114:
33:
24:
456:
80:
310:. It was shown that hypercubane lifetime at room temperature tended to infinity. Therefore, it can be assumed that hypercubane is a kinetically stable molecular system. Among the possible hypercubane decomposition products at high temperatures (more than 1000 K) one can observe polycyclic airscrew-like hydrocarbon C
297:
by removing unnecessary hydrogen atoms and adding the ethylene bridges as well as intercarbon bonds between the sp and sp atoms. To facilitate the future hypercubane spectroscopic identification chemical shifts for both C and H NMR-active nuclei have been calculated by
Pichierri. Two years later, in
137:
InChI=1S/C40H24/c1-2-26-7-9-29-15-11-27-5-3-25(1)4-6-28-12-16-30(10-8-26)20-23-32(22-19-29)24-21-31(17-13-27,18-14-28)39-35(27)33(25)34(26)37(29,35)40(32,39)38(30,34)36(28,33)39/h1-24H
269:. The structure is that of octamethyl cubane—a carbon attached to each corner of cubane itself—having each of those carbon substituents joined to each of its neighbors by an
497:
531:
153:
161:
C/1=C/C\28/C=C\C\39\C=C/C\46/C=C\C17/C=C\C/5%15\C=C/C%13(/C=C2)\C=C/C%11(/C=C3)\C=C/C(/C=C4)(\C=C5)C%12%17C6%10C7%16C8%14C9%10C%11%12C%13%14C%15%16%17
516:
536:
490:
128:
413:
Maslov, Mikhail M.; Katin, Konstantin P. (2016). "High kinetic stability of hypercubane: Tight-binding molecular dynamics study".
302:
simulations, Maslov and Katin demonstrated that hypercubane possessed high thermal stability comparable with the classic cubane C
521:
483:
219:
526:
92:
290:
318:
based on three combined graphene fragments passivated by hydrogen atoms and three isolated acetylene molecules.
415:
375:
339:
286:
463:
294:
424:
384:
109:
46:
335:
299:
273:-1,2-diyl linker to form an outer cage. The edge of each inner core and its outer linker form a
467:
56:
432:
392:
176:
113:
428:
388:
213:
510:
32:
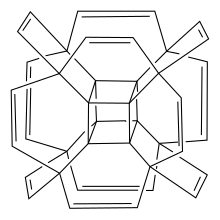
250:. Hypercubane possesses an unconventional geometry of the carbon framework. It has
436:
396:
274:
198:
247:
243:
326:
To date, there has been no method describing the synthesis of hypercubane.
270:
23:
455:
298:
2016, studying the pyrolysis of hypercubane by means of tight-binding
343:
258:
234:
is a hypothetical polycyclic hydrocarbon with the chemical formula C
212:
Except where otherwise noted, data are given for materials in their
366:
Pichierri, Fabio (2014). "Hypercubane: DFT-based prediction of an
79:
69:
285:
Hypercubane was first proposed in 2014 by
Pichierri and studied
97:
293:. The initial model of hypercubane was constructed from
471:
242:. It is a molecular analog of the four-dimensional
361:
359:
55:
491:
8:
408:
406:
498:
484:
112:
15:
355:
158:
133:
108:
373:-symmetric double-shell hydrocarbon".
342:, which have a structure resembling a
140:Key: FFMFUIDOGFAUOP-UHFFFAOYSA-N
7:
452:
450:
532:Polycyclic nonaromatic hydrocarbons
470:. You can help Knowledge (XXG) by
14:
454:
188:
31:
22:
517:Hypothetical chemical compounds
216:(at 25 °C , 100 kPa).
182:
1:
437:10.1016/j.cplett.2015.12.022
397:10.1016/j.cplett.2014.08.032
537:Theoretical chemistry stubs
553:
449:
291:density functional theory
210:
169:
149:
124:
39:
30:
21:
416:Chemical Physics Letters
376:Chemical Physics Letters
340:basic beryllium acetate
257:symmetry like classic
522:Theoretical chemistry
464:theoretical chemistry
462:This article about
429:2016CPL...644..280M
389:2014CPL...612..198P
206: g·mol
18:
527:Molecular geometry
336:Basic zinc acetate
300:molecular dynamics
220:Infobox references
16:
479:
478:
228:Chemical compound
226:
225:
93:CompTox Dashboard
81:Interactive image
544:
500:
493:
486:
458:
451:
441:
440:
410:
401:
400:
363:
295:octamethylcubane
205:
190:
184:
177:Chemical formula
117:
116:
101:
99:
83:
59:
35:
26:
19:
552:
551:
547:
546:
545:
543:
542:
541:
507:
506:
505:
504:
447:
445:
444:
412:
411:
404:
371:
365:
364:
357:
352:
332:
324:
317:
313:
309:
305:
287:computationally
283:
268:
264:
255:
241:
237:
229:
222:
217:
203:
193:
187:
179:
165:
162:
157:
156:
145:
142:
141:
138:
132:
131:
120:
110:DTXSID601337444
102:
95:
86:
73:
62:
49:
12:
11:
5:
550:
548:
540:
539:
534:
529:
524:
519:
509:
508:
503:
502:
495:
488:
480:
477:
476:
459:
443:
442:
402:
369:
354:
353:
351:
348:
347:
346:
331:
328:
323:
320:
315:
311:
307:
303:
282:
279:
266:
262:
253:
239:
235:
227:
224:
223:
218:
214:standard state
211:
208:
207:
201:
195:
194:
191:
185:
180:
175:
172:
171:
167:
166:
164:
163:
160:
152:
151:
150:
147:
146:
144:
143:
139:
136:
135:
127:
126:
125:
122:
121:
119:
118:
105:
103:
91:
88:
87:
85:
84:
76:
74:
67:
64:
63:
61:
60:
52:
50:
45:
42:
41:
37:
36:
28:
27:
13:
10:
9:
6:
4:
3:
2:
549:
538:
535:
533:
530:
528:
525:
523:
520:
518:
515:
514:
512:
501:
496:
494:
489:
487:
482:
481:
475:
473:
469:
465:
460:
457:
453:
448:
438:
434:
430:
426:
422:
418:
417:
409:
407:
403:
398:
394:
390:
386:
382:
378:
377:
372:
362:
360:
356:
349:
345:
341:
337:
334:
333:
329:
327:
321:
319:
301:
296:
292:
288:
280:
278:
276:
272:
260:
256:
249:
245:
233:
221:
215:
209:
202:
200:
197:
196:
181:
178:
174:
173:
168:
159:
155:
148:
134:
130:
123:
115:
111:
107:
106:
104:
94:
90:
89:
82:
78:
77:
75:
71:
66:
65:
58:
54:
53:
51:
48:
44:
43:
38:
34:
29:
25:
20:
472:expanding it
461:
446:
420:
414:
380:
374:
367:
325:
284:
251:
231:
230:
57:1627580-03-3
40:Identifiers
17:Hypercubane
423:: 280–283.
383:: 198–202.
275:cyclohexene
232:Hypercubane
170:Properties
511:Categories
350:References
199:Molar mass
68:3D model (
47:CAS Number
322:Synthesis
248:tesseract
244:hypercube
330:See also
271:ethylene
425:Bibcode
385:Bibcode
281:History
204:504.632
344:5-cell
259:cubane
154:SMILES
466:is a
129:InChI
70:JSmol
468:stub
338:and
433:doi
421:644
393:doi
381:612
289:by
246:or
98:EPA
513::
431:.
419:.
405:^
391:.
379:.
358:^
316:18
312:34
277:.
240:24
236:40
192:24
186:40
499:e
492:t
485:v
474:.
439:.
435::
427::
399:.
395::
387::
370:h
368:O
314:H
308:8
306:H
304:8
267:8
265:H
263:8
261:C
254:h
252:O
238:H
189:H
183:C
100:)
96:(
72:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.