459:
294:
60:
921:
73:
83:
811:
2958:
1443:, also known as the arc process. This process is based upon the oxidation of atmospheric nitrogen by atmospheric oxygen to nitric oxide with a very high temperature electric arc. Yields of up to approximately 4–5% nitric oxide were obtained at 3000 °C, and less at lower temperatures. The nitric oxide was cooled and oxidized by the remaining atmospheric oxygen to nitrogen dioxide, and this was subsequently absorbed in water in a series of
821:
816:
1545:
1705:
916:
47:
2783:
4503:
1168:
1379:. Powder them well, separately, until they are like dust and then place them in a flask. Plug the latter with a palm fibre and attach a glass receiver to it. Then invert the apparatus and heat the upper portion (i.e. the flask containing the mixture) with a gentle fire. There will flow down by reason of the heat an oil like cow's butter.
1602:) remains dissolved in the nitric acid coloring it yellow or even red at higher temperatures. While the pure acid tends to give off white fumes when exposed to air, acid with dissolved nitrogen dioxide gives off reddish-brown vapors, leading to the common names "red fuming nitric acid" and "white fuming nitric acid". Nitrogen oxides (
1456:
tarred lumber to make a compartment for the carbon anode around which the nitric acid is formed. Nitric acid was pumped out from an earthenware pipe that was sunk down to the bottom of the pot. Fresh water was pumped into the top through another earthenware pipe to replace the fluid removed. The interior was filled with
1451:
absorption towers to produce dilute nitric acid. The first towers bubbled the nitrogen dioxide through water and non-reactive quartz fragments. About 20% of the produced oxides of nitrogen remained unreacted so the final towers contained an alkali solution to neutralize the rest. The process was very
1435:
reported the results of extensive distilled water electrolysis experiments concluding that nitric acid was produced at the anode from dissolved atmospheric nitrogen gas. He used a high voltage battery and non-reactive electrodes and vessels such as gold electrode cones that doubled as vessels bridged
1455:
Another early production method was invented by French engineer Albert Nodon around 1913. His method produced nitric acid from electrolysis of calcium nitrate converted by bacteria from nitrogenous matter in peat bogs. An earthenware pot surrounded by limestone was sunk into the peat and staked with
3267:
The standard first-aid treatment for acid spills on the skin is, as for other corrosive agents, irrigation with large quantities of water. Washing is continued for at least 10–15 minutes to cool the tissue surrounding the acid burn and to prevent secondary damage. Contaminated clothing is removed
4501:, Harrar, Jackson E.; Quong, Roland & Rigdon, Lester P. et al., "Large-scale production of anhydrous nitric acid and nitric acid solutions of dinitrogen pentoxide", published April 13, 1987, issued March 13, 2001, assigned to United States Department of Energy
1695:
and water, it obtains a yellow tint. It boils at 83 °C (181 °F). It is usually stored in a glass shatterproof amber bottle with twice the volume of head space to allow for pressure build up, but even with those precautions the bottle must be vented monthly to release pressure.
919:
3169:
are commonly used for cleaning food and dairy equipment primarily to remove precipitated calcium and magnesium compounds (either deposited from the process stream or resulting from the use of hard water during production and cleaning). The phosphoric acid content helps to passivate
3074:, GFAA, and Flame AA, dilute nitric acid (0.5–5.0%) is used as a matrix compound for determining metal traces in solutions. Ultrapure trace metal grade acid is required for such determination, because small amounts of metal ions could affect the result of the analysis.
2287:
group will typically strip a hydrogen from the organic molecule to form water, and the remaining nitro group takes the hydrogen's place. Nitration of organic compounds with nitric acid is the primary method of synthesis of many common explosives, such as
3488:
pp. 140-141, quote: "But among them we find the rudiments of processes which were finally to lead to the discovery of the mineral acids, sulphuric, hydrochloric and nitric. The mineral acids manifest themselves clearly only about three centuries after
3161:
Nitric acid is used either in combination with hydrochloric acid or alone to clean glass cover slips and glass slides for high-end microscopy applications. It is also used to clean glass before silvering when making silver mirrors.
3604:, p. 1002. As Karpenko & Norris note, the uncertain dating of the pseudo-Geber corpus (which was probably written by more than one author) renders the date of its description of nitric acid equally uncertain. According to
1419:'s work to point out that it can be converted from nitric oxide (which he calls "nitrous air"), "combined with an approximately equal volume of the purest part of common air, and with a considerable quantity of water." In 1785
829:
791:
2005:. With these non-active or less electropositive metals the products depend on temperature and the acid concentration. For example, copper reacts with dilute nitric acid at ambient temperatures with a 3:8 stoichiometry:
3419:
1688:(mobile) liquid with a density of 1.512–3 g/cm that solidifies at −42 °C (−44 °F) to form white crystals. Its dynamic viscosity under standard conditions is 0.76 cP. As it decomposes to
922:
1490:. This solution has a boiling temperature of 120.5 °C (249 °F) at 1 atm. It is known as "concentrated nitric acid". The azeotrope of nitric acid and water is a colourless liquid at room temperature.
891:
2687:
at nitric acid's 83 °C boiling point then separates the solid metal-salt residue. The resulting acid solution is the 68.5 % azeotrope, and can be further concentrated (as in industry) with either
944:
1632:
and has a density of 1.50 g/cm. This grade is often used in the explosives industry. It is not as volatile nor as corrosive as the anhydrous acid and has the approximate concentration of 21.4 M.
2798:. This application consumes 75–80% of the 26 million tonnes produced annually (1987). The other main applications are for the production of explosives, nylon precursors, and specialty organic compounds.
2247:(Al) readily dissolve in dilute nitric acid, the concentrated acid forms a metal-oxide layer that protects the bulk of the metal from further oxidation. The formation of this protective layer is called
1460:. Cast iron cathodes were sunk into the peat surrounding it. Resistance was about 3 ohms per cubic meter and the power supplied was around 10 volts. Production from one deposit was 800 tons per year.
895:
3555:
p. 1002, quote: " dating the discovery of nitric acid is likewise uncertain. It is estimated that this discovery took place after 1300 A passage from the second part of Pseudo-Geber's
3077:
It is also typically used in the digestion process of turbid water samples, sludge samples, solid samples as well as other types of unique samples which require elemental analysis via
1581:
This reaction may give rise to some non-negligible variations in the vapor pressure above the liquid because the nitrogen oxides produced dissolve partly or completely in the acid.
920:
1279:, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as pigments in inks and dyes. Nitric acid is also commonly used as a
3337:
999:
1181:
887:
3408:
2296:(TNT). As very many less stable byproducts are possible, these reactions must be carefully thermally controlled, and the byproducts removed to isolate the desired product.
4543:
2194:
Being a powerful oxidizing agent, nitric acid reacts with many non-metallic compounds, sometimes explosively. Depending on the acid concentration, temperature and the
2424:, respectively. Although it reacts with graphite and amorphous carbon, it does not react with diamond; it can separate diamond from the graphite that it oxidizes.
1898:
Nitric acid reacts with most metals, but the details depend on the concentration of the acid and the nature of the metal. Dilute nitric acid behaves as a typical
59:
1778:
value is usually reported as less than −1. This means that the nitric acid in diluted solution is fully dissociated except in extremely acidic solutions. The p
1653:
fuming nitric acid, either white inhibited fuming nitric acid (IWFNA), or red inhibited fuming nitric acid (IRFNA), can be made by the addition of 0.6 to 0.7%
5874:
4965:
1646:) leaving the solution with a reddish-brown color. Due to the dissolved nitrogen dioxide, the density of red fuming nitric acid is lower at 1.490 g/cm.
508:
1669:
White fuming nitric acid, pure nitric acid or WFNA, is very close to anhydrous nitric acid. It is available as 99.9% nitric acid by assay, or about 24
4161:
3871:
3764:
3205:. The resulting nitrates are converted to various complexes that can be reacted and extracted selectively in order to separate the metals from each other.
3501:
Science and
Civilisation in China. Volume 5, Chemistry and Chemical Technology. Part IV, Spagyrical Discovery and Invention: Apparatus, Theories and Gifts
3001:. These forms include red fuming nitric acid, white fuming nitric acid, mixtures with sulfuric acid, and these forms with HF inhibitor. IRFNA (inhibited
2440:. This test is carried out by adding concentrated nitric acid to the substance being tested, and then heating the mixture. If proteins that contain
2183:
can be easily oxidized and dissolved by nitric acid, leading to colour changes of the gold-alloy surface. Nitric acid is used as a cheap means in
3264:. These yellow stains turn orange when neutralized. Systemic effects are unlikely, and the substance is not considered a carcinogen or mutagen.
2614:. Historically, higher acid concentrations were also produced by dissolving additional nitrogen dioxide in the acid, but the last plant in the
4814:
O’Neal, Carol L; Crouch, Dennis J; Fatah, Alim A (April 2000). "Validation of twelve chemical spot tests for the detection of drugs of abuse".
3516:
p. 195, quote: "It is generally accepted that mineral acids were quite unknown both to the ancients in the West and to the Arabic alchemists."
875:
4664:
4631:
4585:
4400:
4364:
4339:
4267:
4044:
3737:
3532:
3509:
3463:
3392:
760:
82:
2829:
6585:
4560:
3727:
3710:
3577:
3588:
was written and the seventeenth century, the mineral acids–sulfuric, hydrochloric, nitric, and the mixture of the latter two, called
3539:
p. 59, quote: "The text is given here in full because of the prevailing notion that
Islamic chemists did not produce mineral acids."
4468:
3844:
473:
72:
3158:, is used for etching metals to reveal the microstructure. ISO 14104 is one of the standards detailing this well known procedure.
5867:
4958:
2251:. Typical passivation concentrations range from 20% to 50% by volume. Metals that are passivated by concentrated nitric acid are
2206:. As a general rule, oxidizing reactions occur primarily with the concentrated acid, favoring the formation of nitrogen dioxide (
1718:
The two terminal N–O bonds are nearly equivalent and relatively short, at 1.20 and 1.21 Å. This can be explained by theories of
7928:
7943:
7933:
4082:
3090:
1858:
reactions. Since nitric acid has both acidic and basic properties, it can undergo an autoprotolysis reaction, similar to the
2621:
More recently, electrochemical means have been developed to produce anhydrous acid from concentrated nitric acid feedstock.
4720:
Fischer, A. H.; Jacobson, K. A.; Rose, J.; Zeller, R. (1 May 2008). "Preparation of Slides and
Coverslips for Microscopy".
4417:
4927:
3628:"Min al-kīmiyāʾ ad alchimiam. The Transmission of Alchemy from the Arab-Muslim World to the Latin West in the Middle Ages"
3043:
883:
1188:
5835:
1233:. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86%
869:
406:
7913:
5860:
4951:
2586:
Dissolved nitrogen oxides are either stripped (in the case of white fuming nitric acid) or remain in solution to form
1673:. One specification for white fuming nitric acid is that it has a maximum of 2% water and a maximum of 0.5% dissolved
437:
186:
4541:
Thiemann, Michael; Scheibler, Erich; Wiegand, Karl
Wilhelm (2000). "Nitric Acid, Nitrous Acid, and Nitrogen Oxides".
1440:
930:
7918:
4448:
3830:
3658:
that were sometimes attributed to al-Razi; see
Moureau 2020, p. 107 (no. 5), p. 114 (no. 20), pp. 114–115 (no. 21).
1769:
1023:
1010:
649:
2043:. With more concentrated nitric acid, nitrogen dioxide is produced directly in a reaction with 1:4 stoichiometry:
7609:
7095:
4622:
Eaton, Andrew D.; Greenberg, Arnold E.; Rice, Eugene W.; Clesceri, Lenore S.; Franson, Mary Ann H., eds. (2005).
3198:
3047:
2460:. Respective local skin color changes are indicative of inadequate safety precautions when handling nitric acid.
1768:
at ambient temperatures. There is some disagreement over the value of the acid dissociation constant, though the
301:
3936:
3788:. Vol. 1. Cushing/Whitney Medical Library, Yale University. London, H. Colburn, and R. Bentley. p. 40.
810:
7938:
7870:
7322:
4874:
3779:
3542:
1859:
1748:
351:
1467:
for the efficient production of ammonia was introduced in 1913, nitric acid production from ammonia using the
3050:. Its ability to dissolve certain metals selectively or be a solvent for many metal salts makes it useful in
7693:
7669:
7633:
7353:
7310:
5659:
2248:
680:
7140:
1229:. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into
815:
289:
7923:
7858:
7834:
7741:
7574:
7369:
6578:
6369:
5606:
3683:
3002:
2587:
2437:
1636:
1620:
1249:
736:
357:
7782:
2629:
Laboratory-scale nitric acid syntheses abound. Most take inspiration from the industrial techniques.
7822:
7810:
7758:
7657:
7645:
7471:
7427:
7338:
7286:
7207:
7159:
7129:
7083:
6893:
6881:
6774:
6445:
6348:
5964:
5738:
5343:
3455:
3108:
1956:
1723:
1719:
1408:
1351:
879:
843:
820:
803:
626:
102:
3930:
1452:
energy intensive and was rapidly displaced by the
Ostwald process once cheap ammonia became available.
251:
7491:
4498:
4160:
Allan, D. R.; Marshall, W. G.; Francis, D. J.; Oswald, I. D. H.; Pulham, C. R.; Spanswick, C. (2010).
3299:
He goes on to point out that "nitrous air" is the reverse, or "nitric acid deprived of air and water."
1471:
overtook production from the
Birkeland–Eyde process. This method of production is still in use today.
7729:
7705:
7681:
7451:
7117:
7059:
7027:
6857:
6380:
6361:
6159:
6147:
5435:
4768:
4304:
4211:
4129:
4090:
2998:
1831:
1658:
1132:
1127:
6563:
4895:
2452:, the color turns orange. These color changes are caused by nitrated aromatic rings in the protein.
1411:
devised a process to obtain nitric acid by distilling potassium nitrate with sulfuric acid. In 1776
7846:
7770:
7717:
7420:
7408:
7183:
6939:
6388:
6353:
6104:
5513:
5477:
5368:
5280:
5023:
5013:
4937:
1372:
1122:
454:
193:
152:
6466:
4239:
A comprehensive database of published data on equilibrium constants of metal complexes and ligands
1423:
determined its precise composition and showed that it could be synthesized by passing a stream of
7462:
7393:
6970:
6951:
6928:
6650:
6490:
6482:
6396:
6259:
6096:
5771:
5626:
5539:
5495:
5285:
4974:
4796:
4474:
4460:
4004:
3957:
3679:
3557:
3518:
3479:
3063:
2937:
2700:
2503:
2453:
2125:
1855:
1331:
1327:
1323:
1204:
609:
83 °C (181 °F; 356 K) 68% solution boils at 121 °C (250 °F; 394 K)
3652:
as the work's author, but this is likely a conflation with several other Latin treatises called
3618:(died before 1236) but perhaps translated by him from the Arabic. One of the manuscripts of the
3022:
Nitric acid can be used to convert metals to oxidized forms, such as converting copper metal to
2593:
Commercial grade nitric acid solutions are usually between 52% and 68% nitric acid by mass, the
1532:
An older density scale is occasionally seen, with concentrated nitric acid specified as 42
211:
4870:
3042:). These salts can be used to purify gold and other metals beyond 99.9% purity by processes of
7621:
7381:
7071:
7045:
6963:
6916:
6823:
6813:
6762:
6750:
6717:
6677:
6637:
6571:
6272:
5691:
5673:
5553:
5379:
5197:
5192:
5178:
5090:
5085:
5018:
4831:
4737:
4660:
4627:
4581:
4556:
4464:
4396:
4392:
4386:
4360:
4335:
4263:
4184:
4040:
4036:
4026:
3840:
3733:
3573:
3563:
3528:
3505:
3499:
3459:
3388:
3027:
2825:
2813:
2704:
2693:
2218:
2164:
2112:
1933:
1654:
1412:
1142:
1099:
1050:
4295:Ōsawa, Eiji (December 2007). "Recent progress and perspectives in single-digit nanodiamond".
4060:
3567:
3522:
1395:(both 13th century). These works describe the distillation of a mixture containing niter and
7439:
7037:
7012:
6905:
6845:
6806:
6798:
6732:
6725:
6704:
6541:
6320:
6215:
6140:
6012:
5824:
5528:
5449:
5325:
5242:
5125:
5050:
5045:
5034:
4823:
4786:
4776:
4729:
4548:
4456:
4312:
4219:
4176:
4137:
4098:
3649:
3639:
3490:
3447:
3245:
3148:
3143:
The corrosive effects of nitric acid are exploited for some specialty applications, such as
3104:
3039:
2817:
2795:
2507:
2372:
2312:
2198:
involved, the end products can be variable. Reaction takes place with all metals except the
2138:
2040:
1416:
1396:
1388:
1341:
1268:
1245:
1147:
1104:
699:
615:
536:
4162:"The crystal structures of the low-temperature and high-pressure polymorphs of nitric acid"
4076:
Cox, A. P.; Ellis, M. C.; Attfield, C. J.; Ferris, A. C. (1994). "Microwave spectrum of DNO
3865:
3758:
2957:
415:
7298:
7263:
7195:
7171:
7020:
6994:
6869:
6831:
6684:
6629:
6503:
6305:
6203:
5720:
5621:
5463:
5207:
5202:
5115:
3229:
3221:
3166:
3123:
In a low concentration (approximately 10%), nitric acid is often used to artificially age
3112:
2899:
2833:
2469:
2457:
2399:
2293:
2283:, nitric acid reacts with many organic materials, and the reactions may be explosive. The
2157:
and platinum-group metals do not react with nitric acid, though pure gold does react with
1468:
1420:
1280:
320:
162:
46:
4933:
3915:
2483:
The
Ostwald process' technical innovation is the proper conditions under which anhydrous
1056:
271:
4772:
4601:
4308:
4215:
4133:
4094:
3998:
3951:
1704:
1544:
231:
7794:
7597:
7586:
7499:
7271:
7241:
7234:
7152:
6742:
6457:
6412:
6404:
6084:
6071:
6049:
5948:
5782:
5702:
5631:
5295:
5075:
4706:
ISO 14104:2017 - Gears - Surface temper etch inspection after grinding, chemical method
4653:
4059:
Wolfram
Research, Inc., Wolfram|Alpha Knowledgebase, Champaign, IL (2022) —
3495:
3351:
3132:
3131:. The color produced is a grey-gold very much like very old wax- or oil-finished wood (
3023:
2652:
2280:
2195:
2188:
2150:
1556:
or light decomposition and for this reason it was often stored in brown glass bottles:
1533:
1384:
1368:
1272:
1230:
1159:
1137:
1094:
857:
667:
639:
458:
293:
4827:
4202:
Cox, A. P.; Riveros, J. M. (1965). "Microwave
Spectrum and Structure of Nitric Acid".
3569:
Atoms and Alchemy: Chymistry and the Experimental Origins of the Scientific Revolution
7907:
7249:
6669:
6521:
6420:
6283:
6267:
6238:
5927:
5796:
5645:
5564:
5354:
5266:
5252:
5232:
5164:
5130:
5095:
4985:
4800:
4518:
4478:
4258:
Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 15: The group 15 elements".
4102:
3975:
3860:
3834:
3753:
3271:
Being a strong oxidizing agent, nitric acid can react violently with many compounds.
3225:
2978:
2922:
2689:
2637:
2615:
2602:
2473:
2412:
2289:
2214:
2103:
1843:
1789:
1661:
in metal tanks. The fluoride creates a metal fluoride layer that protects the metal.
1464:
1439:
The industrial production of nitric acid from atmospheric air began in 1905 with the
1297:
604:
594:
282:
4850:
3783:
6982:
6529:
6474:
6428:
6292:
6116:
6092:
6054:
5914:
5810:
5756:
5592:
5578:
5421:
5407:
5305:
5135:
5105:
5055:
4999:
4791:
3887:
3615:
3280:
3051:
2982:
2949:
2684:
2488:
2316:
2222:
2180:
2032:
1979:
1448:
1432:
1424:
1305:
1226:
1076:
31:
4316:
1734:. The third N–O bond is elongated because its O atom is bonded to H atom, with a
6436:
6222:
6135:
5212:
5065:
4236:
4032:
3171:
2974:
2941:
2821:
2703:
gives nitrogen dioxide and oxygen gases; these are then passed through water or
2598:
2445:
2199:
1765:
1739:
1735:
1731:
1727:
1457:
1392:
1264:
987:
963:
379:
3909:
3800:
951:
6044:
5393:
5222:
4922:
4691:
4142:
4117:
3333:
3257:
3031:
2791:
2782:
2492:
2441:
2390:
2381:
2299:
Reaction with non-metallic elements, with the exceptions of nitrogen, oxygen,
2176:
2159:
2075:
1444:
1300:
such as nitric acid is generally believed to go back to 13th-century European
553:
262:
4552:
3875:. Vol. 19 (11th ed.). Cambridge University Press. pp. 711–712.
3768:. Vol. 19 (11th ed.). Cambridge University Press. pp. 711–712.
6517:
6247:
6190:
6030:
5936:
3214:
2807:
2594:
2477:
2300:
2268:
2244:
1907:
1903:
1685:
1681:
1480:
1276:
1260:
1223:
849:
426:
4835:
4741:
4188:
3608:, p. 62, recipes for the preparation of nitric acid also occur in the
4704:
2315:
as acids with the formation of nitrogen dioxide for concentrated acid and
1639:, or RFNA, contains substantial quantities of dissolved nitrogen dioxide (
853:
6525:
6211:
6022:
5906:
5883:
5300:
4733:
3643:
3561:
was long considered to be the earliest known recipe for sulfuric acid ".
3202:
3178:
2994:
2284:
2260:
2236:
2099:
2095:
2071:
1915:
1670:
1212:
1209:
950:
943:
936:
929:
902:
3976:"The Production of Nitrates by the Direct Electrolysis of Peat Deposits"
2448:
rings are present, the mixture turns yellow. Upon adding a base such as
6696:
6595:
6547:
6535:
6511:
6178:
6123:
6066:
6039:
6017:
6007:
5922:
5898:
3261:
3233:
3144:
3086:
3082:
3071:
2945:
2841:
2633:
2484:
2449:
2433:
2308:
2304:
2184:
2091:
1301:
672:
584:
366:
302:
125:
4223:
3627:
3165:
Commercially available aqueous blends of 5–30% nitric acid and 15–40%
4943:
4523:
4180:
3078:
3067:
3006:
2977:, is produced on a large scale by oxidation of "KA oil"—a mixture of
2894:
Either concentrated sulfuric acid or oleum absorbs the excess water.
2363:
2264:
2256:
2172:
2168:
2083:
2079:
2036:
2002:
1998:
1215:
719:
242:
4756:
3836:
The Edge of Objectivity: An Essay in the History of Scientific Ideas
1158:
Except where otherwise noted, data are given for materials in their
17:
5852:
4781:
2070:
Upon reaction with nitric acid, most metals give the corresponding
1747:
and NOH planes are tilted away from each other by 2°) and there is
1304:. The conventional view is that nitric acid was first described in
340:
5999:
5994:
5986:
5956:
4637:
3864:
3757:
3253:
3241:
3237:
3194:
3155:
3128:
2970:
2914:
2883:
2781:
2535:
2203:
1738:
of 1.41 Å in the gas phase. The molecule is slightly aplanar (the
1730:
character in these two bonds, causing them to be shorter than N–O
1703:
1543:
390:
222:
192:
185:
175:
3093:. Typically these digestions use a 50% solution of the purchased
2213:). However, the powerful oxidizing properties of nitric acid are
937:
6553:
4282:
3524:
Science and Technology in Islam: Technology and applied sciences
3249:
3217:
3124:
3035:
2790:
The main industrial use of nitric acid is for the production of
2436:
to form yellow nitrated products. This reaction is known as the
2252:
2240:
2154:
1911:
1899:
1553:
1376:
1038:
861:
574:
331:
6567:
5856:
4947:
4685:
Muraoka, Hisashi (1995) "Silicon wafer cleaning fluid with HNO
3839:. Princeton, NJ: Princeton University Press. pp. 223–24.
3182:
2860:
2087:
1788:
Nitric acid can act as a base with respect to an acid such as
1428:
1085:
1070:
3418:. Fisher Scientific International. 23 March 2015. p. 2.
4624:
Standard Methods For the Examination of Water and Wastewater
3279:
Nitric acid is one of the most common types of acid used in
2820:. A mixture of nitric and sulfuric acids introduces a nitro
2476:
are extremely efficient, requiring only air and natural gas
2217:
in nature, but sometimes its oxidation reactions are rather
1548:
Fuming nitric acid contaminated with yellow nitrogen dioxide
442:
81:
71:
2816:, industrial and otherwise, the nitro group is a versatile
4418:"The Ostwald Process & Catalytic Oxidation of Ammonia"
2311:
other than iodine, usually oxidizes them to their highest
3185:, giving a variety of colours depending on the alkaloid.
914:
1248:
present, fuming nitric acid is further characterized as
4761:
Publications of the Astronomical Society of the Pacific
4080:, and average structures of nitric and nitrous acids".
3268:
immediately and the underlying skin washed thoroughly.
1176:
4928:
National Pollutant Inventory – Nitric Acid Fact Sheet
3802:
An Illustrated History of Alchemy and Early Chemistry
3624:
mentions that it was translated by Michael Scot; see
3338:
National Institute for Occupational Safety and Health
3107:, nitric acid is used as a chemical doping agent for
4519:"H2O2-mediated electrosynthesis of nitrate from air"
4442:
4440:
4438:
3691:
3668:
3498:; Ping-Yü, Ho; Gwei-Djen, Lu; Sivin, Nathan (1980).
3369:(2nd ed.), Ithaca, NY: Cornell University Press
2187:
shops to quickly spot low-gold alloys (< 14
1358:
1345:
1335:
6502:
6341:
6083:
5979:
5891:
4896:"Freeze mob to highlight the issue of acid attacks"
4626:(21 ed.). American Public Health Association.
4118:"Structure cristalline de l'acide nitrique anhydre"
3726:Ḥasan, Aḥmad Yūsuf; Hill, Donald Routledge (1986).
1363:
attributed to Jabir has been translated as follows:
4652:
3005:) was one of three liquid fuel components for the
2993:Nitric acid has been used in various forms as the
2794:. Nitric acid is neutralized with ammonia to give
2560:The net reaction is maximal oxidation of ammonia:
2502:). The nitric oxide is then oxidized, often with
1997:Nitric acid can oxidize non-active metals such as
1785:value rises to 1 at a temperature of 250 °C.
3712:Discovery: A Monthly Popular Journal of Knowledge
1625:Commercial-grade fuming nitric acid contains 98%
1330:, the nitric acid also occurs in various earlier
210:
378:
4453:Kirk-Othmer Encyclopedia of Chemical Technology
2538:to nitric acid and the nitric oxide feedstock:
918:
161:
4544:Ullmann's Encyclopedia of Industrial Chemistry
3601:
3201:methods, where it can dissolve many different
3154:A solution of nitric acid, water and alcohol,
2221:non-favored. The presence of small amounts of
1493:Two solid hydrates are known: the monohydrate
6579:
5868:
4959:
3174:against corrosion by the dilute nitric acid.
1399:, which they call "eau forte" (aqua fortis).
1383:Nitric acid is also found in post-1300 works
8:
4461:10.1002/0471238961.1409201803120118.a01.pub3
4388:Chemical and process technology encyclopedia
4380:
4378:
4376:
4357:A practical course in agricultural chemistry
4253:
4251:
4249:
4247:
4245:
4000:The Manufacture of Chemicals by Electrolysis
3932:Industrial Nitrogen Compounds and Explosives
3541:Karpenko, Vladimír; Norris, John A. (2002).
2163:, a mixture of concentrated nitric acid and
1259:Nitric acid is the primary reagent used for
86:Resonance space-filling model of nitric acid
3969:
3967:
3929:Martin, Geoffrey; Barbour, William (1915).
3903:
3901:
3732:. Cambridge University Press. p. 147.
3653:
3619:
3609:
3177:Nitric acid can be used as a spot test for
3151:or cleaning silicon wafers in electronics.
2468:Industrial nitric acid production uses the
1764:Nitric acid is normally considered to be a
6586:
6572:
6564:
5875:
5861:
5853:
4966:
4952:
4944:
4536:
4534:
3888:"On Some Chemical Agencies of Electricity"
3729:Islamic Technology: An Illustrated History
3584:p. 98, quote: " between the time when the
3026:. It can also be used in combination with
2232:) greatly increases the rate of reaction.
457:
292:
270:
38:
4790:
4780:
4141:
3697:
3674:
3648:p. 115 (no. 22). Al-Hassan 2001 mentions
3614:, a Latin treatise usually attributed to
3605:
3504:. Cambridge: Cambridge University Press.
3442:
3440:
3387:. Houghton Mifflin Company. p. A22.
2601:to 98% can be achieved with concentrated
2191:) and to rapidly assess the gold purity.
1479:Commercially available nitric acid is an
414:
6776:
6710:
6706:
6671:
6652:
5838:
5827:
5817:
5813:
5803:
5799:
5789:
5785:
5774:
5763:
5759:
5749:
5745:
5741:
5731:
5727:
5723:
5713:
5709:
5705:
5694:
5684:
5680:
5676:
5666:
5662:
5652:
5648:
5638:
5634:
5613:
5609:
5599:
5595:
5585:
5581:
5571:
5567:
5556:
5546:
5542:
5531:
5520:
5516:
5506:
5502:
5498:
5488:
5484:
5480:
5470:
5466:
5456:
5452:
5442:
5438:
5428:
5424:
5414:
5410:
5400:
5396:
5386:
5382:
5371:
5361:
5357:
5346:
5336:
5332:
5328:
5318:
5308:
5288:
5273:
5269:
5259:
5255:
5245:
5235:
5225:
5215:
5185:
5181:
5171:
5167:
5157:
5153:
5149:
5142:
5138:
5118:
5108:
5098:
5078:
5068:
5058:
5037:
5026:
5006:
5002:
4992:
4988:
4155:
4153:
3572:. Chicago: University of Chicago Press.
3332:NIOSH Pocket Guide to Chemical Hazards.
3111:, and in purification processes for raw
3096:
2928:
2924:
2916:
2909:
2905:
2901:
2885:
2878:
2874:
2870:
2866:
2862:
2856:
2851:
2847:
2843:
2770:
2766:
2762:
2758:
2745:
2741:
2737:
2733:
2725:
2721:
2717:
2713:
2699:Alternatively, thermal decomposition of
2677:
2673:
2669:
2665:
2661:
2647:
2643:
2609:
2605:
2578:
2574:
2570:
2566:
2552:
2548:
2544:
2527:
2523:
2513:
2498:
2419:
2415:
2406:
2402:
2393:
2384:
2375:
2366:
2354:
2350:
2346:
2337:
2333:
2329:
2325:
2228:
2209:
2141:
2132:
2128:
2119:
2115:
2106:
2062:
2058:
2023:
2019:
2015:
2011:
1990:
1985:
1981:
1975:
1967:
1962:
1958:
1952:
1944:
1939:
1935:
1929:
1918:
1885:
1872:
1868:
1849:
1814:
1810:
1806:
1802:
1798:
1742:
1711:
1691:
1676:
1642:
1628:
1605:
1598:
1594:
1587:
1574:
1570:
1566:
1562:
1520:
1512:
1508:
1496:
1486:
1236:
1218:
544:
4071:
4069:
3892:The Collected Works of Sir Humphry Davy
3311:
3292:
2802:Precursor to organic nitrogen compounds
1708:Two major resonance representations of
513:
478:
453:
356:
6619:
6594:Salts and covalent derivatives of the
4923:NIOSH Pocket Guide to Chemical Hazards
3425:from the original on 10 September 2022
3327:
3325:
3323:
3321:
3319:
3317:
3315:
2534:The dioxide then disproportionates in
599:−42 °C (−44 °F; 231 K)
283:
7563:
4871:"Nitric acid: Toxicological overview"
3825:
3823:
3543:"Vitriol in the History of Chemistry"
2618:ceased using that process in 2012.
1483:with water at a concentration of 68%
485:Key: GRYLNZFGIOXLOG-UHFFFAOYSA-N
250:
230:
7:
6606:
4938:densities, molarities and molalities
3956:. D. Van Nostrand Company. pp.
3953:The Fixation of Atmospheric Nitrogen
3378:
3376:
3260:yellow due to its reaction with the
2035:produced may react with atmospheric
1312:("On the Discovery of Truth", after
4385:Considine, Douglas M., ed. (1974).
3935:. Crosby Lockwood and Son. p.
3690:p. 60. For the claim regarding the
3256:). Concentrated nitric acid stains
2830:electrophilic aromatic substitution
2167:. However, some less noble metals (
1540:Contamination with nitrogen dioxide
1275:are shock- and thermally-sensitive
495:Key: GRYLNZFGIOXLOG-UHFFFAOYAO
369:
339:
76:Ball-and-stick model of nitric acid
4755:Curtis, Heber D. (February 1911).
4689:, HF, HCl, surfactant, and water"
4391:. New York: McGraw-Hill. pp.
3914:. Longmans, Green and Co. p.
3224:. The major hazard posed by it is
2362:Concentrated nitric acid oxidizes
1902:in its reaction with most metals.
1340:("Chest of Wisdom") attributed to
25:
3244:), which consequently decomposes
3034:to dissolve noble metals such as
2595:maximum distillable concentration
2456:is formed when the acid contacts
1657:(HF). This fluoride is added for
1350:attributed to the Fatimid caliph
482:InChI=1S/HNO3/c2-1(3)4/h(H,2,3,4)
3193:Nitric acid plays a key role in
2956:
2179:relatively poor in gold such as
1684:nitric acid is a colorless, low-
1475:Physical and chemical properties
1252:at concentrations above 86%, or
1166:
819:
814:
809:
492:InChI=1/HNO3/c2-1(3)4/h(H,2,3,4)
58:
45:
4636:. Also available on CD-ROM and
4204:The Journal of Chemical Physics
4003:. D. Van Nostrand Co. pp.
3493:, in the works of Europeans ".
1591:) and/or dinitrogen tetroxide (
1162:(at 25 °C , 100 kPa).
4816:Forensic Science International
4757:"Methods of Silvering Mirrors"
3091:atomic absorption spectroscopy
1611:) are soluble in nitric acid.
1:
4894:Rees, Anna (1 October 2013).
4828:10.1016/S0379-0738(99)00235-2
4330:Sherman, Henry Clapp (2007).
4317:10.1016/j.diamond.2007.08.008
4297:Diamond and Related Materials
4028:Lange's Handbook of Chemistry
3894:. Vol. 5. pp. 1–12.
3667:For the claims regarding the
1313:
1256:at concentrations above 95%.
1244:. Depending on the amount of
1004:(US health exposure limits):
27:Highly corrosive mineral acid
4722:Cold Spring Harbor Protocols
4580:. Rutgers University Press.
4517:Dong, Kai (April 19, 2024).
4103:10.1016/0022-2860(93)08008-R
3100:mixed with Type 1 DI Water.
2651:) — for example,
2472:. The combined Ostwald and
1751:about the N–OH single bond.
4849:May, Paul (November 2007).
4334:. Read Books. p. 315.
4332:Methods of organic analysis
3831:Gillispie, Charles Coulston
3692:
3669:
3626:Moureau, Sébastien (2020).
3383:Zumdahl, Steven S. (2009).
2832:. Many explosives, such as
2786:Nitric acid in a laboratory
2707:as in the Ostwald process:
1854:, is the active reagent in
1359:
1346:
1336:
976:or concentration (LD, LC):
67:
7960:
7565:
4359:. Read Books. p. 76.
3911:Modern Inorganic Chemistry
3688:. Oxford: Clarendon Press.
3602:Karpenko & Norris 2002
3385:Chemical Principles 6th Ed
3139:Etchant and cleaning agent
2805:
1618:
1552:Nitric acid is subject to
29:
6621:
6603:
4981:
4262:(3rd ed.). Pearson.
4143:10.1107/S0365110X51000404
3452:Chemistry of the Elements
3450:; Earnshaw, Alan (1997).
3199:nuclear fuel reprocessing
3189:Nuclear fuel reprocessing
2836:, are prepared this way:
2275:Reactions with non-metals
1156:
1062:
998:
972:
790:
785:
729:
529:
504:
469:
145:
111:
101:
96:
66:
57:
44:
4875:Health Protection Agency
4553:10.1002/14356007.a17_293
4171:(Submitted manuscript).
3980:London Electrical Review
3886:Davy, John, ed. (1839).
3785:The history of chemistry
3484:The Origins of Chemistry
3149:pickling stainless steel
2944:, allowing synthesis of
2432:Nitric acid reacts with
1860:self-ionization of water
1254:white fuming nitric acid
870:Precautionary statements
30:Not to be confused with
4792:2027/mdp.39015018047608
4547:. Weinheim: Wiley-VCH.
4355:Knowles, Frank (2007).
3872:Encyclopædia Britannica
3799:Katz, David A. (2008).
3765:Encyclopædia Britannica
3592:, had been discovered."
3367:The Proton in Chemistry
3048:selective precipitation
2948:compounds from various
2936:The nitro group can be
2636:salts metathesize with
2175:, ...) present in some
1375:and two parts of Yemen
1310:De inventione veritatis
1271:. While some resulting
1240:, it is referred to as
681:Magnetic susceptibility
7929:Photographic chemicals
4940:of aqueous nitric acid
4576:Clark, John D (1972).
4122:Acta Crystallographica
3908:Mellor, J. W. (1918).
3654:
3620:
3610:
3109:organic semiconductors
3003:red fuming nitric acid
2787:
2588:red fuming nitric acid
2438:xanthoproteic reaction
2345:3 C (graphite) + 4 HNO
1715:
1637:Red fuming nitric acid
1621:Red fuming nitric acid
1584:The nitrogen dioxide (
1549:
1441:Birkeland–Eyde process
1381:
1322:However, according to
1281:strong oxidizing agent
1250:red fuming nitric acid
994:138 ppm (rat, 30 min)
925:
589:1.51 g/cm, 1.41 g/cm
87:
77:
7944:Nitrogen(V) compounds
7934:Drug testing reagents
4692:U.S. patent 5,635,463
4655:Hand-applied finishes
4651:Jewitt, Jeff (1997).
3997:Hale, Arthur (1919).
3950:Knox, Joseph (1914).
3655:Liber Luminis luminum
3621:Liber Luminis luminum
3611:Liber Luminis luminum
3456:Butterworth-Heinemann
3448:Greenwood, Norman N.
2999:liquid-fueled rockets
2785:
1894:Reactions with metals
1707:
1700:Structure and bonding
1665:Anhydrous nitric acid
1547:
1409:Johann Rudolf Glauber
1407:In the 17th century,
1369:pure flowers of nitre
1365:
1352:al-Hakim bi-Amr Allah
1344:(8th century) or the
924:
85:
75:
4734:10.1101/pdb.prot4988
4116:Luzzati, V. (1951).
3715:. John Murray. 1924.
3486:. London: Oldbourne.
3458:. pp. 465–471.
3365:Bell, R. P. (1973),
3228:, as it carries out
2625:Laboratory synthesis
2324:C (graphite) + 4 HNO
1832:Equilibrium constant
1760:Acid-base properties
1659:corrosion resistance
1263:– the addition of a
1133:Dinitrogen pentoxide
1128:Dinitrogen tetroxide
988:median concentration
907:(fire diamond)
4773:1911PASP...23...13C
4728:(6): pdb.prot4988.
4309:2007DRM....16.2018O
4260:Inorganic Chemistry
4216:1965JChPh..42.3106C
4134:1951AcCry...4..120L
4095:1994JMoSt.320...91C
4025:Dean, John (1992).
3866:"Nitric Acid"
3759:"Nitric Acid"
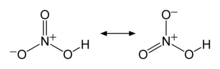
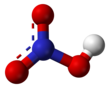
3685:Makers Of Chemistry
3680:Holmyard, John Eric
3519:Al-Hassan, Ahmad Y.
3480:Multhauf, Robert P.
3409:"Safety Data Sheet"
3275:Use in acid attacks
2985:—with nitric acid.
2202:series and certain
1749:restricted rotation
1517:and the trihydrate
1505:or oxonium nitrate
1367:Take five parts of
1123:Dinitrogen trioxide
1032:ST 4 ppm (10 mg/m)
1017:TWA 2 ppm (5 mg/m)
616:Solubility in water
579:Acrid, suffocating
561: g·mol
41:
7914:Hydrogen compounds
4975:Hydrogen compounds
3586:Summa perfectionis
3564:Newman, William R.
3558:Summa perfectionis
3352:"nitric acid_msds"
3064:elemental analysis
3058:Analytical reagent
2826:aromatic compounds
2788:
2701:copper(II) nitrate
2632:A wide variety of
2504:atmospheric oxygen
2454:Xanthoproteic acid
2428:Xanthoproteic test
2102:are oxidized into
1856:aromatic nitration
1716:
1615:Fuming nitric acid
1550:
1436:by damp asbestos.
1385:falsely attributed
1357:The recipe in the
1328:Ahmad Y. al-Hassan
1324:Eric John Holmyard
1267:, typically to an
1242:fuming nitric acid
1231:oxides of nitrogen
1205:inorganic compound
1189:Infobox references
1063:Related compounds
1041:(Immediate danger)
1030:TWA 2 ppm (5 mg/m)
926:
88:
78:
39:
7919:Nitrogen oxoacids
7901:
7900:
7895:
7894:
6561:
6560:
5850:
5849:
4666:978-1-56158-154-2
4659:. Taunton Press.
4633:978-0-87553-047-5
4587:978-0-8135-0725-5
4455:. pp. 1–37.
4402:978-0-07-012423-3
4366:978-1-4067-4583-2
4341:978-1-4086-2802-7
4303:(12): 2018–2022.
4269:978-0-13-175553-6
4237:IUPAC SC-Database
4224:10.1063/1.1696387
4175:(15): 3736–3743.
4061:via Wolfram|Alpha
4046:978-0-07-016194-8
3974:Dary, G. (1913).
3739:978-92-3-102294-4
3534:978-92-3-103831-0
3511:978-0-521-08573-1
3465:978-0-08-037941-8
3394:978-0-618-94690-7
3213:Nitric acid is a
3089:, GFAA and flame
3044:recrystallization
3028:hydrochloric acid
2989:Rocket propellant
2969:The precursor to
2965:Use as an oxidant
2814:organic synthesis
2705:hydrogen peroxide
2694:magnesium nitrate
2319:for dilute acid.
2279:Being a powerful
2165:hydrochloric acid
1655:hydrogen fluoride
1413:Antoine Lavoisier
1371:, three parts of
1296:The discovery of
1222:. It is a highly
1207:with the formula
1197:Chemical compound
1195:
1194:
1143:Nitrogen monoxide
1115:Related compounds
1100:Potassium nitrate
1051:Safety data sheet
844:Hazard statements
569:Colorless liquid
438:CompTox Dashboard
194:Interactive image
187:Interactive image
92:
91:
16:(Redirected from
7951:
6789:
6787:
6786:
6783:
6714:
6675:
6665:
6663:
6662:
6659:
6607:
6588:
6581:
6574:
6565:
6551:
6539:
6515:
6504:Oxidation states
6333:
6332:
6301:
6300:
6235:
6234:
6199:
6198:
6187:
6186:
6132:
6131:
5877:
5870:
5863:
5854:
5842:
5831:
5820:
5806:
5792:
5778:
5767:
5752:
5734:
5716:
5698:
5687:
5669:
5655:
5641:
5617:
5602:
5588:
5574:
5560:
5549:
5535:
5524:
5509:
5491:
5473:
5459:
5445:
5431:
5417:
5403:
5389:
5375:
5364:
5350:
5339:
5321:
5311:
5291:
5276:
5262:
5248:
5238:
5228:
5218:
5188:
5174:
5160:
5145:
5121:
5111:
5101:
5081:
5071:
5061:
5041:
5030:
5009:
4995:
4968:
4961:
4954:
4945:
4934:surface tensions
4911:
4910:
4908:
4906:
4891:
4885:
4884:
4882:
4881:
4867:
4861:
4860:
4858:
4857:
4846:
4840:
4839:
4811:
4805:
4804:
4794:
4784:
4752:
4746:
4745:
4717:
4711:
4710:
4701:
4695:
4694:
4683:
4677:
4676:
4674:
4673:
4658:
4648:
4642:
4641:
4640:by subscription.
4619:
4613:
4612:
4610:
4609:
4602:"BOMARC Summary"
4598:
4592:
4591:
4573:
4567:
4566:
4538:
4529:
4528:
4514:
4508:
4507:
4506:
4502:
4495:
4489:
4488:
4486:
4485:
4444:
4433:
4432:
4430:
4428:
4413:
4407:
4406:
4382:
4371:
4370:
4352:
4346:
4345:
4327:
4321:
4320:
4292:
4286:
4285:standard A967-05
4280:
4274:
4273:
4255:
4240:
4234:
4228:
4227:
4199:
4193:
4192:
4181:10.1039/B923975H
4166:
4157:
4148:
4147:
4145:
4113:
4107:
4106:
4073:
4064:
4057:
4051:
4050:
4022:
4016:
4015:
4013:
4012:
3994:
3988:
3987:
3971:
3962:
3961:
3947:
3941:
3940:
3926:
3920:
3919:
3905:
3896:
3895:
3883:
3877:
3876:
3868:
3857:
3851:
3850:
3827:
3818:
3817:
3815:
3813:
3807:
3796:
3790:
3789:
3776:
3770:
3769:
3761:
3750:
3744:
3743:
3723:
3717:
3716:
3707:
3701:
3695:
3693:Taʿwīdh al-Ḥākim
3689:
3672:
3665:
3659:
3657:
3650:Abu Bakr al-Razi
3647:
3623:
3613:
3599:
3593:
3583:
3554:
3538:
3515:
3487:
3476:
3470:
3469:
3454:(2nd ed.).
3444:
3435:
3434:
3432:
3430:
3424:
3413:
3405:
3399:
3398:
3380:
3371:
3370:
3362:
3356:
3355:
3348:
3342:
3341:
3329:
3300:
3297:
3147:in printmaking,
3113:carbon nanotubes
3105:electrochemistry
3099:
3040:chloroauric acid
3018:Metal processing
2960:
2932:
2890:
2818:functional group
2796:ammonium nitrate
2773:
2755:
2751:
2748:
2728:
2680:
2650:
2612:
2582:
2556:
2530:
2516:
2508:nitrogen dioxide
2501:
2491:(NO) instead of
2458:epithelial cells
2422:
2409:
2396:
2387:
2378:
2369:
2358:
2341:
2313:oxidation states
2231:
2212:
2144:
2135:
2122:
2109:
2086:; for instance,
2066:
2056:
2055:
2052:
2041:nitrogen dioxide
2027:
1993:
1970:
1947:
1921:
1889:
1883:
1882:
1879:
1853:
1830:
1826:
1825:
1824:
1821:
1745:
1722:; the two major
1714:
1694:
1679:
1645:
1631:
1610:
1601:
1590:
1577:
1528:
1516:
1504:
1489:
1417:Joseph Priestley
1389:Albert the Great
1362:
1349:
1347:Taʿwīdh al-Ḥākim
1342:Jabir ibn Hayyan
1339:
1318:
1315:
1292:Medieval alchemy
1269:organic molecule
1246:nitrogen dioxide
1239:
1221:
1179:
1173:
1170:
1169:
1148:Nitrogen dioxide
1105:Ammonium nitrate
953:
946:
939:
932:
917:
897:
893:
889:
885:
881:
877:
863:
859:
855:
851:
823:
818:
813:
777:
750:
730:Thermochemistry
713:1.397 (16.5 °C)
700:Refractive index
693:
691:
644:48 mmHg (20 °C)
560:
547:
537:Chemical formula
462:
461:
446:
444:
418:
382:
371:
360:
343:
321:Gmelin Reference
304:
296:
285:
274:
254:
234:
214:
196:
189:
165:
132:Hydrogen nitrate
68:
62:
52:Pure nitric acid
49:
42:
21:
7959:
7958:
7954:
7953:
7952:
7950:
7949:
7948:
7939:Oxidizing acids
7904:
7903:
7902:
7897:
7896:
7878:
7874:
7866:
7862:
7854:
7850:
7842:
7838:
7830:
7826:
7818:
7814:
7806:
7802:
7798:
7790:
7786:
7778:
7774:
7766:
7762:
7749:
7745:
7737:
7733:
7725:
7721:
7713:
7709:
7701:
7697:
7689:
7685:
7677:
7673:
7665:
7661:
7653:
7649:
7641:
7637:
7629:
7625:
7617:
7613:
7605:
7601:
7596:
7594:
7590:
7582:
7578:
7507:
7503:
7495:
7479:
7475:
7466:
7461:
7459:
7455:
7447:
7443:
7435:
7431:
7426:
7424:
7416:
7412:
7407:
7405:
7401:
7397:
7389:
7385:
7377:
7373:
7365:
7361:
7357:
7346:
7342:
7334:
7330:
7326:
7318:
7314:
7306:
7302:
7294:
7290:
7279:
7275:
7267:
7257:
7253:
7245:
7240:
7238:
7227:
7223:
7219:
7215:
7211:
7203:
7199:
7191:
7187:
7179:
7175:
7167:
7163:
7158:
7156:
7148:
7144:
7139:
7137:
7133:
7125:
7121:
7107:
7103:
7099:
7091:
7087:
7079:
7075:
7067:
7063:
7053:
7049:
7041:
7026:
7024:
7016:
7002:
6998:
6990:
6986:
6978:
6974:
6969:
6967:
6959:
6955:
6947:
6943:
6938:
6936:
6932:
6924:
6920:
6915:
6913:
6909:
6901:
6897:
6889:
6885:
6877:
6873:
6865:
6861:
6853:
6849:
6839:
6835:
6827:
6812:
6810:
6802:
6784:
6781:
6780:
6778:
6773:
6772:
6770:
6766:
6758:
6754:
6746:
6731:
6729:
6721:
6712:
6708:
6703:
6702:
6700:
6692:
6688:
6683:
6681:
6676:
6673:
6668:
6660:
6657:
6656:
6654:
6649:
6645:
6641:
6633:
6614:
6599:
6592:
6562:
6557:
6545:
6533:
6509:
6498:
6494:
6486:
6478:
6470:
6461:
6453:
6449:
6440:
6432:
6424:
6416:
6408:
6400:
6392:
6384:
6374:
6365:
6357:
6337:
6331:
6328:
6327:
6326:
6324:
6317:
6313:
6309:
6299:
6296:
6295:
6294:
6287:
6280:
6276:
6263:
6255:
6251:
6242:
6233:
6230:
6229:
6228:
6226:
6219:
6207:
6197:
6194:
6193:
6192:
6185:
6182:
6181:
6180:
6175:
6167:
6163:
6155:
6151:
6144:
6130:
6127:
6126:
6125:
6120:
6112:
6108:
6100:
6079:
6075:
6062:
6058:
6034:
6026:
6003:
5990:
5975:
5969:
5960:
5952:
5944:
5940:
5931:
5918:
5910:
5902:
5887:
5881:
5851:
5846:
5840:
5836:
5829:
5825:
5819:
5815:
5811:
5805:
5801:
5797:
5791:
5787:
5783:
5776:
5772:
5765:
5761:
5757:
5751:
5747:
5743:
5739:
5733:
5729:
5725:
5721:
5715:
5711:
5707:
5703:
5696:
5692:
5686:
5682:
5678:
5674:
5668:
5664:
5660:
5654:
5650:
5646:
5640:
5636:
5632:
5615:
5611:
5607:
5601:
5597:
5593:
5587:
5583:
5579:
5573:
5569:
5565:
5558:
5554:
5548:
5544:
5540:
5533:
5529:
5522:
5518:
5514:
5508:
5504:
5500:
5496:
5490:
5486:
5482:
5478:
5472:
5468:
5464:
5458:
5454:
5450:
5444:
5440:
5436:
5430:
5426:
5422:
5416:
5412:
5408:
5402:
5398:
5394:
5388:
5384:
5380:
5373:
5369:
5363:
5359:
5355:
5348:
5344:
5338:
5334:
5330:
5326:
5320:
5316:
5310:
5306:
5290:
5286:
5275:
5271:
5267:
5261:
5257:
5253:
5247:
5243:
5237:
5233:
5227:
5223:
5217:
5213:
5187:
5183:
5179:
5173:
5169:
5165:
5159:
5155:
5151:
5147:
5144:
5140:
5136:
5120:
5116:
5110:
5106:
5100:
5096:
5080:
5076:
5070:
5066:
5060:
5056:
5039:
5035:
5028:
5024:
5008:
5004:
5000:
4994:
4990:
4986:
4977:
4972:
4919:
4914:
4904:
4902:
4893:
4892:
4888:
4879:
4877:
4869:
4868:
4864:
4855:
4853:
4848:
4847:
4843:
4813:
4812:
4808:
4754:
4753:
4749:
4719:
4718:
4714:
4703:
4702:
4698:
4690:
4688:
4684:
4680:
4671:
4669:
4667:
4650:
4649:
4645:
4634:
4621:
4620:
4616:
4607:
4605:
4600:
4599:
4595:
4588:
4575:
4574:
4570:
4563:
4540:
4539:
4532:
4516:
4515:
4511:
4504:
4497:
4496:
4492:
4483:
4481:
4471:
4446:
4445:
4436:
4426:
4424:
4415:
4414:
4410:
4403:
4384:
4383:
4374:
4367:
4354:
4353:
4349:
4342:
4329:
4328:
4324:
4294:
4293:
4289:
4281:
4277:
4270:
4257:
4256:
4243:
4235:
4231:
4201:
4200:
4196:
4164:
4159:
4158:
4151:
4115:
4114:
4110:
4089:(1–2): 91–106.
4083:J. Mol. Struct.
4079:
4075:
4074:
4067:
4058:
4054:
4047:
4031:(14 ed.).
4024:
4023:
4019:
4010:
4008:
3996:
3995:
3991:
3973:
3972:
3965:
3949:
3948:
3944:
3928:
3927:
3923:
3907:
3906:
3899:
3885:
3884:
3880:
3859:
3858:
3854:
3847:
3829:
3828:
3821:
3811:
3809:
3805:
3798:
3797:
3793:
3780:Thomson, Thomas
3778:
3777:
3773:
3752:
3751:
3747:
3740:
3725:
3724:
3720:
3709:
3708:
3704:
3678:
3670:Ṣundūq al-ḥikma
3666:
3662:
3625:
3600:
3596:
3580:
3562:
3553:(12): 997–1005.
3540:
3535:
3517:
3512:
3496:Needham, Joseph
3494:
3478:
3477:
3473:
3466:
3446:
3445:
3438:
3428:
3426:
3422:
3411:
3407:
3406:
3402:
3395:
3382:
3381:
3374:
3364:
3363:
3359:
3350:
3349:
3345:
3331:
3330:
3313:
3309:
3304:
3303:
3298:
3294:
3289:
3277:
3230:acid hydrolysis
3222:oxidizing agent
3220:and a powerful
3211:
3191:
3167:phosphoric acid
3141:
3121:
3098:
3094:
3060:
3020:
3015:
2991:
2967:
2930:
2926:
2918:
2911:
2907:
2903:
2898:
2887:
2880:
2876:
2872:
2868:
2864:
2858:
2853:
2849:
2845:
2840:
2810:
2804:
2780:
2772:
2768:
2764:
2760:
2756:
2753:
2749:
2747:
2743:
2739:
2735:
2731:
2727:
2723:
2719:
2715:
2711:
2679:
2675:
2671:
2667:
2663:
2659:
2649:
2645:
2641:
2627:
2611:
2607:
2603:
2580:
2576:
2572:
2568:
2564:
2554:
2550:
2546:
2542:
2529:
2525:
2521:
2515:
2511:
2500:
2496:
2474:Haber processes
2470:Ostwald process
2466:
2430:
2421:
2417:
2413:
2408:
2404:
2400:
2395:
2391:
2386:
2382:
2377:
2373:
2368:
2364:
2356:
2352:
2348:
2344:
2339:
2335:
2331:
2327:
2323:
2294:trinitrotoluene
2277:
2230:
2226:
2211:
2207:
2153:, such as pure
2151:precious metals
2143:
2139:
2134:
2130:
2126:
2121:
2117:
2113:
2108:
2104:
2064:
2060:
2053:
2050:
2049:
2048:Cu + 4 H + 2 NO
2047:
2025:
2021:
2017:
2013:
2009:
1992:
1987:
1983:
1977:
1973:
1969:
1964:
1960:
1954:
1950:
1946:
1941:
1937:
1931:
1927:
1920:
1916:
1896:
1887:
1880:
1877:
1876:
1874:
1870:
1866:
1851:
1847:
1828:
1822:
1819:
1818:
1816:
1812:
1808:
1804:
1800:
1796:
1784:
1776:
1762:
1757:
1744:
1740:
1724:canonical forms
1713:
1709:
1702:
1693:
1689:
1678:
1674:
1667:
1644:
1640:
1630:
1626:
1623:
1617:
1609:
1603:
1600:
1596:
1592:
1589:
1585:
1576:
1572:
1568:
1564:
1560:
1542:
1526:
1522:
1518:
1514:
1510:
1506:
1502:
1498:
1494:
1488:
1484:
1477:
1469:Ostwald process
1425:electric sparks
1421:Henry Cavendish
1405:
1360:Ṣundūq al-ḥikma
1337:Ṣundūq al-ḥikma
1316:
1294:
1289:
1273:nitro compounds
1238:
1234:
1220:
1208:
1198:
1191:
1186:
1185:
1184: ?)
1175:
1171:
1167:
1163:
1152:
1116:
1109:
1088:
1073:
1042:
1031:
1027:
1014:
991:
985:
958:
957:
956:
955:
948:
941:
934:
927:
923:
915:
872:
846:
832:
806:
778:
775:
769:
765:
762:
761:Std enthalpy of
751:
748:
741:
738:
722:
710:
708:
689:
687:
684:
658:
618:
558:
546:
542:
539:
525:
522:
517:
512:
511:
500:
497:
496:
493:
487:
486:
483:
477:
476:
465:
447:
440:
421:
401:
385:
372:
346:
323:
314:
277:
257:
237:
217:
199:
179:
168:
155:
141:
140:
136:Acidum nitricum
122:Spirit of niter
107:
53:
50:
35:
28:
23:
22:
15:
12:
11:
5:
7957:
7955:
7947:
7946:
7941:
7936:
7931:
7926:
7921:
7916:
7906:
7905:
7899:
7898:
7893:
7892:
7889:
7886:
7883:
7880:
7876:
7872:
7868:
7864:
7860:
7856:
7852:
7848:
7844:
7840:
7836:
7832:
7828:
7824:
7820:
7816:
7812:
7808:
7804:
7800:
7796:
7792:
7788:
7784:
7780:
7776:
7772:
7768:
7764:
7760:
7756:
7752:
7751:
7747:
7743:
7739:
7735:
7731:
7727:
7723:
7719:
7715:
7711:
7707:
7703:
7699:
7695:
7691:
7687:
7683:
7679:
7675:
7671:
7667:
7663:
7659:
7655:
7651:
7647:
7643:
7639:
7635:
7631:
7627:
7623:
7619:
7615:
7611:
7607:
7603:
7599:
7592:
7588:
7584:
7580:
7576:
7572:
7568:
7567:
7564:
7561:
7560:
7557:
7554:
7551:
7548:
7545:
7542:
7539:
7536:
7533:
7530:
7527:
7524:
7521:
7518:
7515:
7512:
7509:
7505:
7501:
7497:
7493:
7488:
7487:
7484:
7481:
7477:
7473:
7469:
7464:
7457:
7453:
7449:
7445:
7441:
7437:
7433:
7429:
7422:
7418:
7414:
7410:
7403:
7399:
7395:
7391:
7387:
7383:
7379:
7375:
7371:
7367:
7363:
7359:
7355:
7351:
7348:
7344:
7340:
7336:
7332:
7328:
7324:
7320:
7316:
7312:
7308:
7304:
7300:
7296:
7292:
7288:
7284:
7281:
7277:
7273:
7269:
7265:
7260:
7259:
7255:
7251:
7247:
7243:
7236:
7232:
7229:
7225:
7221:
7217:
7213:
7209:
7205:
7201:
7197:
7193:
7189:
7185:
7181:
7177:
7173:
7169:
7165:
7161:
7154:
7150:
7146:
7142:
7135:
7131:
7127:
7123:
7119:
7115:
7112:
7109:
7105:
7101:
7097:
7093:
7089:
7085:
7081:
7077:
7073:
7069:
7065:
7061:
7057:
7055:
7051:
7047:
7043:
7039:
7034:
7033:
7030:
7022:
7018:
7014:
7010:
7007:
7004:
7000:
6996:
6992:
6988:
6984:
6980:
6976:
6972:
6965:
6961:
6957:
6953:
6949:
6945:
6941:
6934:
6930:
6926:
6922:
6918:
6911:
6907:
6903:
6899:
6895:
6891:
6887:
6883:
6879:
6875:
6871:
6867:
6863:
6859:
6855:
6851:
6847:
6843:
6841:
6837:
6833:
6829:
6825:
6820:
6819:
6816:
6808:
6804:
6800:
6796:
6793:
6790:
6768:
6764:
6760:
6756:
6752:
6748:
6744:
6739:
6738:
6735:
6727:
6723:
6719:
6715:
6698:
6694:
6690:
6686:
6679:
6666:
6647:
6643:
6639:
6635:
6631:
6626:
6625:
6622:
6620:
6618:
6616:
6612:
6605:
6604:
6601:
6600:
6593:
6591:
6590:
6583:
6576:
6568:
6559:
6558:
6508:
6506:
6500:
6499:
6497:
6496:
6492:
6488:
6484:
6480:
6476:
6472:
6468:
6464:
6459:
6455:
6451:
6447:
6443:
6438:
6434:
6430:
6426:
6422:
6418:
6414:
6410:
6406:
6402:
6398:
6394:
6390:
6386:
6382:
6378:
6372:
6367:
6363:
6359:
6355:
6351:
6345:
6343:
6339:
6338:
6336:
6335:
6329:
6322:
6315:
6311:
6307:
6303:
6297:
6290:
6285:
6278:
6274:
6270:
6261:
6257:
6253:
6249:
6245:
6240:
6231:
6224:
6217:
6209:
6205:
6201:
6195:
6183:
6173:
6169:
6165:
6161:
6157:
6153:
6149:
6142:
6138:
6128:
6118:
6114:
6110:
6106:
6102:
6098:
6089:
6087:
6081:
6080:
6078:
6077:
6073:
6069:
6064:
6060:
6056:
6052:
6047:
6042:
6037:
6032:
6028:
6024:
6020:
6015:
6010:
6005:
6001:
5997:
5992:
5988:
5983:
5981:
5977:
5976:
5974:
5973:
5967:
5962:
5958:
5954:
5950:
5946:
5942:
5938:
5934:
5929:
5925:
5920:
5916:
5912:
5908:
5904:
5900:
5895:
5893:
5889:
5888:
5882:
5880:
5879:
5872:
5865:
5857:
5848:
5847:
5845:
5844:
5833:
5822:
5808:
5794:
5780:
5769:
5754:
5736:
5718:
5700:
5689:
5671:
5657:
5643:
5629:
5624:
5619:
5604:
5590:
5576:
5562:
5551:
5537:
5526:
5511:
5493:
5475:
5461:
5447:
5433:
5419:
5405:
5391:
5377:
5366:
5352:
5341:
5323:
5313:
5303:
5298:
5293:
5283:
5278:
5264:
5250:
5240:
5230:
5220:
5210:
5205:
5200:
5195:
5190:
5176:
5162:
5133:
5128:
5123:
5113:
5103:
5093:
5088:
5083:
5073:
5063:
5053:
5048:
5043:
5032:
5021:
5016:
5011:
4997:
4982:
4979:
4978:
4973:
4971:
4970:
4963:
4956:
4948:
4942:
4941:
4930:
4925:
4918:
4917:External links
4915:
4913:
4912:
4886:
4862:
4841:
4822:(3): 189–201.
4806:
4782:10.1086/122040
4747:
4712:
4696:
4686:
4678:
4665:
4643:
4632:
4614:
4593:
4586:
4568:
4562:978-3527306732
4561:
4530:
4509:
4490:
4469:
4447:Wiley (2020).
4434:
4416:Foist, Laura.
4408:
4401:
4372:
4365:
4347:
4340:
4322:
4287:
4275:
4268:
4241:
4229:
4194:
4149:
4128:(2): 120–131.
4108:
4077:
4065:
4052:
4045:
4017:
3989:
3963:
3942:
3921:
3897:
3878:
3863:, ed. (1911).
3861:Chisholm, Hugh
3852:
3845:
3819:
3791:
3771:
3756:, ed. (1911).
3754:Chisholm, Hugh
3745:
3738:
3718:
3702:
3698:Al-Hassan 2001
3677:, p. 62;
3675:Al-Hassan 2001
3660:
3606:Al-Hassan 2001
3594:
3579:978-0226576961
3578:
3547:Chemické listy
3533:
3510:
3471:
3464:
3436:
3400:
3393:
3372:
3357:
3343:
3310:
3308:
3305:
3302:
3301:
3291:
3290:
3288:
3285:
3276:
3273:
3226:chemical burns
3210:
3207:
3190:
3187:
3172:ferrous alloys
3140:
3137:
3133:wood finishing
3120:
3117:
3059:
3056:
3024:cupric nitrate
3019:
3016:
3014:
3011:
2990:
2987:
2966:
2963:
2962:
2961:
2934:
2933:
2892:
2891:
2803:
2800:
2779:
2776:
2775:
2774:
2729:
2720:→ 2 CuO + 4 NO
2682:
2681:
2653:sodium nitrate
2626:
2623:
2584:
2583:
2558:
2557:
2532:
2531:
2465:
2462:
2429:
2426:
2360:
2359:
2342:
2281:oxidizing acid
2276:
2273:
2196:reducing agent
2146:respectively.
2068:
2067:
2029:
2028:
1995:
1994:
1971:
1948:
1895:
1892:
1891:
1890:
1840:
1839:
1782:
1774:
1761:
1758:
1756:
1753:
1701:
1698:
1666:
1663:
1619:Main article:
1616:
1613:
1579:
1578:
1541:
1538:
1524:
1500:
1476:
1473:
1427:through moist
1404:
1401:
1373:Cyprus vitriol
1293:
1290:
1288:
1285:
1196:
1193:
1192:
1187:
1165:
1164:
1160:standard state
1157:
1154:
1153:
1151:
1150:
1145:
1140:
1138:Nitrogen oxide
1135:
1130:
1125:
1119:
1117:
1114:
1111:
1110:
1108:
1107:
1102:
1097:
1095:Sodium nitrate
1091:
1089:
1083:
1080:
1079:
1074:
1068:
1065:
1064:
1060:
1059:
1054:
1047:
1046:
1043:
1037:
1034:
1033:
1028:
1022:
1019:
1018:
1015:
1009:
1006:
1005:
996:
995:
992:
983:
981:
978:
977:
970:
969:
968:Non-flammable
966:
960:
959:
949:
942:
935:
928:
913:
912:
911:
910:
908:
899:
898:
896:P305+P351+P338
892:P304+P340+P310
888:P303+P361+P353
873:
868:
865:
864:
847:
842:
839:
838:
833:
828:
825:
824:
807:
802:
799:
798:
788:
787:
783:
782:
779:
773:
767:
759:
756:
755:
754:146 J/(mol·K)
752:
746:
735:
732:
731:
727:
726:
725:2.17 ± 0.02 D
723:
718:
715:
714:
711:
706:
698:
695:
694:
692:10 cm/mol
685:
679:
676:
675:
670:
668:Conjugate base
664:
663:
660:
656:
646:
645:
642:
640:Vapor pressure
636:
635:
632:
623:
622:
619:
614:
611:
610:
607:
601:
600:
597:
591:
590:
587:
581:
580:
577:
571:
570:
567:
563:
562:
556:
550:
549:
540:
535:
532:
531:
527:
526:
524:
523:
520:
518:
515:
507:
506:
505:
502:
501:
499:
498:
494:
491:
490:
488:
484:
481:
480:
472:
471:
470:
467:
466:
464:
463:
450:
448:
436:
433:
432:
429:
423:
422:
420:
419:
411:
409:
403:
402:
400:
399:
395:
393:
387:
386:
384:
383:
375:
373:
365:
362:
361:
354:
348:
347:
345:
344:
336:
334:
328:
327:
324:
319:
316:
315:
313:
312:
308:
306:
298:
297:
287:
279:
278:
276:
275:
267:
265:
259:
258:
256:
255:
247:
245:
239:
238:
236:
235:
227:
225:
219:
218:
216:
215:
207:
205:
201:
200:
198:
197:
190:
182:
180:
173:
170:
169:
167:
166:
158:
156:
151:
148:
147:
143:
142:
139:
138:
133:
130:
123:
120:
114:
113:
109:
108:
105:
99:
98:
94:
93:
90:
89:
79:
64:
63:
55:
54:
51:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
7956:
7945:
7942:
7940:
7937:
7935:
7932:
7930:
7927:
7925:
7924:Mineral acids
7922:
7920:
7917:
7915:
7912:
7911:
7909:
7890:
7887:
7884:
7881:
7879:
7869:
7867:
7857:
7855:
7845:
7843:
7833:
7831:
7821:
7819:
7809:
7807:
7793:
7791:
7781:
7779:
7769:
7767:
7757:
7754:
7753:
7750:
7740:
7738:
7728:
7726:
7716:
7714:
7704:
7702:
7692:
7690:
7680:
7678:
7668:
7666:
7656:
7654:
7644:
7642:
7632:
7630:
7620:
7618:
7608:
7606:
7595:
7585:
7583:
7573:
7570:
7569:
7562:
7558:
7555:
7552:
7549:
7546:
7543:
7540:
7537:
7534:
7531:
7528:
7525:
7522:
7519:
7516:
7513:
7510:
7508:
7498:
7496:
7490:
7489:
7485:
7482:
7480:
7470:
7468:
7460:
7450:
7448:
7438:
7436:
7425:
7419:
7417:
7406:
7392:
7390:
7380:
7378:
7368:
7366:
7352:
7349:
7347:
7337:
7335:
7321:
7319:
7309:
7307:
7297:
7295:
7285:
7282:
7280:
7270:
7268:
7262:
7261:
7258:
7248:
7246:
7239:
7233:
7230:
7228:
7206:
7204:
7194:
7192:
7182:
7180:
7170:
7168:
7157:
7151:
7149:
7138:
7128:
7126:
7116:
7113:
7110:
7108:
7094:
7092:
7082:
7080:
7070:
7068:
7058:
7056:
7054:
7044:
7042:
7036:
7035:
7031:
7029:
7025:
7019:
7017:
7011:
7008:
7005:
7003:
6993:
6991:
6981:
6979:
6968:
6962:
6960:
6950:
6948:
6937:
6927:
6925:
6914:
6904:
6902:
6892:
6890:
6880:
6878:
6868:
6866:
6856:
6854:
6844:
6842:
6840:
6830:
6828:
6822:
6821:
6817:
6815:
6811:
6805:
6803:
6797:
6794:
6791:
6788:
6771:
6761:
6759:
6749:
6747:
6741:
6740:
6736:
6734:
6730:
6724:
6722:
6716:
6713:
6701:
6695:
6693:
6682:
6674:
6667:
6664:
6648:
6646:
6636:
6634:
6628:
6627:
6623:
6617:
6615:
6609:
6608:
6602:
6597:
6589:
6584:
6582:
6577:
6575:
6570:
6569:
6566:
6555:
6550:
6549:
6543:
6538:
6537:
6531:
6527:
6523:
6519:
6514:
6513:
6507:
6505:
6501:
6495:
6489:
6487:
6481:
6479:
6473:
6471:
6465:
6463:
6456:
6454:
6444:
6442:
6435:
6433:
6427:
6425:
6419:
6417:
6411:
6409:
6403:
6401:
6395:
6393:
6387:
6385:
6379:
6376:
6375:
6368:
6366:
6360:
6358:
6352:
6350:
6347:
6346:
6344:
6340:
6334:
6318:
6304:
6302:
6291:
6289:
6281:
6271:
6269:
6265:
6258:
6256:
6246:
6244:
6236:
6220:
6213:
6210:
6208:
6202:
6200:
6188:
6176:
6170:
6168:
6158:
6156:
6145:
6139:
6137:
6133:
6121:
6115:
6113:
6103:
6101:
6094:
6091:
6090:
6088:
6086:
6082:
6076:
6070:
6068:
6065:
6063:
6053:
6051:
6048:
6046:
6043:
6041:
6038:
6036:
6029:
6027:
6021:
6019:
6016:
6014:
6011:
6009:
6006:
6004:
5998:
5996:
5993:
5991:
5985:
5984:
5982:
5978:
5971:
5970:
5963:
5961:
5955:
5953:
5947:
5945:
5935:
5933:
5926:
5924:
5921:
5919:
5913:
5911:
5905:
5903:
5897:
5896:
5894:
5890:
5885:
5878:
5873:
5871:
5866:
5864:
5859:
5858:
5855:
5843:
5834:
5832:
5823:
5821:
5809:
5807:
5795:
5793:
5781:
5779:
5770:
5768:
5755:
5753:
5737:
5735:
5719:
5717:
5701:
5699:
5690:
5688:
5672:
5670:
5658:
5656:
5644:
5642:
5630:
5628:
5625:
5623:
5620:
5618:
5605:
5603:
5591:
5589:
5577:
5575:
5563:
5561:
5552:
5550:
5538:
5536:
5527:
5525:
5512:
5510:
5494:
5492:
5476:
5474:
5462:
5460:
5448:
5446:
5434:
5432:
5420:
5418:
5406:
5404:
5392:
5390:
5378:
5376:
5367:
5365:
5353:
5351:
5342:
5340:
5324:
5322:
5314:
5312:
5304:
5302:
5299:
5297:
5294:
5292:
5284:
5282:
5279:
5277:
5265:
5263:
5251:
5249:
5241:
5239:
5231:
5229:
5221:
5219:
5211:
5209:
5206:
5204:
5201:
5199:
5196:
5194:
5191:
5189:
5177:
5175:
5163:
5161:
5134:
5132:
5129:
5127:
5124:
5122:
5114:
5112:
5104:
5102:
5094:
5092:
5089:
5087:
5084:
5082:
5074:
5072:
5064:
5062:
5054:
5052:
5049:
5047:
5044:
5042:
5033:
5031:
5022:
5020:
5017:
5015:
5012:
5010:
4998:
4996:
4984:
4983:
4980:
4976:
4969:
4964:
4962:
4957:
4955:
4950:
4949:
4946:
4939:
4935:
4932:Calculators:
4931:
4929:
4926:
4924:
4921:
4920:
4916:
4901:
4897:
4890:
4887:
4876:
4872:
4866:
4863:
4852:
4851:"Nitric acid"
4845:
4842:
4837:
4833:
4829:
4825:
4821:
4817:
4810:
4807:
4802:
4798:
4793:
4788:
4783:
4778:
4774:
4770:
4766:
4762:
4758:
4751:
4748:
4743:
4739:
4735:
4731:
4727:
4723:
4716:
4713:
4708:
4707:
4700:
4697:
4693:
4682:
4679:
4668:
4662:
4657:
4656:
4647:
4644:
4639:
4635:
4629:
4625:
4618:
4615:
4604:. BILLONY.COM
4603:
4597:
4594:
4589:
4583:
4579:
4572:
4569:
4564:
4558:
4554:
4550:
4546:
4545:
4537:
4535:
4531:
4526:
4525:
4520:
4513:
4510:
4500:
4494:
4491:
4480:
4476:
4472:
4470:9780471484943
4466:
4462:
4458:
4454:
4450:
4449:"Nitric acid"
4443:
4441:
4439:
4435:
4423:
4419:
4412:
4409:
4404:
4398:
4394:
4390:
4389:
4381:
4379:
4377:
4373:
4368:
4362:
4358:
4351:
4348:
4343:
4337:
4333:
4326:
4323:
4318:
4314:
4310:
4306:
4302:
4298:
4291:
4288:
4284:
4279:
4276:
4271:
4265:
4261:
4254:
4252:
4250:
4248:
4246:
4242:
4238:
4233:
4230:
4225:
4221:
4217:
4213:
4209:
4205:
4198:
4195:
4190:
4186:
4182:
4178:
4174:
4170:
4169:Dalton Trans.
4163:
4156:
4154:
4150:
4144:
4139:
4135:
4131:
4127:
4124:(in French).
4123:
4119:
4112:
4109:
4104:
4100:
4096:
4092:
4088:
4085:
4084:
4072:
4070:
4066:
4062:
4056:
4053:
4048:
4042:
4038:
4034:
4030:
4029:
4021:
4018:
4006:
4002:
4001:
3993:
3990:
3985:
3981:
3977:
3970:
3968:
3964:
3959:
3955:
3954:
3946:
3943:
3938:
3934:
3933:
3925:
3922:
3917:
3913:
3912:
3904:
3902:
3898:
3893:
3889:
3882:
3879:
3874:
3873:
3867:
3862:
3856:
3853:
3848:
3846:0-691-02350-6
3842:
3838:
3837:
3832:
3826:
3824:
3820:
3804:
3803:
3795:
3792:
3787:
3786:
3781:
3775:
3772:
3767:
3766:
3760:
3755:
3749:
3746:
3741:
3735:
3731:
3730:
3722:
3719:
3714:
3713:
3706:
3703:
3700:, p. 62.
3699:
3694:
3687:
3686:
3681:
3676:
3671:
3664:
3661:
3656:
3651:
3645:
3644:2078.1/211340
3641:
3637:
3633:
3629:
3622:
3617:
3612:
3607:
3603:
3598:
3595:
3591:
3587:
3581:
3575:
3571:
3570:
3565:
3560:
3559:
3552:
3548:
3544:
3536:
3530:
3526:
3525:
3520:
3513:
3507:
3503:
3502:
3497:
3492:
3485:
3481:
3475:
3472:
3467:
3461:
3457:
3453:
3449:
3443:
3441:
3437:
3421:
3417:
3416:fishersci.com
3410:
3404:
3401:
3396:
3390:
3386:
3379:
3377:
3373:
3368:
3361:
3358:
3353:
3347:
3344:
3339:
3335:
3328:
3326:
3324:
3322:
3320:
3318:
3316:
3312:
3306:
3296:
3293:
3286:
3284:
3282:
3274:
3272:
3269:
3265:
3263:
3259:
3255:
3251:
3247:
3246:living tissue
3243:
3239:
3235:
3231:
3227:
3223:
3219:
3216:
3208:
3206:
3204:
3200:
3196:
3188:
3186:
3184:
3180:
3175:
3173:
3168:
3163:
3159:
3157:
3152:
3150:
3146:
3138:
3136:
3134:
3130:
3126:
3118:
3116:
3114:
3110:
3106:
3101:
3092:
3088:
3084:
3080:
3075:
3073:
3069:
3065:
3057:
3055:
3053:
3049:
3045:
3041:
3037:
3033:
3029:
3025:
3017:
3012:
3010:
3008:
3004:
3000:
2996:
2988:
2986:
2984:
2980:
2979:cyclohexanone
2976:
2972:
2964:
2959:
2955:
2954:
2953:
2951:
2950:nitrobenzenes
2947:
2943:
2939:
2931:
2920:
2912:
2897:
2896:
2895:
2889:
2881:
2854:
2839:
2838:
2837:
2835:
2831:
2827:
2824:onto various
2823:
2819:
2815:
2809:
2801:
2799:
2797:
2793:
2784:
2777:
2730:
2710:
2709:
2708:
2706:
2702:
2697:
2695:
2691:
2690:sulfuric acid
2686:
2658:
2657:
2656:
2654:
2639:
2638:sulfuric acid
2635:
2630:
2624:
2622:
2619:
2617:
2616:United States
2613:
2600:
2596:
2591:
2589:
2563:
2562:
2561:
2541:
2540:
2539:
2537:
2520:
2519:
2518:
2509:
2505:
2494:
2490:
2486:
2481:
2479:
2475:
2471:
2463:
2461:
2459:
2455:
2451:
2447:
2443:
2439:
2435:
2427:
2425:
2423:
2410:
2397:
2388:
2379:
2370:
2343:
2322:
2321:
2320:
2318:
2314:
2310:
2306:
2302:
2297:
2295:
2291:
2290:nitroglycerin
2286:
2282:
2274:
2272:
2270:
2266:
2262:
2258:
2254:
2250:
2246:
2242:
2238:
2233:
2224:
2220:
2216:
2215:thermodynamic
2205:
2201:
2197:
2192:
2190:
2186:
2182:
2178:
2174:
2170:
2166:
2162:
2161:
2156:
2152:
2147:
2145:
2136:
2123:
2110:
2101:
2097:
2093:
2089:
2085:
2081:
2077:
2073:
2046:
2045:
2044:
2042:
2038:
2034:
2008:
2007:
2006:
2004:
2000:
1988:
1972:
1965:
1949:
1942:
1926:
1925:
1924:
1922:
1913:
1909:
1905:
1901:
1893:
1865:
1864:
1863:
1861:
1857:
1845:
1844:nitronium ion
1837:
1833:
1795:
1794:
1793:
1791:
1790:sulfuric acid
1786:
1781:
1777:
1773:
1767:
1759:
1754:
1752:
1750:
1746:
1737:
1733:
1729:
1725:
1721:
1706:
1699:
1697:
1687:
1683:
1672:
1664:
1662:
1660:
1656:
1652:
1647:
1638:
1634:
1622:
1614:
1612:
1608:
1582:
1559:
1558:
1557:
1555:
1546:
1539:
1537:
1535:
1530:
1491:
1482:
1474:
1472:
1470:
1466:
1465:Haber process
1461:
1459:
1453:
1450:
1446:
1445:packed column
1442:
1437:
1434:
1430:
1426:
1422:
1418:
1414:
1410:
1402:
1400:
1398:
1397:green vitriol
1394:
1390:
1386:
1380:
1378:
1374:
1370:
1364:
1361:
1355:
1354:(985–1021).
1353:
1348:
1343:
1338:
1333:
1329:
1325:
1320:
1311:
1307:
1303:
1299:
1298:mineral acids
1291:
1286:
1284:
1282:
1278:
1274:
1270:
1266:
1262:
1257:
1255:
1251:
1247:
1243:
1232:
1228:
1225:
1217:
1214:
1211:
1206:
1202:
1190:
1183:
1178:
1161:
1155:
1149:
1146:
1144:
1141:
1139:
1136:
1134:
1131:
1129:
1126:
1124:
1121:
1120:
1118:
1113:
1112:
1106:
1103:
1101:
1098:
1096:
1093:
1092:
1090:
1087:
1082:
1081:
1078:
1075:
1072:
1067:
1066:
1061:
1058:
1055:
1052:
1049:
1048:
1044:
1040:
1036:
1035:
1029:
1026:(Recommended)
1025:
1021:
1020:
1016:
1013:(Permissible)
1012:
1008:
1007:
1003:
1002:
997:
993:
989:
980:
979:
975:
971:
967:
965:
962:
961:
954:
947:
940:
933:
909:
906:
905:
901:
900:
874:
871:
867:
866:
848:
845:
841:
840:
837:
834:
831:
827:
826:
822:
817:
812:
808:
805:
801:
800:
796:
794:
789:
784:
780:
772:
764:
758:
757:
753:
745:
740:
734:
733:
728:
724:
721:
720:Dipole moment
717:
716:
712:
705:
701:
697:
696:
686:
682:
678:
677:
674:
671:
669:
666:
665:
661:
655:
651:
648:
647:
643:
641:
638:
637:
633:
631:
630:
625:
624:
620:
617:
613:
612:
608:
606:
605:Boiling point
603:
602:
598:
596:
595:Melting point
593:
592:
588:
586:
583:
582:
578:
576:
573:
572:
568:
565:
564:
557:
555:
552:
551:
541:
538:
534:
533:
528:
519:
514:
510:
503:
489:
479:
475:
468:
460:
456:
455:DTXSID5029685
452:
451:
449:
439:
435:
434:
430:
428:
425:
424:
417:
413:
412:
410:
408:
405:
404:
397:
396:
394:
392:
389:
388:
381:
377:
376:
374:
368:
364:
363:
359:
355:
353:
350:
349:
342:
338:
337:
335:
333:
330:
329:
325:
322:
318:
317:
310:
309:
307:
305:
300:
299:
295:
291:
288:
286:
284:ECHA InfoCard
281:
280:
273:
269:
268:
266:
264:
261:
260:
253:
249:
248:
246:
244:
241:
240:
233:
229:
228:
226:
224:
221:
220:
213:
209:
208:
206:
203:
202:
195:
191:
188:
184:
183:
181:
177:
172:
171:
164:
160:
159:
157:
154:
150:
149:
144:
137:
134:
131:
129:
128:
124:
121:
119:
116:
115:
110:
104:
100:
95:
84:
80:
74:
70:
69:
65:
61:
56:
48:
43:
37:
33:
19:
6610:
6552:(a strongly
6546:
6534:
6510:
6370:
6171:
5965:
5837:H[Co(CO)
5315:
4903:. Retrieved
4899:
4889:
4878:. Retrieved
4865:
4854:. Retrieved
4844:
4819:
4815:
4809:
4764:
4760:
4750:
4725:
4721:
4715:
4705:
4699:
4681:
4670:. Retrieved
4654:
4646:
4623:
4617:
4606:. Retrieved
4596:
4577:
4571:
4542:
4522:
4512:
4493:
4482:. Retrieved
4452:
4425:. Retrieved
4421:
4411:
4387:
4356:
4350:
4331:
4325:
4300:
4296:
4290:
4278:
4259:
4232:
4207:
4203:
4197:
4172:
4168:
4125:
4121:
4111:
4086:
4081:
4055:
4027:
4020:
4009:. Retrieved
3999:
3992:
3986:: 1020–1021.
3983:
3979:
3952:
3945:
3931:
3924:
3910:
3891:
3881:
3870:
3855:
3835:
3810:. Retrieved
3808:. p. 23
3801:
3794:
3784:
3774:
3763:
3748:
3728:
3721:
3711:
3705:
3684:
3663:
3635:
3631:
3616:Michael Scot
3597:
3589:
3585:
3568:
3556:
3550:
3546:
3523:
3500:
3483:
3474:
3451:
3427:. Retrieved
3415:
3403:
3384:
3366:
3360:
3346:
3295:
3281:acid attacks
3278:
3270:
3266:
3240:) and fats (
3212:
3192:
3176:
3164:
3160:
3153:
3142:
3122:
3102:
3076:
3061:
3052:gold parting
3021:
2992:
2983:cyclohexanol
2968:
2935:
2893:
2811:
2789:
2698:
2685:Distillation
2683:
2631:
2628:
2620:
2592:
2585:
2559:
2533:
2489:nitric oxide
2482:
2467:
2431:
2361:
2353:+ 4 NO + 2 H
2317:nitric oxide
2298:
2278:
2234:
2223:nitrous acid
2200:noble metals
2193:
2181:colored gold
2158:
2148:
2069:
2033:nitric oxide
2030:
2022:+ 2 NO + 4 H
2010:3 Cu + 8 HNO
1996:
1897:
1841:
1835:
1787:
1779:
1771:
1763:
1732:single bonds
1717:
1668:
1650:
1648:
1635:
1624:
1606:
1583:
1580:
1551:
1531:
1492:
1478:
1462:
1454:
1449:plate column
1438:
1433:Humphry Davy
1406:
1382:
1366:
1356:
1334:such as the
1332:Arabic works
1321:
1309:
1306:pseudo-Geber
1295:
1258:
1253:
1241:
1227:mineral acid
1200:
1199:
1077:Nitrous acid
1000:
973:
903:
835:
792:
781:−207 kJ/mol
770:
743:
703:
653:
628:
391:RTECS number
146:Identifiers
135:
126:
117:
112:Other names
40:Nitric acid
36:
32:nitrous acid
4767:(135): 13.
4210:(9): 3106.
4035:. pp.
4033:McGraw-Hill
3119:Woodworking
3054:processes.
2975:adipic acid
2942:amine group
2940:to give an
2822:substituent
2792:fertilizers
2599:dehydration
2442:amino acids
2301:noble gases
2249:passivation
2219:kinetically
2177:gold alloys
2057:→ Cu + 2 NO
1766:strong acid
1736:bond length
1728:double bond
1431:. In 1806,
1393:Ramon Llull
1317: 1300
1265:nitro group
1201:Nitric acid
974:Lethal dose
964:Flash point
830:Signal word
566:Appearance
530:Properties
358:Nitric+acid
290:100.028.832
232:CHEBI:48107
118:Aqua fortis
106:Nitric acid
7908:Categories
4880:2011-12-07
4856:2009-05-28
4672:2009-05-28
4608:2009-05-28
4499:US 6200456
4484:2023-08-09
4011:2019-09-15
3812:21 October
3638:: 87–141.
3632:Micrologus
3590:aqua regia
3527:. UNESCO.
3307:References
3258:human skin
3197:and other
3032:aqua regia
3013:Niche uses
2806:See also:
2597:. Further
2493:dinitrogen
2478:feedstocks
2464:Production
2243:(Fe), and
2160:aqua regia
2076:metalloids
1974:Zn + 2 HNO
1951:Mn + 2 HNO
1928:Mg + 2 HNO
1817:O] + 2 HSO
1813:] + [H
1726:show some
1403:Modern era
1277:explosives
804:Pictograms
554:Molar mass
416:411VRN1TV4
263:ChemSpider
252:ChEMBL1352
174:3D model (
153:CAS Number
103:IUPAC name
5519:[PtCl
4801:120665136
4578:Ignition!
4479:260923593
4427:5 January
4422:Study.com
4037:2.79–2.80
3429:4 October
3215:corrosive
3203:actinides
3179:alkaloids
3009:missile.
2808:Nitration
2551:O → 2 HNO
2487:burns to
2269:aluminium
2245:aluminium
2235:Although
2082:give the
2014:→ 3 Cu(NO
1914:liberate
1908:manganese
1904:Magnesium
1871:⇌ [NO
1809:⇌ [NO
1755:Reactions
1720:resonance
1686:viscosity
1682:Anhydrous
1651:inhibited
1511:O][NO
1481:azeotrope
1463:Once the
1261:nitration
1224:corrosive
1057:ICSC 0183
795:labelling
763:formation
737:Std molar
621:Miscible
427:UN number
398:QU5775000
311:231-714-2
303:EC Number
163:7697-37-2
127:Eau forte
6319: /
6282: /
6266: /
6237: /
6221: /
6214: /
6189: /
6177: /
6146: /
6134: /
6122: /
6095: /
5995:>C=NR
5892:Hydrides
5884:Nitrogen
5612:[SiF
5036:H[BF
4900:RESET.to
4836:10725655
4742:21356831
4189:20354626
3833:(1960).
3782:(1830).
3682:(1931).
3566:(2006).
3521:(2001).
3482:(1966).
3420:Archived
3340:(NIOSH).
3234:proteins
2995:oxidizer
2522:2 NO + O
2446:aromatic
2434:proteins
2309:halogens
2285:hydroxyl
2261:chromium
2237:chromium
2072:nitrates
2039:to give
1569:O + 4 NO
1523:·3H
904:NFPA 704
786:Hazards
683:(χ)
521:ON(=O)=O
7566:
6596:nitrate
6342:Halides
5980:Organic
5886:species
4905:25 June
4769:Bibcode
4305:Bibcode
4212:Bibcode
4130:Bibcode
4091:Bibcode
3491:al-Razi
3334:"#0447"
3262:keratin
3145:etching
3087:ICP-AES
3083:ICP-OES
3072:ICP-AES
2946:aniline
2938:reduced
2855:+ 3 HNO
2769:→ 2 HNO
2740:O → HNO
2712:2 Cu(NO
2676:+ NaHSO
2634:nitrate
2485:ammonia
2450:ammonia
2305:silicon
2185:jewelry
2074:. Some
1848:[NO
1554:thermal
1499:·H
1302:alchemy
1287:History
1182:what is
1180: (
1086:cations
1045:25 ppm
739:entropy
673:Nitrate
650:Acidity
585:Density
548:
516:(=O)(O)
367:PubChem
7783:PaO(NO
7463:BiO(NO
7311:TaO(NO
7084:NbO(NO
6556:oxide)
6554:acidic
6085:Oxides
4936:, and
4834:
4799:
4740:
4663:
4638:online
4630:
4584:
4559:
4524:Nature
4505:
4477:
4467:
4399:
4393:769–72
4363:
4338:
4266:
4187:
4043:
3843:
3736:
3696:, see
3673:, see
3576:
3531:
3508:
3462:
3391:
3248:(e.g.
3209:Safety
3079:ICP-MS
3068:ICP-MS
3007:BOMARC
2754:
2750:
2526:→ 2 NO
2411:, and
2380:, and
2349:→ 3 CO
2332:+ 4 NO
2307:, and
2267:, and
2265:nickel
2257:cobalt
2239:(Cr),
2204:alloys
2189:karats
2137:, and
2098:, and
2084:oxides
2080:metals
2037:oxygen
2003:silver
1999:copper
1910:, and
1875:] + NO
1829:
1507:[H
1415:cited
1203:is an
1177:verify
1174:
1084:Other
1071:anions
1069:Other
1053:(SDS)
836:Danger
634:−0.13
559:63.012
509:SMILES
341:D02313
243:ChEMBL
212:B00068
204:3DMet
97:Names
7871:Cf(NO
7859:Bk(NO
7847:Cm(NO
7835:Am(NO
7823:Pu(NO
7811:Np(NO
7771:Th(NO
7759:Ac(NO
7742:Yb(NO
7730:Tm(NO
7718:Er(NO
7706:Ho(NO
7694:Dy(NO
7682:Tb(NO
7670:Gd(NO
7658:Eu(NO
7646:Sm(NO
7634:Pm(NO
7622:Nd(NO
7610:Pr(NO
7598:Ce(NO
7587:Ce(NO
7575:La(NO
7500:Ra(NO
7472:Po(NO
7452:Bi(NO
7440:Pb(NO
7428:Tl(NO
7409:Hg(NO
7382:Au(NO
7370:Pt(NO
7299:Hf(NO
7287:Lu(NO
7272:Ba(NO
7250:Xe(NO
7196:Sn(NO
7184:In(NO
7172:Cd(NO
7160:Ag(NO
7141:Pd(NO
7130:Pd(NO
7118:Rh(NO
7072:Zr(NO
7046:Sr(NO
6995:Ga(NO
6983:Zn(NO
6971:Cu(NO
6952:Ni(NO
6940:Co(NO
6929:Co(NO
6917:Fe(NO
6906:Fe(NO
6894:Mn(NO
6882:Cr(NO
6870:VO(NO
6858:Ti(NO
6846:Sc(NO
6832:Ca(NO
6807:ClONO
6775:Al(NO
6763:Al(NO
6751:Mg(NO
6718:HOONO
6638:Be(NO
6524:, 0,
6216:(HON)
6000:-CONR
5287:NaHCO
4797:S2CID
4475:S2CID
4165:(PDF)
3806:(PDF)
3423:(PDF)
3412:(PDF)
3287:Notes
3254:flesh
3242:ester
3238:amide
3232:with
3195:PUREX
3181:like
3156:nital
3129:maple
2971:nylon
2744:+ HNO
2672:→ HNO
2573:→ HNO
2569:+ 2 O
2536:water
2506:, to
2444:with
2389:into
2336:+ 2 H
2149:Some
2061:+ 2 H
1980:Zn(NO
1957:Mn(NO
1934:Mg(NO
1867:2 HNO
1801:+ 2 H
1671:molar
1565:→ 2 H
1561:4 HNO
1534:Baumé
1001:NIOSH
688:−1.99
662:−1.4
474:InChI
431:2031
326:1576
223:ChEBI
176:JSmol
7492:FrNO
7421:TlNO
7358:O(NO
7264:CsNO
7216:(OH)
7153:AgNO
7060:Y(NO
7038:RbNO
7021:BrNO
7013:+SeO
6964:CuNO
6743:NaNO
6670:RONO
6651:B(NO
6630:LiNO
6483:NHBr
6475:NHCl
6268:ONOO
6097:(NO)
6050:HNCS
6045:HNCO
6040:HOCN
6023:(CN)
5627:HNCS
5622:HSCN
5296:HNCO
5244:HMnO
5131:HCNO
5117:HClO
5107:HClO
5097:HClO
5091:HClO
5077:HBrO
5067:HBrO
5057:HBrO
5051:HBrO
5014:HArF
4907:2021
4832:PMID
4738:PMID
4726:2008
4661:ISBN
4628:ISBN
4582:ISBN
4557:ISBN
4465:ISBN
4429:2019
4397:ISBN
4361:ISBN
4336:ISBN
4283:ASTM
4264:ISBN
4185:PMID
4041:ISBN
3960:–50.
3841:ISBN
3814:2023
3734:ISBN
3574:ISBN
3529:ISBN
3506:ISBN
3460:ISBN
3431:2022
3389:ISBN
3252:and
3250:skin
3218:acid
3127:and
3125:pine
3046:and
3038:(as
3036:gold
2981:and
2921:→ 2
2882:+ 3
2778:Uses
2757:2 NO
2732:2 NO
2696:.
2660:NaNO
2555:+ NO
2543:3 NO
2480:.
2328:→ CO
2292:and
2253:iron
2241:iron
2155:gold
2078:and
2031:The
2001:and
1912:zinc
1900:acid
1842:The
1838:≈ 22
1458:coke
1391:and
1377:alum
1326:and
1039:IDLH
884:P280
880:P220
876:P210
862:H331
858:H314
854:H290
850:H272
627:log
575:Odor
407:UNII
352:MeSH
332:KEGG
18:HNO3
7891:No
7888:Md
7885:Fm
7882:Es
7799:(NO
7755:**
7559:Og
7556:Ts
7553:Lv
7550:Mc
7547:Fl
7544:Nh
7541:Cn
7538:Rg
7535:Ds
7532:Mt
7529:Hs
7526:Bh
7523:Sg
7520:Db
7517:Rf
7514:Lr
7511:**
7486:Rn
7483:At
7398:(NO
7350:Os
7339:ReO
7327:(NO
7242:+IO
7235:INO
7231:Te
7220:(NO
7114:Ru
7111:Tc
7100:(NO
7096:MoO
7032:Kr
7028:+Br
7009:As
7006:Ge
6824:KNO
6818:Ar
6814:+Cl
6799:+SO
6792:Si
6737:Ne
6726:FNO
6678:+CO
6624:He
6611:HNO
6598:ion
6544:,
6491:NHI
6467:NHF
6421:BrN
6413:ClN
6389:NBr
6381:NCl
6377:(?)
6288:NOO
6252:NNO
6212:HNO
6172:HNO
6148:(NO
6117:HNO
6072:-NO
6067:-NO
6035:NCN
6013:HCN
6008:-CN
5972:(?)
5816:TiO
5802:TeO
5788:TeO
5598:SiO
5584:SeO
5570:SeO
5360:NSO
5345:HNO
5317:HNO
5307:HNO
5301:HNO
5281:HNC
5272:MoO
5258:MnO
5234:HIO
5224:HIO
5214:HIO
5208:HIO
5198:HFO
5141:CrO
5126:HCN
5086:HCl
5046:HBr
5025:HSO
5019:HAt
5005:AsO
4991:AsO
4824:doi
4820:109
4787:hdl
4777:doi
4730:doi
4549:doi
4457:doi
4313:doi
4220:doi
4177:doi
4138:doi
4099:doi
4087:320
4007:–32
3916:509
3640:hdl
3183:LSD
3135:).
3103:In
3095:HNO
3066:by
3062:In
3030:as
2997:in
2869:(NO
2834:TNT
2828:by
2812:In
2761:+ H
2736:+ H
2724:+ O
2692:or
2664:+ H
2590:.
2577:+ H
2547:+ H
2517:):
2392:HIO
2227:HNO
2140:TiO
2105:SnO
1989:+ H
1966:+ H
1943:+ H
1884:+ H
1797:HNO
1710:HNO
1649:An
1627:HNO
1573:+ O
1519:HNO
1495:HNO
1485:HNO
1447:or
1429:air
1387:to
1319:).
1308:'s
1235:HNO
1024:REL
1011:PEL
793:GHS
774:298
747:298
543:HNO
443:EPA
380:944
370:CID
272:919
7910::
7795:UO
7571:*
7394:Hg
7364:10
7354:Ir
7343:NO
7323:WO
7283:*
7208:Sb
6795:P
6733:+F
6709:NO
6705:NH
6697:NO
6685:+C
6548:+5
6542:+4
6540:,
6536:+3
6532:,
6530:+2
6528:,
6526:+1
6522:−1
6520:,
6518:−2
6516:,
6512:−3
6462:Cl
6458:NH
6437:NH
6429:IN
6405:FN
6397:NI
6371:NF
6362:NF
6354:NF
6349:NF
6293:NO
6277:NO
6273:HO
6264:NO
6260:HO
6204:NO
6191:NO
6179:NO
6141:NO
6136:NO
6124:NO
6093:NO
6055:CH
6018:CN
5987:NR
5966:NH
5949:HN
5932:OH
5928:NH
5915:NH
5907:NH
5899:NH
5830:Po
5777:Te
5762:SO
5758:CF
5665:SO
5651:SO
5637:SO
5559:Se
5507:10
5469:PO
5455:PO
5441:PO
5203:HI
5193:HF
5184:CS
5170:CO
5152:Cr
4898:.
4873:.
4830:.
4818:.
4795:.
4785:.
4775:.
4765:23
4763:.
4759:.
4736:.
4724:.
4555:.
4533:^
4521:.
4473:.
4463:.
4451:.
4437:^
4420:.
4395:.
4375:^
4311:.
4301:16
4299:.
4244:^
4218:.
4208:42
4206:.
4183:.
4173:39
4167:.
4152:^
4136:.
4120:.
4097:.
4068:^
4039:.
4005:30
3984:73
3982:.
3978:.
3966:^
3958:45
3937:21
3900:^
3890:.
3869:.
3822:^
3762:.
3636:28
3634:.
3630:.
3551:96
3549:.
3545:.
3439:^
3414:.
3375:^
3336:.
3314:^
3283:.
3115:.
3085:,
3081:,
3070:,
2973:,
2952::
2927:SO
2913:+
2877:CH
2859:→
2850:CH
2752:or
2668:SO
2655::
2646:SO
2608:SO
2565:NH
2512:NO
2418:SO
2405:PO
2398:,
2371:,
2303:,
2271:.
2263:,
2259:,
2255:,
2208:NO
2173:Cu
2171:,
2169:Ag
2127:Sb
2124:,
2114:As
2111:,
2100:Ti
2096:Sb
2094:,
2092:As
2090:,
2088:Sn
1978:→
1955:→
1932:→
1923::
1906:,
1862::
1846:,
1834::
1805:SO
1792::
1741:NO
1690:NO
1680:.
1675:NO
1641:NO
1604:NO
1586:NO
1536:.
1529:.
1314:c.
1283:.
984:50
982:LC
952:OX
894:,
890:,
886:,
882:,
878:,
860:,
856:,
852:,
797::
766:(Δ
659:)
652:(p
7877:3
7875:)
7873:3
7865:3
7863:)
7861:3
7853:3
7851:)
7849:3
7841:3
7839:)
7837:3
7829:4
7827:)
7825:3
7817:4
7815:)
7813:3
7805:2
7803:)
7801:3
7797:2
7789:3
7787:)
7785:3
7777:4
7775:)
7773:3
7765:3
7763:)
7761:3
7748:3
7746:)
7744:3
7736:3
7734:)
7732:3
7724:3
7722:)
7720:3
7712:3
7710:)
7708:3
7700:3
7698:)
7696:3
7688:3
7686:)
7684:3
7676:3
7674:)
7672:3
7664:3
7662:)
7660:3
7652:3
7650:)
7648:3
7640:3
7638:)
7636:3
7628:3
7626:)
7624:3
7616:3
7614:)
7612:3
7604:4
7602:)
7600:3
7593:3
7591:)
7589:3
7581:3
7579:)
7577:3
7506:2
7504:)
7502:3
7494:3
7478:4
7476:)
7474:3
7467:)
7465:3
7458:3
7456:)
7454:3
7446:2
7444:)
7442:3
7434:3
7432:)
7430:3
7423:3
7415:2
7413:)
7411:3
7404:2
7402:)
7400:3
7396:2
7388:3
7386:)
7384:3
7376:2
7374:)
7372:3
7362:)
7360:3
7356:3
7345:3
7341:3
7333:2
7331:)
7329:3
7325:2
7317:3
7315:)
7313:3
7305:4
7303:)
7301:3
7293:3
7291:)
7289:3
7278:2
7276:)
7274:3
7266:3
7256:2
7254:)
7252:3
7244:3
7237:3
7226:2
7224:)
7222:3
7218:2
7214:4
7212:O
7210:4
7202:4
7200:)
7198:3
7190:3
7188:)
7186:3
7178:2
7176:)
7174:3
7166:2
7164:)
7162:3
7155:3
7147:4
7145:)
7143:3
7136:2
7134:)
7132:3
7124:3
7122:)
7120:3
7106:2
7104:)
7102:3
7098:2
7090:3
7088:)
7086:3
7078:4
7076:)
7074:3
7066:3
7064:)
7062:3
7052:2
7050:)
7048:3
7040:3
7023:3
7015:3
7001:3
6999:)
6997:3
6989:2
6987:)
6985:3
6977:2
6975:)
6973:3
6966:3
6958:2
6956:)
6954:3
6946:3
6944:)
6942:3
6935:2
6933:)
6931:3
6923:3
6921:)
6919:3
6912:2
6910:)
6908:3
6900:2
6898:)
6896:3
6888:3
6886:)
6884:3
6876:3
6874:)
6872:3
6864:4
6862:)
6860:3
6852:3
6850:)
6848:3
6838:2
6836:)
6834:3
6826:3
6809:2
6801:4
6785:4
6782:−
6779:)
6777:3
6769:3
6767:)
6765:3
6757:2
6755:)
6753:3
6745:3
6728:3
6720:2
6711:3
6707:4
6699:3
6691:4
6689:O
6687:2
6680:3
6672:2
6661:4
6658:−
6655:)
6653:3
6644:2
6642:)
6640:3
6632:3
6613:3
6587:e
6580:t
6573:v
6493:2
6485:2
6477:2
6469:2
6460:2
6452:2
6450:F
6448:2
6446:N
6441:F
6439:2
6431:3
6423:3
6415:3
6407:3
6399:3
6391:3
6383:3
6373:5
6364:3
6356:2
6330:3
6325:O
6323:2
6321:N
6316:4
6314:O
6312:2
6310:N
6308:4
6306:H
6298:4
6286:2
6284:O
6279:2
6275:2
6262:2
6254:2
6250:2
6248:H
6243:O
6241:2
6239:N
6232:2
6227:O
6225:2
6223:N
6218:2
6206:3
6196:2
6184:3
6174:3
6166:5
6164:O
6162:2
6160:N
6154:2
6152:)
6150:2
6143:2
6129:2
6119:2
6111:3
6109:O
6107:2
6105:N
6099:2
6074:2
6061:2
6059:N
6057:2
6033:2
6031:H
6025:2
6002:2
5989:3
5968:5
5959:3
5957:N
5951:3
5943:4
5941:H
5939:2
5937:N
5930:2
5923:N
5917:2
5909:4
5901:3
5876:e
5869:t
5862:v
5841:]
5839:4
5828:2
5826:H
5818:4
5814:4
5812:H
5804:6
5800:6
5798:H
5790:3
5786:2
5784:H
5775:2
5773:H
5766:H
5764:3
5760:3
5750:8
5748:O
5746:2
5744:S
5742:2
5740:H
5732:7
5730:O
5728:2
5726:S
5724:2
5722:H
5714:6
5712:O
5710:2
5708:S
5706:2
5704:H
5697:O
5695:3
5693:H
5685:3
5683:O
5681:2
5679:S
5677:2
5675:H
5667:5
5663:2
5661:H
5653:4
5649:2
5647:H
5639:3
5635:2
5633:H
5616:]
5614:6
5610:2
5608:H
5600:4
5596:4
5594:H
5586:4
5582:2
5580:H
5572:3
5568:2
5566:H
5557:2
5555:H
5547:2
5545:S
5543:2
5541:H
5534:S
5532:2
5530:H
5523:]
5521:6
5517:2
5515:H
5505:O
5503:3
5501:P
5499:5
5497:H
5489:7
5487:O
5485:2
5483:P
5481:4
5479:H
5471:4
5467:3
5465:H
5457:3
5453:3
5451:H
5443:2
5439:3
5437:H
5429:5
5427:O
5425:2
5423:H
5415:4
5413:O
5411:2
5409:H
5401:3
5399:O
5397:2
5395:H
5387:2
5385:O
5383:2
5381:H
5374:O
5372:2
5370:H
5362:3
5358:3
5356:H
5349:S
5347:5
5337:2
5335:O
5333:2
5331:N
5329:2
5327:H
5319:3
5309:2
5289:3
5274:4
5270:2
5268:H
5260:4
5256:2
5254:H
5246:4
5236:4
5226:3
5216:2
5186:3
5182:2
5180:H
5172:3
5168:2
5166:H
5158:7
5156:O
5154:2
5150:2
5148:H
5146:/
5143:4
5139:2
5137:H
5119:4
5109:3
5099:2
5079:4
5069:3
5059:2
5040:]
5038:4
5029:F
5027:3
5007:4
5003:3
5001:H
4993:3
4989:3
4987:H
4967:e
4960:t
4953:v
4909:.
4883:.
4859:.
4838:.
4826::
4803:.
4789::
4779::
4771::
4744:.
4732::
4709:.
4687:3
4675:.
4611:.
4590:.
4565:.
4551::
4527:.
4487:.
4459::
4431:.
4405:.
4369:.
4344:.
4319:.
4315::
4307::
4272:.
4226:.
4222::
4214::
4191:.
4179::
4146:.
4140::
4132::
4126:4
4105:.
4101::
4093::
4078:3
4063:.
4049:.
4014:.
3939:.
3918:.
3849:.
3816:.
3742:.
3646:.
3642::
3582:.
3537:.
3514:.
3468:.
3433:.
3397:.
3354:.
3236:(
3097:3
2929:4
2925:2
2923:H
2919:O
2917:2
2915:H
2910:7
2908:O
2906:2
2904:S
2902:2
2900:H
2888:O
2886:2
2884:H
2879:3
2875:3
2873:)
2871:2
2867:2
2865:H
2863:6
2861:C
2857:3
2852:3
2848:5
2846:H
2844:6
2842:C
2771:3
2767:2
2765:O
2763:2
2759:2
2746:3
2742:2
2738:2
2734:2
2726:2
2722:2
2718:2
2716:)
2714:3
2678:4
2674:3
2670:4
2666:2
2662:3
2648:4
2644:2
2642:H
2640:(
2610:4
2606:2
2604:H
2581:O
2579:2
2575:3
2571:2
2567:3
2553:3
2549:2
2545:2
2528:2
2524:2
2514:2
2510:(
2499:2
2497:N
2495:(
2420:4
2416:2
2414:H
2407:4
2403:3
2401:H
2394:3
2385:8
2383:S
2376:4
2374:P
2367:2
2365:I
2357:O
2355:2
2351:2
2347:3
2340:O
2338:2
2334:2
2330:2
2326:3
2229:2
2225:(
2210:2
2142:2
2133:5
2131:O
2129:2
2120:5
2118:O
2116:2
2107:2
2065:O
2063:2
2059:2
2054:3
2051:−
2026:O
2024:2
2020:2
2018:)
2016:3
2012:3
1991:2
1986:2
1984:)
1982:3
1976:3
1968:2
1963:2
1961:)
1959:3
1953:3
1945:2
1940:2
1938:)
1936:3
1930:3
1919:2
1917:H
1888:O
1886:2
1881:3
1878:−
1873:2
1869:3
1852:]
1850:2
1836:K
1827:;
1823:4
1820:−
1815:3
1811:2
1807:4
1803:2
1799:3
1783:a
1780:K
1775:a
1772:K
1770:p
1743:2
1712:3
1692:2
1677:2
1643:2
1629:3
1607:x
1599:4
1597:O
1595:2
1593:N
1588:2
1575:2
1571:2
1567:2
1563:3
1527:O
1525:2
1521:3
1515:]
1513:3
1509:3
1503:O
1501:2
1497:3
1487:3
1237:3
1219:3
1216:O
1213:N
1210:H
1172:Y
990:)
986:(
945:2
938:0
931:3
776:)
771:H
768:f
749:)
744:S
742:(
709:)
707:D
704:n
702:(
690:×
657:a
654:K
629:P
545:3
445:)
441:(
178:)
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.