemistry stubs
761:
102:named after its inventor,
446:Metal–air electrochemical
354:
37:This article includes a
66:more precise citations.
748:Semipermeable membrane
537:Lithium–iron–phosphate
228:History of the battery
98:was an early electric
21:
619:Rechargeable alkaline
297:Electrochemical cells
259:Practical Electricity
257:Ayrton, W.E. (1911).
249:Principles of Physics
223:List of battery types
110:, and consisted of a
19:
829:Disposable batteries
776:analytical chemistry
599:Nickel–metal hydride
108:William Robert Grove
774:This article about
609:Polysulfide–bromide
451:Nickel oxyhydroxide
343:Thermogalvanic cell
197:By the time of the
106:physical scientist
372:(non-rechargeable)
316:Concentration cell
199:American Civil War
39:list of references
22:
791:
790:
756:
755:
185:than the earlier
92:
91:
84:
20:Grove cell (1897)
841:
812:
805:
798:
770:
763:
552:Lithium–titanate
497:
373:
360:
321:Electric battery
290:
283:
276:
267:
262:
253:
203:nitrogen dioxide
128:in concentrated
87:
80:
76:
73:
67:
62:this article by
53:inline citations
32:
31:
24:
849:
848:
844:
843:
842:
840:
839:
838:
819:
818:
817:
816:
759:
757:
752:
691:
670:
663:
584:Nickel–hydrogen
542:Lithium–polymer
498:
495:
494:
485:
374:
371:
370:
361:
352:
299:
294:
256:
245:
242:
219:
208:
195:
175:
167:
163:
159:
155:
151:
147:
138:
88:
77:
71:
68:
57:
43:related reading
33:
29:
12:
11:
5:
847:
845:
837:
836:
831:
821:
820:
815:
814:
807:
800:
792:
789:
788:
771:
754:
753:
751:
750:
745:
740:
735:
730:
725:
720:
715:
710:
705:
699:
697:
693:
692:
690:
689:
684:
679:
677:Atomic battery
673:
671:
668:
665:
664:
662:
661:
656:
651:
649:Vanadium redox
646:
641:
636:
631:
626:
624:Silver–cadmium
621:
616:
611:
606:
601:
596:
594:Nickel–lithium
591:
586:
581:
579:Nickel–cadmium
576:
571:
566:
561:
556:
555:
554:
549:
547:Lithium–sulfur
544:
539:
534:
524:
519:
518:
517:
507:
501:
499:
496:(rechargeable)
492:Secondary cell
490:
487:
486:
484:
483:
478:
473:
468:
463:
458:
453:
448:
443:
438:
433:
428:
423:
418:
416:Edison–Lalande
413:
408:
403:
398:
393:
388:
383:
377:
375:
366:
363:
362:
355:
353:
351:
350:
345:
340:
335:
334:
333:
331:Trough battery
328:
318:
313:
307:
305:
301:
300:
295:
293:
292:
285:
278:
270:
264:
263:
254:
241:
238:
237:
236:
230:
225:
218:
215:
206:
194:
191:
174:
171:
170:
169:
165:
161:
157:
153:
149:
145:
137:
134:
90:
89:
47:external links
36:
34:
27:
13:
10:
9:
6:
4:
3:
2:
846:
835:
832:
830:
827:
826:
824:
813:
808:
806:
801:
799:
794:
793:
787:
785:
781:
777:
772:
769:
765:
760:
749:
746:
744:
741:
739:
736:
734:
731:
729:
726:
724:
721:
719:
716:
714:
711:
709:
706:
704:
701:
700:
698:
694:
688:
685:
683:
680:
678:
675:
674:
672:
666:
660:
657:
655:
652:
650:
647:
645:
642:
640:
639:Sodium–sulfur
637:
635:
632:
630:
627:
625:
622:
620:
617:
615:
614:Potassium ion
612:
610:
607:
605:
602:
600:
597:
595:
592:
590:
587:
585:
582:
580:
577:
575:
572:
570:
567:
565:
562:
560:
557:
553:
550:
548:
545:
543:
540:
538:
535:
533:
530:
529:
528:
525:
523:
520:
516:
513:
512:
511:
508:
506:
503:
502:
500:
493:
488:
482:
479:
477:
474:
472:
469:
467:
464:
462:
459:
457:
454:
452:
449:
447:
444:
442:
439:
437:
434:
432:
431:Lithium metal
429:
427:
424:
422:
419:
417:
414:
412:
409:
407:
404:
402:
399:
397:
394:
392:
389:
387:
386:Aluminium–air
384:
382:
379:
378:
376:
369:
364:
359:
349:
346:
344:
341:
339:
336:
332:
329:
327:
324:
323:
322:
319:
317:
314:
312:
311:Galvanic cell
309:
308:
306:
302:
298:
291:
286:
284:
279:
277:
272:
271:
268:
260:
255:
251:
250:
244:
243:
239:
234:
231:
229:
226:
224:
221:
220:
216:
214:
212:
211:Daniell cells
204:
200:
193:Disadvantages
192:
190:
188:
184:
180:
172:
143:
142:
141:
135:
133:
131:
127:
124:
120:
119:sulfuric acid
116:
113:
109:
105:
101:
97:
86:
83:
75:
65:
61:
55:
54:
48:
44:
40:
35:
26:
25:
18:
784:expanding it
773:
758:
654:Zinc–bromine
461:Silver oxide
420:
396:Chromic acid
368:Primary cell
348:Voltaic pile
326:Flow battery
258:
248:
196:
187:Daniell cell
176:
139:
136:Cell details
100:primary cell
95:
93:
78:
69:
58:Please help
50:
743:Salt bridge
728:Electrolyte
659:Zinc–cerium
644:Solid state
629:Silver–zinc
604:Nickel–zinc
589:Nickel–iron
564:Molten salt
532:Dual carbon
527:Lithium ion
522:Lithium–air
481:Zinc–carbon
456:Silicon–air
436:Lithium–air
233:Bunsen cell
130:nitric acid
64:introducing
823:Categories
696:Cell parts
687:Solar cell
669:Other cell
634:Sodium ion
505:Automotive
117:in dilute
96:Grove cell
72:April 2022
733:Half-cell
723:Electrode
682:Fuel cell
559:Metal–air
510:Lead–acid
426:Leclanché
338:Fuel cell
179:telegraph
713:Catalyst
574:Nanowire
569:Nanopore
515:gel–VRLA
476:Zinc–air
381:Alkaline
217:See also
164:O + 2 NO
123:platinum
718:Cathode
471:Zamboni
441:Mercury
406:Daniell
183:voltage
152:+ 2 HNO
126:cathode
60:improve
708:Binder
466:Weston
391:Bunsen
156:⇌ ZnSO
144:Zn + H
121:and a
778:is a
703:Anode
421:Grove
401:Clark
304:Types
240:Notes
160:+ 2 H
115:anode
104:Welsh
45:, or
780:stub
738:Ions
112:zinc
94:The
411:Dry
205:(NO
173:Use
825::
213:.
148:SO
49:,
41:,
811:e
804:t
797:v
786:.
289:e
282:t
275:v
207:2
168:↑
166:2
162:2
158:4
154:3
150:4
146:2
85:)
79:(
74:)
70:(
56:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.