1108:
source (natural live food) as well. Oxyhemocyanin and blood glucose levels were higher in shrimp housed in outdoor ponds. It was also found that blood metabolite levels tended to be lower in low activity level species, such as crabs, lobsters, and the indoor shrimp when compared to the outdoor shrimp. This correlation is possibly indicative of the morphological and physiological evolution of crustaceans. The levels of these blood proteins and metabolites appear to be dependent on energetic demands and availability of those energy sources.
865:
997:
931:
432:
273:
40:
1093:. KLH has been shown to be a significant treatment against the proliferations of breast cancer, pancreas cancer, and prostate cancer cells when delivered in vitro. Keyhole limpet hemocyanin inhibits growth of human Barrett's esophageal cancer through both apoptic and nonapoptic mechanisms of cell death.
825:
hemocyanin was arranged in protein sub-complexes of 6 subunits (hexamer) each with one oxygen binding site; binding of oxygen on one unit in the complex would increase the affinity of the neighboring units. Each hexamer complex was arranged together to form a larger complex of dozens of hexamers. In
1107:
found that the levels of hemocyanin, oxyhemocyanin in particular, are affected by the diet. The study compared oxyhemocyanin levels in the blood of white shrimp housed in an indoor pond with a commercial diet with that of white shrimp housed in an outdoor pond with a more readily available protein
793:
living in cold environments with low oxygen pressure. Under these circumstances hemoglobin oxygen transportation is less efficient than hemocyanin oxygen transportation. Nevertheless, there are also terrestrial arthropods using hemocyanin, notably spiders and scorpions, that live in warm climates.
752:
The evolutionary changes within the phylogeny of the hemocyanin superfamily are closely related to the emergence of these different proteins in various species. The understanding of proteins within this superfamily would not be well understood without the extensive studies of hemocyanin in
1051:
Much work has been devoted to preparing synthetic analogues of the active site of hemocyanin. One such model, which features a pair of copper centers bridged side-on by peroxo ligand, shows ν(O-O) at 741 cm and a UV-Vis spectrum with absorbances at 349 and 551 nm. Both of these
978:
or proenzymes) must be activated first. This is done by removing the amino acid that blocks the entrance channel to the active site when the proenzyme is not active. There is currently no other known modifications necessary to activate the proenzyme and enable catalytic activity.
2082:
Kitajima N, Fujisawa K, Fujimoto C, Morooka Y, Hashimoto S, Kitagawa T, et al. (1992). "A new model for dioxygen binding in hemocyanin. Synthesis, characterization, and molecular structure of the μ-η2:η2 peroxo dinuclear copper(II) complexes, 2(O2) (R = i-Pr and Ph)".
943:
2153:
Kodera M, Katayama K, Tachi Y, Kano K, Hirota S, Fujinami S, et al. (1999). "Crystal
Structure and Reversible O2-Binding of a Room Temperature Stable μ-η2:η2-Peroxodicopper(II) Complex of a Sterically Hindered Hexapyridine Dinucleating Ligand".
826:
one study, cooperative binding was found to be dependent on hexamers being arranged together in the larger complex, suggesting cooperative binding between hexamers. Hemocyanin oxygen-binding profile is also affected by dissolved salt ion levels and
1827:
Gai, Zuoqi; Matsuno, Asuka; Kato, Koji; Kato, Sanae; Khan, Md
Rafiqul Islam; Shimizu, Takeshi; Yoshioka, Takeya; Kato, Yuki; Kishimura, Hideki; Kanno, Gaku; Miyabe, Yoshikatsu; Terada, Tohru; Tanaka, Yoshikazu; Yao, Min (2015).
723:
Phenoloxidase are copper containing tyrosinases. These proteins are involved in the process of sclerotization of arthropod cuticle, in wound healing, and humoral immune defense. Phenoloxidase is synthesized by
1630:
Rannulu NS, Rodgers MT (March 2005). "Solvation of copper ions by imidazole: structures and sequential binding energies of Cu+(imidazole)x, x = 1-4. Competition between ion solvation and hydrogen bonding".
1082:
hemocyanin showed antitumor effects: prolonged survival, decreased tumor growth and incidence, and lack of toxic effects and may have a potential use in future immunotherapy for superficial bladder cancer.
987:
activity, but with slowed kinetics from greater steric bulk at the active site. Partial denaturation actually improves hemocyanin's phenol oxidase activity by providing greater access to the active site.
761:
Although the respiratory function of hemocyanin is similar to that of hemoglobin, there are a significant number of differences in its molecular structure and mechanism. Whereas hemoglobin carries its
2243:
Pascual C, Gaxiola G, Rosas C (2003). "Blood metabolites and hemocyanin of the white shrimp, Litopenaeus vannamei: The effect of culture conditions and a comparison with other crustacean species".
2208:
McFadden DW, Riggs DR, Jackson BJ, Vona-Davis L (November 2003). "Keyhole limpet hemocyanin, a novel immune stimulant with promising anticancer activity in
Barrett's esophageal adenocarcinoma".
821:
of 1.6–3.0. Hill coefficients vary depending on species and laboratory measurement settings. Hemoglobin, for comparison, has a Hill coefficient of usually 2.8–3.0. In these cases of
2110:
Gaykema WP, Hol WG, Vereijken JM, Soeter NM, Bak HJ, Beintema JJ (1984). "3.2 Å structure of the copper-containing, oxygen-carrying protein
Panulirus interruptus haemocyanin".
1052:
measurements agree with the experimental observations for oxyHc. The Cu-Cu separation in the model complex is 3.56 Å, that of oxyhemocyanin is ca. 3.6 Å (deoxyHc: ca. 4.6 Å).
3082:
745:
Pseudohemocyanin and cryptocyanins genetic sequences are closely related to hemocyanins in crustaceans. These proteins have a similar structure and function, but lack the
543:
390:
154:
1992:
Decker H, Schweikardt T, Nillius D, Salzbrunn U, Jaenicke E, Tuczek F (August 2007). "Similar enzyme activation and catalysis in hemocyanins and tyrosinases".
1676:"Influence of temperature, hypercapnia, and development on the relative expression of different hemocyanin isoforms in the common cuttlefish Sepia officinalis"
872:
hemocyanin. It is a homodecamer of five dimers arranged into a 31 nm diameter cylinder. Each monomer has a string of eight individual subunits each with a Cu
922:
they are about 625. In the large complexes there is a variety of variant chains, all about the same length; pure components do not usually self-assemble.
853:
depending on species; the dimer or hexamer complex is likewise arranged in chains or clusters with weights exceeding 1500 kDa. The subunits are usually
801:
and are roughly one-fourth as efficient as hemoglobin at transporting oxygen per amount of blood. Hemoglobin binds oxygen cooperatively due to steric
2401:
2336:
1889:
1751:
Perton FG, Beintema JJ, Decker H (May 1997). "Influence of antibody binding on oxygen binding behavior of
Panulirus interruptus hemocyanin".
491:
338:
102:
1246:"Complete sequence of the 24-mer hemocyanin of the tarantula Eurypelma californicum. Structure and intramolecular evolution of the subunits"
861:
with two variant subunit types. Because of the large size of hemocyanin, it is usually found free-floating in the blood, unlike hemoglobin.
1915:"Complete subunit sequences, structure and evolution of the 6 x 6-mer hemocyanin from the common house centipede, Scutigera coleoptrata"
1957:
Decker H, Tuczek F (August 2000). "Tyrosinase/catecholoxidase activity of hemocyanins: structural basis and molecular mechanism".
1338:
1038:
1287:"Crystallization and preliminary analysis of crystals of the 24-meric hemocyanin of the emperor scorpion (Pandinus imperator)"
1716:
Sterner R, Vogl T, Hinz HJ, Penz F, Hoff R, Föll R, Decker H (May 1995). "Extreme thermostability of tarantula hemocyanin".
563:
410:
174:
3122:
983:
differences determine the type of catalytic activity that the hemocyanin is able to perform. Hemocyanin also exhibits
2394:
789:
rings of six histidine residues. It has been noted that species using hemocyanin for oxygen transportation include
3117:
1122:
1086:
436:
crystallographic analysis of oxygenated and deoxygenated states of arthropod hemocyanin shows unusual differences
1532:"Cryptocyanin, a crustacean molting protein: evolutionary link with arthropod hemocyanins and insect hexamerins"
3112:
3102:
2469:
980:
551:
398:
162:
3107:
2547:
2528:
2491:
1872:
van Holde KE, Miller KI (1995). "Hemocyanins". In
Anfinsen CB, Richards FM, Edsall JT, Eisenberg DS (eds.).
1117:
1062:
674:
697:
1101:
A 2003 study of the effect of culture conditions of blood metabolites and hemocyanin of the white shrimp
2387:
1044:
869:
738:
Hexamerins are storage proteins commonly found in insects. These proteins are synthesized by the larval
684:
279:
785:
residues. Each hemocyanin monomer holds a pair of copper(I) cations in place via interactions with the
547:
394:
158:
709:
2672:
2658:
2644:
2630:
2119:
1640:
1543:
1298:
1103:
809:, which increases hemoglobin's affinity for oxygen when partially oxygenated. In some hemocyanins of
504:
351:
115:
2938:
2908:
2832:
2474:
1411:
Burmester T (February 2002). "Origin and evolution of arthropod hemocyanins and related proteins".
1137:
822:
798:
794:
The molecule is conformationally stable and fully functioning at temperatures up to 90 degrees C.
713:
2922:
2604:
2260:
2135:
1479:
1454:
Cerenius L, Söderhäll K (April 2004). "The prophenoloxidase-activating system in invertebrates".
1436:
1223:
1019:
1675:
705:
2903:
2798:
2359:
2332:
2315:
2225:
2061:
2009:
1974:
1936:
1895:
1885:
1851:
1809:
1768:
1733:
1698:
1656:
1612:
1571:
1471:
1428:
1393:
1326:
1267:
1215:
1176:
906:
have an 8-hexamer (i. e. 48-chain) hemocyanin. Simple hexamers are found in the spiny lobster
882:
864:
538:
385:
181:
149:
2808:
2767:
2697:
2379:
2305:
2295:
2252:
2217:
2190:
2163:
2127:
2092:
2051:
2043:
2001:
1966:
1926:
1877:
1841:
1799:
1760:
1725:
1690:
1648:
1602:
1561:
1551:
1510:
1463:
1420:
1383:
1316:
1306:
1257:
1207:
1168:
1006:
971:
818:
778:
679:
653:
644:
1830:"Crystal Structure of the 3.8-MDa Respiratory Supermolecule Hemocyanin at 3.0 Å Resolution"
1674:
Strobel A, Hu MY, Gutowska MA, Lieb B, Lucassen M, Melzner F, et al. (December 2012).
530:
377:
141:
3062:
2917:
1607:
1590:
1388:
1371:
1089:(KLH) is an immune stimulant derived from circulating glycoproteins of the marine mollusk
903:
806:
802:
615:
622:, hemocyanins are not confined in blood cells, but are instead suspended directly in the
2123:
1644:
1547:
1413:
Journal of
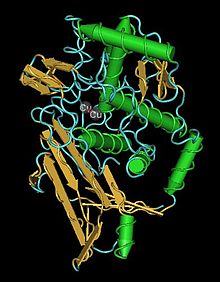
Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology
1302:
854:
688:. Also, larval storage proteins in many insects appear to be derived from hemocyanins.
2964:
2959:
2310:
2283:
2056:
2031:
2030:
Elwell CE, Gagnon NL, Neisen BD, Dhar D, Spaeth AD, Yee GM, Tolman WB (February 2017).
1321:
1286:
1067:
984:
810:
649:
595:
1970:
1881:
1804:
1787:
1764:
3096:
3069:
3028:
2943:
2750:
2532:
2515:
2454:
2368:
1931:
1914:
1729:
1566:
1531:
1467:
898:
is made up of 4 hexamers or 24 peptide chains. A hemocyanin from the house centipede
858:
846:
842:
701:
496:
343:
107:
2264:
1483:
1440:
1346:
1227:
930:
894:
Hexamers are characteristic of arthropod hemocyanins. A hemocyanin of the tarantula
460:
307:
71:
3057:
2998:
2874:
2860:
2846:
2736:
2711:
2139:
919:
833:
Hemocyanin is made of many individual subunit proteins, each of which contains two
591:
526:
373:
137:
2221:
996:
942:
1311:
1244:
Voit R, Feldmaier-Fuchs G, Schweikardt T, Decker H, Burmester T (December 2000).
1196:"The pre-history of hemocyanin. The discovery of copper in the blood of molluscs"
966:
residues, called "type 3" copper-binding coordination centers, as do the enzymes
472:
319:
83:
27:
Proteins that transport oxygen throughout the bodies of some invertebrate animals
3005:
2418:
2047:
915:
790:
619:
2194:
2005:
1536:
Proceedings of the
National Academy of Sciences of the United States of America
3052:
3010:
2913:
2436:
2256:
1846:
1829:
1424:
1195:
1172:
1127:
967:
959:
814:
729:
669:
665:
661:
614:
in frequency of use as an oxygen transport molecule. Unlike the hemoglobin in
611:
2032:"Copper-Oxygen Complexes Revisited: Structures, Spectroscopy, and Reactivity"
1372:"Evolution of arthropod hemocyanins and insect storage proteins (hexamerins)"
1219:
2993:
2300:
1132:
963:
786:
782:
766:
623:
2353:
2319:
2229:
2065:
2013:
1978:
1940:
1855:
1702:
1683:
Journal of
Experimental Zoology. Part A, Ecological Genetics and Physiology
1660:
1616:
1575:
1556:
1515:
1498:
1475:
1432:
1330:
1271:
1262:
1245:
1180:
1159:
Coates CJ, Nairn J (July 2014). "Diverse immune functions of hemocyanins".
17:
1913:
Kusche K, Hembach A, Hagner-Holler S, Gebauer W, Burmester T (July 2003).
1899:
1813:
1772:
1737:
1397:
500:
431:
347:
272:
111:
739:
657:
652:
in 1878. The presence of copper in molluscs was detected even earlier by
467:
314:
78:
2096:
39:
2410:
2373:
2363:
1211:
975:
850:
732:
725:
717:
587:
484:
479:
331:
326:
95:
90:
45:
2167:
886:
2427:
2131:
1694:
1652:
834:
774:
746:
603:
599:
558:
405:
169:
249:
243:
236:
230:
224:
218:
212:
206:
199:
193:
187:
2479:
1075:
995:
941:
929:
863:
742:
and are associated with molting cycles or nutritional conditions.
627:
2588:
2576:
2571:
2503:
2414:
2282:
Rehm P, Pick C, Borner J, Markl J, Burmester T (February 2012).
974:. In both cases inactive precursors to the enzymes (also called
770:
762:
631:
520:
455:
367:
302:
131:
66:
2383:
1034:
is bound in a symmetric environment (ν(O-O) is not IR-allowed).
2818:
2559:
1014:
Spectroscopy of oxyhemocyanin shows several salient features:
1591:"Molecular evolution of the arthropod hemocyanin superfamily"
672:, and utilized by some land arthropods such as the tarantula
630:
change between the colorless Cu(I) deoxygenated form and the
2358:
Overview of all the structural information available in the
44:
Single oxygenated functional unit from the hemocyanin of an
1499:"Hemolymph Proteins and Molting in Crustaceans and Insects"
827:
1060:
The hemocyanin found in the blood of the
Chilean abalone,
2284:"The diversity and evolution of chelicerate hemocyanins"
2181:
Atala A (2006). "This Month in Investigative Urology".
1097:
Case studies: environmental impact on hemocyanin levels
958:
Hemocyanin is homologous to the phenol oxidases (e.g.
1788:"The structure of arthropod and mollusc hemocyanins"
1370:
Beintema JJ, Stam WT, Hazes B, Smidt MP (May 1994).
1041:-silent indicating the absence of unpaired electrons
590:
that transport oxygen throughout the bodies of some
3045:
3019:
2984:
2977:
2952:
2931:
2896:
2789:
2729:
2690:
2623:
2612:
2603:
2526:
2451:
2444:
2435:
2426:
2354:
3D hemocyanin structures in the EM Data Bank (EMDB)
2331:. Saarbrücken: VDM Verlag Dr. Müller. p. 160.
1285:Jaenicke E, Pairet B, Hartmann H, Decker H (2012).
557:
537:
519:
514:
490:
478:
466:
454:
446:
441:
424:
404:
384:
366:
361:
337:
325:
313:
301:
293:
288:
265:
180:
168:
148:
130:
125:
101:
89:
77:
65:
57:
52:
32:
1530:Terwilliger NB, Dangott L, Ryan M (March 1999).
1339:"The blue blood of the emperor scorpion x-rayed"
938:(each Cu center is a cation, charges not shown).
277:Crystal structure of hexameric haemocyanin from
1952:
1950:
1876:. Vol. 47. Academic Press. pp. 1–81.
1010:). The purple coloring is caused by hemocyanin.
950:-bound form of a hemocyanin active site (the Cu
918:are about 660 amino acid residues long, and in
1867:
1865:
1154:
1152:
2395:
8:
934:A hemocyanin active site in the absence of O
2077:
2075:
1239:
1237:
2981:
2620:
2609:
2448:
2441:
2432:
2402:
2388:
2380:
511:
430:
358:
271:
122:
38:
3083:disorders of globin and globulin proteins
2309:
2299:
2055:
2025:
2023:
1930:
1845:
1803:
1606:
1565:
1555:
1514:
1387:
1320:
1310:
1261:
837:atoms and can bind one oxygen molecule (O
2156:Journal of the American Chemical Society
2085:Journal of the American Chemical Society
1161:Developmental and Comparative Immunology
1066:, has immunotherapeutic effects against
954:center is a dication, charge not shown).
817:, cooperative binding is observed, with
1194:Ghiretti-Magaldi A, Ghiretti F (1992).
1148:
902:is made up of 6 hexamers or 36 chains.
797:Most hemocyanins bind with oxygen non-
656:in 1833. Hemocyanins are found in the
421:
262:
29:
1608:10.1093/oxfordjournals.molbev.a003792
1389:10.1093/oxfordjournals.molbev.a040129
7:
1343:Johannes Gutenberg-Universität Mainz
602:atoms that reversibly bind a single
33:Hemocyanin, copper containing domain
2329:Scorpion Hemocyanin: The blue blood
1792:The Journal of Biological Chemistry
1633:Physical Chemistry Chemical Physics
1250:The Journal of Biological Chemistry
1070:in murine models. Mice primed with
868:The 3.8 MDa structure of molluscan
845:(kDa). Subunits may be arranged in
642:Hemocyanin was first discovered in
283:refined at 3.2 angstroms resolution
25:
1078:(MBT-2) cells. Mice treated with
777:atoms of hemocyanin are bound as
728:and are activated by cleaving an
1932:10.1046/j.1432-1033.2003.03664.x
1919:European Journal of Biochemistry
1468:10.1111/j.0105-2896.2004.00116.x
841:). Each subunit weighs about 75
1595:Molecular Biology and Evolution
1376:Molecular Biology and Evolution
1345:. June 22, 2012. Archived from
1074:before implantation of bladder
1959:Trends in Biochemical Sciences
1:
2222:10.1016/j.amjsurg.2003.08.002
1971:10.1016/S0968-0004(00)01602-9
1882:10.1016/S0065-3233(08)60545-8
1874:Advances in Protein Chemistry
1805:10.1016/S0021-9258(19)41469-5
1765:10.1016/S0014-5793(97)00269-X
1589:Burmester T (February 2001).
1118:Atlantic horseshoe crab blood
515:Available protein structures:
362:Available protein structures:
126:Available protein structures:
1730:10.1016/0014-5793(95)00341-6
1312:10.1371/journal.pone.0032548
266:Hemocyanin, all-alpha domain
2210:American Journal of Surgery
2048:10.1021/acs.chemrev.6b00636
1047:shows ν(O-O) of 755 cm
962:) since both proteins have
610:). They are second only to
3139:
2195:10.1016/j.juro.2006.09.002
2006:10.1016/j.gene.2007.02.051
813:and some other species of
692:The hemocyanin superfamily
425:Hemocyanin, ig-like domain
3078:
2327:Ali SA, Abbasi A (2011).
2257:10.1007/s00227-002-0995-2
1847:10.1016/j.str.2015.09.008
1425:10.1007/s00360-001-0247-7
1173:10.1016/j.dci.2014.01.021
1123:Keyhole limpet hemocyanin
1087:Keyhole limpet hemocyanin
696:The arthropod hemocyanin
510:
429:
357:
270:
121:
37:
2288:BMC Evolutionary Biology
634:Cu(II) oxygenated form.
2372:(Hemocyanin II) at the
2301:10.1186/1471-2148-12-19
1497:Terwilliger NB (1999).
1063:Concholepas concholepas
757:Structure and mechanism
720:) hexamerin receptors.
626:. Oxygenation causes a
2183:The Journal of Urology
1557:10.1073/pnas.96.5.2013
1263:10.1074/jbc.M005442200
1011:
955:
939:
896:Eurypelma californicum
891:
675:Eurypelma californicum
1786:Waxman L (May 1975).
1456:Immunological Reviews
1045:Infrared spectroscopy
1000:The underside of the
999:
945:
933:
908:Panulirus interruptus
900:Scutigera coleoptrata
870:Japanese flying squid
867:
685:Scutigera coleoptrata
280:Panulirus interruptus
3123:Respiratory pigments
1516:10.1093/icb/39.3.589
1104:Litopenaeus vannamei
1004:of a red rock crab (
914:. Peptide chains in
912:Bathynomus giganteus
682:, and the centipede
638:Species distribution
2939:Glycated hemoglobin
2909:Carbaminohemoglobin
2124:1984Natur.309...23G
2097:10.1021/ja00030a025
1645:2005PCCP....7.1014R
1548:1999PNAS...96.2013T
1303:2012PLoSO...732548J
1256:(50): 39339–39344.
1138:Respiratory pigment
1091:Megathura crenulata
992:Spectral properties
823:cooperative binding
1503:American Zoologist
1212:10.1007/BF01919143
1056:Anticancer effects
1020:Raman spectroscopy
1012:
956:
940:
926:Catalytic activity
892:
3090:
3089:
3041:
3040:
3037:
3036:
2973:
2972:
2904:Carboxyhemoglobin
2892:
2891:
2785:
2784:
2599:
2598:
2338:978-3-639-33725-9
2168:10.1021/ja992295q
1925:(13): 2860–2868.
1891:978-0-12-034247-1
1840:(12): 2204–2212.
1798:(10): 3796–3806.
819:Hill coefficients
779:prosthetic groups
710:pseudohemocyanins
573:
572:
569:
568:
564:structure summary
420:
419:
416:
415:
411:structure summary
261:
260:
257:
256:
175:structure summary
16:(Redirected from
3130:
3118:Immunostimulants
2982:
2621:
2610:
2449:
2442:
2433:
2404:
2397:
2390:
2381:
2342:
2323:
2313:
2303:
2269:
2268:
2240:
2234:
2233:
2205:
2199:
2198:
2178:
2172:
2171:
2150:
2144:
2143:
2132:10.1038/309023a0
2107:
2101:
2100:
2079:
2070:
2069:
2059:
2042:(3): 2059–2107.
2036:Chemical Reviews
2027:
2018:
2017:
2000:(1–2): 183–191.
1989:
1983:
1982:
1954:
1945:
1944:
1934:
1910:
1904:
1903:
1869:
1860:
1859:
1849:
1824:
1818:
1817:
1807:
1783:
1777:
1776:
1748:
1742:
1741:
1713:
1707:
1706:
1695:10.1002/jez.1743
1680:
1671:
1665:
1664:
1653:10.1039/b418141g
1639:(5): 1014–1025.
1627:
1621:
1620:
1610:
1586:
1580:
1579:
1569:
1559:
1542:(5): 2013–2018.
1527:
1521:
1520:
1518:
1494:
1488:
1487:
1451:
1445:
1444:
1408:
1402:
1401:
1391:
1367:
1361:
1358:
1356:
1354:
1334:
1324:
1314:
1282:
1276:
1275:
1265:
1241:
1232:
1231:
1191:
1185:
1184:
1156:
1033:
1032:
1031:
1007:Cancer productus
972:catechol oxidase
889:
680:emperor scorpion
654:Bartolomeo Bizio
645:Octopus vulgaris
582:and abbreviated
512:
434:
422:
359:
275:
263:
252:
246:
239:
233:
227:
221:
215:
209:
202:
196:
190:
123:
42:
30:
21:
3138:
3137:
3133:
3132:
3131:
3129:
3128:
3127:
3113:Copper proteins
3103:Metalloproteins
3093:
3092:
3091:
3086:
3074:
3063:Cytochrome P450
3033:
3015:
2969:
2948:
2927:
2918:Deoxyhemoglobin
2888:
2884:
2880:
2870:
2866:
2856:
2852:
2842:
2838:
2828:
2824:
2814:
2804:
2781:
2777:
2773:
2763:
2759:
2754:
2746:
2742:
2725:
2721:
2717:
2707:
2703:
2686:
2682:
2678:
2673:HbE Portland II
2668:
2664:
2654:
2650:
2640:
2636:
2615:
2595:
2522:
2453:Alpha locus on
2422:
2408:
2350:
2345:
2339:
2326:
2281:
2277:
2275:Further reading
2272:
2242:
2241:
2237:
2207:
2206:
2202:
2180:
2179:
2175:
2162:(47): 11006–7.
2152:
2151:
2147:
2109:
2108:
2104:
2081:
2080:
2073:
2029:
2028:
2021:
1991:
1990:
1986:
1956:
1955:
1948:
1912:
1911:
1907:
1892:
1871:
1870:
1863:
1826:
1825:
1821:
1785:
1784:
1780:
1750:
1749:
1745:
1715:
1714:
1710:
1678:
1673:
1672:
1668:
1629:
1628:
1624:
1588:
1587:
1583:
1529:
1528:
1524:
1496:
1495:
1491:
1453:
1452:
1448:
1410:
1409:
1405:
1369:
1368:
1364:
1352:
1350:
1337:
1284:
1283:
1279:
1243:
1242:
1235:
1206:(10): 971–972.
1193:
1192:
1188:
1158:
1157:
1150:
1146:
1114:
1099:
1058:
1030:
1027:
1026:
1025:
1023:
994:
953:
949:
937:
928:
910:and the isopod
904:Horseshoe crabs
881:
879:
875:
840:
811:horseshoe crabs
807:protein complex
805:changes in the
781:coordinated by
759:
749:binding sites.
700:is composed of
694:
640:
616:red blood cells
609:
596:metalloproteins
594:animals. These
437:
284:
248:
242:
235:
229:
223:
217:
211:
205:
198:
192:
186:
48:
28:
23:
22:
15:
12:
11:
5:
3136:
3134:
3126:
3125:
3120:
3115:
3110:
3108:Blood proteins
3105:
3095:
3094:
3088:
3087:
3079:
3076:
3075:
3073:
3072:
3067:
3066:
3065:
3060:
3049:
3047:
3043:
3042:
3039:
3038:
3035:
3034:
3032:
3031:
3025:
3023:
3017:
3016:
3014:
3013:
3008:
3003:
3002:
3001:
2990:
2988:
2979:
2975:
2974:
2971:
2970:
2968:
2967:
2965:Erythrocruorin
2962:
2956:
2954:
2950:
2949:
2947:
2946:
2941:
2935:
2933:
2929:
2928:
2926:
2925:
2923:Sulfhemoglobin
2920:
2911:
2906:
2900:
2898:
2894:
2893:
2890:
2889:
2887:
2886:
2882:
2878:
2872:
2868:
2864:
2858:
2854:
2850:
2844:
2840:
2836:
2830:
2826:
2822:
2816:
2812:
2806:
2802:
2795:
2793:
2787:
2786:
2783:
2782:
2780:
2779:
2775:
2771:
2765:
2761:
2757:
2752:
2748:
2744:
2740:
2733:
2731:
2727:
2726:
2724:
2723:
2719:
2715:
2709:
2705:
2701:
2694:
2692:
2688:
2687:
2685:
2684:
2680:
2676:
2670:
2666:
2662:
2659:HbE Portland I
2656:
2652:
2648:
2642:
2638:
2634:
2627:
2625:
2618:
2607:
2601:
2600:
2597:
2596:
2594:
2593:
2592:
2591:
2581:
2580:
2579:
2574:
2564:
2563:
2562:
2552:
2551:
2550:
2539:
2537:
2524:
2523:
2521:
2520:
2519:
2518:
2508:
2507:
2506:
2496:
2495:
2494:
2484:
2483:
2482:
2477:
2472:
2461:
2459:
2446:
2439:
2430:
2424:
2423:
2409:
2407:
2406:
2399:
2392:
2384:
2378:
2377:
2356:
2349:
2348:External links
2346:
2344:
2343:
2337:
2324:
2278:
2276:
2273:
2271:
2270:
2251:(4): 735–745.
2245:Marine Biology
2235:
2216:(5): 552–555.
2200:
2173:
2145:
2118:(5963): 23–9.
2102:
2091:(4): 1277–91.
2071:
2019:
1984:
1965:(8): 392–397.
1946:
1905:
1890:
1861:
1819:
1778:
1759:(2): 124–126.
1743:
1708:
1689:(8): 511–523.
1666:
1622:
1601:(2): 184–195.
1581:
1522:
1509:(3): 589–599.
1489:
1462:(1): 116–126.
1446:
1403:
1382:(3): 493–503.
1362:
1360:
1359:
1349:on May 6, 2022
1277:
1233:
1186:
1147:
1145:
1142:
1141:
1140:
1135:
1130:
1125:
1120:
1113:
1110:
1098:
1095:
1080:C. concholepas
1072:C. concholepas
1068:bladder cancer
1057:
1054:
1049:
1048:
1042:
1035:
1028:
993:
990:
985:phenol oxidase
981:Conformational
951:
947:
935:
927:
924:
880:binding site.
877:
873:
838:
758:
755:
702:phenoloxidases
693:
690:
650:Leon Fredericq
639:
636:
607:
578:(also spelled
571:
570:
567:
566:
561:
555:
554:
541:
535:
534:
524:
517:
516:
508:
507:
494:
488:
487:
482:
476:
475:
470:
464:
463:
458:
452:
451:
448:
444:
443:
439:
438:
435:
427:
426:
418:
417:
414:
413:
408:
402:
401:
388:
382:
381:
371:
364:
363:
355:
354:
341:
335:
334:
329:
323:
322:
317:
311:
310:
305:
299:
298:
295:
291:
290:
286:
285:
276:
268:
267:
259:
258:
255:
254:
184:
178:
177:
172:
166:
165:
152:
146:
145:
135:
128:
127:
119:
118:
105:
99:
98:
93:
87:
86:
81:
75:
74:
69:
63:
62:
59:
55:
54:
50:
49:
43:
35:
34:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
3135:
3124:
3121:
3119:
3116:
3114:
3111:
3109:
3106:
3104:
3101:
3100:
3098:
3085:
3084:
3077:
3071:
3070:Methemalbumin
3068:
3064:
3061:
3059:
3056:
3055:
3054:
3051:
3050:
3048:
3044:
3030:
3029:Leghemoglobin
3027:
3026:
3024:
3022:
3018:
3012:
3009:
3007:
3004:
3000:
2997:
2996:
2995:
2992:
2991:
2989:
2987:
2983:
2980:
2976:
2966:
2963:
2961:
2960:Chlorocruorin
2958:
2957:
2955:
2951:
2945:
2944:Methemoglobin
2942:
2940:
2937:
2936:
2934:
2930:
2924:
2921:
2919:
2915:
2914:Oxyhemoglobin
2912:
2910:
2907:
2905:
2902:
2901:
2899:
2895:
2876:
2873:
2862:
2859:
2848:
2845:
2834:
2831:
2820:
2817:
2810:
2807:
2800:
2797:
2796:
2794:
2792:
2788:
2769:
2766:
2755:
2749:
2738:
2735:
2734:
2732:
2728:
2713:
2710:
2699:
2696:
2695:
2693:
2689:
2674:
2671:
2660:
2657:
2646:
2643:
2632:
2629:
2628:
2626:
2622:
2619:
2617:
2611:
2608:
2606:
2602:
2590:
2587:
2586:
2585:
2582:
2578:
2575:
2573:
2570:
2569:
2568:
2565:
2561:
2558:
2557:
2556:
2553:
2549:
2546:
2545:
2544:
2541:
2540:
2538:
2536:
2534:
2530:
2525:
2517:
2514:
2513:
2512:
2509:
2505:
2502:
2501:
2500:
2497:
2493:
2490:
2489:
2488:
2485:
2481:
2478:
2476:
2473:
2471:
2468:
2467:
2466:
2463:
2462:
2460:
2458:
2456:
2450:
2447:
2443:
2440:
2438:
2434:
2431:
2429:
2425:
2420:
2416:
2413:that contain
2412:
2405:
2400:
2398:
2393:
2391:
2386:
2385:
2382:
2375:
2371:
2370:
2365:
2361:
2357:
2355:
2352:
2351:
2347:
2340:
2334:
2330:
2325:
2321:
2317:
2312:
2307:
2302:
2297:
2293:
2289:
2285:
2280:
2279:
2274:
2266:
2262:
2258:
2254:
2250:
2246:
2239:
2236:
2231:
2227:
2223:
2219:
2215:
2211:
2204:
2201:
2196:
2192:
2189:(6): 2335–6.
2188:
2184:
2177:
2174:
2169:
2165:
2161:
2157:
2149:
2146:
2141:
2137:
2133:
2129:
2125:
2121:
2117:
2113:
2106:
2103:
2098:
2094:
2090:
2086:
2078:
2076:
2072:
2067:
2063:
2058:
2053:
2049:
2045:
2041:
2037:
2033:
2026:
2024:
2020:
2015:
2011:
2007:
2003:
1999:
1995:
1988:
1985:
1980:
1976:
1972:
1968:
1964:
1960:
1953:
1951:
1947:
1942:
1938:
1933:
1928:
1924:
1920:
1916:
1909:
1906:
1901:
1897:
1893:
1887:
1883:
1879:
1875:
1868:
1866:
1862:
1857:
1853:
1848:
1843:
1839:
1835:
1831:
1823:
1820:
1815:
1811:
1806:
1801:
1797:
1793:
1789:
1782:
1779:
1774:
1770:
1766:
1762:
1758:
1754:
1747:
1744:
1739:
1735:
1731:
1727:
1723:
1719:
1712:
1709:
1704:
1700:
1696:
1692:
1688:
1684:
1677:
1670:
1667:
1662:
1658:
1654:
1650:
1646:
1642:
1638:
1634:
1626:
1623:
1618:
1614:
1609:
1604:
1600:
1596:
1592:
1585:
1582:
1577:
1573:
1568:
1563:
1558:
1553:
1549:
1545:
1541:
1537:
1533:
1526:
1523:
1517:
1512:
1508:
1504:
1500:
1493:
1490:
1485:
1481:
1477:
1473:
1469:
1465:
1461:
1457:
1450:
1447:
1442:
1438:
1434:
1430:
1426:
1422:
1419:(2): 95–107.
1418:
1414:
1407:
1404:
1399:
1395:
1390:
1385:
1381:
1377:
1373:
1366:
1363:
1348:
1344:
1340:
1336:
1335:
1332:
1328:
1323:
1318:
1313:
1308:
1304:
1300:
1297:(3): e32548.
1296:
1292:
1288:
1281:
1278:
1273:
1269:
1264:
1259:
1255:
1251:
1247:
1240:
1238:
1234:
1229:
1225:
1221:
1217:
1213:
1209:
1205:
1201:
1197:
1190:
1187:
1182:
1178:
1174:
1170:
1166:
1162:
1155:
1153:
1149:
1143:
1139:
1136:
1134:
1131:
1129:
1126:
1124:
1121:
1119:
1116:
1115:
1111:
1109:
1106:
1105:
1096:
1094:
1092:
1088:
1084:
1081:
1077:
1073:
1069:
1065:
1064:
1055:
1053:
1046:
1043:
1040:
1036:
1021:
1017:
1016:
1015:
1009:
1008:
1003:
998:
991:
989:
986:
982:
977:
973:
969:
965:
961:
944:
932:
925:
923:
921:
917:
913:
909:
905:
901:
897:
888:
884:
871:
866:
862:
860:
859:heterogeneous
856:
852:
848:
844:
836:
831:
829:
824:
820:
816:
812:
808:
804:
800:
799:cooperatively
795:
792:
788:
784:
780:
776:
773:groups), the
772:
768:
764:
756:
754:
750:
748:
743:
741:
736:
734:
731:
727:
721:
719:
715:
714:cryptocyanins
711:
707:
703:
699:
691:
689:
687:
686:
681:
677:
676:
671:
667:
663:
659:
655:
651:
647:
646:
637:
635:
633:
629:
625:
621:
617:
613:
605:
601:
597:
593:
589:
585:
581:
577:
565:
562:
560:
556:
553:
549:
545:
542:
540:
536:
532:
528:
525:
522:
518:
513:
509:
506:
502:
498:
495:
493:
489:
486:
483:
481:
477:
474:
471:
469:
465:
462:
459:
457:
453:
449:
445:
440:
433:
428:
423:
412:
409:
407:
403:
400:
396:
392:
389:
387:
383:
379:
375:
372:
369:
365:
360:
356:
353:
349:
345:
342:
340:
336:
333:
330:
328:
324:
321:
318:
316:
312:
309:
306:
304:
300:
296:
292:
287:
282:
281:
274:
269:
264:
251:
245:
241:
240:C:136-393
238:
232:
226:
222:D:136-393
220:
214:
208:
201:
195:
189:
185:
183:
179:
176:
173:
171:
167:
164:
160:
156:
153:
151:
147:
143:
139:
136:
133:
129:
124:
120:
117:
113:
109:
106:
104:
100:
97:
94:
92:
88:
85:
82:
80:
76:
73:
70:
68:
64:
60:
56:
51:
47:
41:
36:
31:
19:
3080:
3058:Cytochrome b
3020:
2999:Metmyoglobin
2985:
2790:
2616:development:
2613:
2583:
2566:
2554:
2542:
2527:
2510:
2498:
2486:
2464:
2452:
2419:hemoproteins
2367:
2328:
2291:
2287:
2248:
2244:
2238:
2213:
2209:
2203:
2186:
2182:
2176:
2159:
2155:
2148:
2115:
2111:
2105:
2088:
2084:
2039:
2035:
1997:
1993:
1987:
1962:
1958:
1922:
1918:
1908:
1873:
1837:
1833:
1822:
1795:
1791:
1781:
1756:
1753:FEBS Letters
1752:
1746:
1721:
1718:FEBS Letters
1717:
1711:
1686:
1682:
1669:
1636:
1632:
1625:
1598:
1594:
1584:
1539:
1535:
1525:
1506:
1502:
1492:
1459:
1455:
1449:
1416:
1412:
1406:
1379:
1375:
1365:
1351:. Retrieved
1347:the original
1342:
1294:
1290:
1280:
1253:
1249:
1203:
1199:
1189:
1167:(1): 43–55.
1164:
1160:
1102:
1100:
1090:
1085:
1079:
1071:
1061:
1059:
1050:
1013:
1005:
1001:
957:
920:chelicerates
911:
907:
899:
895:
893:
832:
803:conformation
796:
760:
753:arthropods.
751:
744:
737:
722:
695:
683:
673:
664:, including
643:
641:
598:contain two
592:invertebrate
583:
580:haemocyanins
579:
575:
574:
450:Hemocyanin_C
297:Hemocyanin_N
278:
247:C:136-393
234:C:136-393
228:B:136-393
216:A:136-393
204:
203::110-373
61:Hemocyanin_M
3006:Neuroglobin
2932:Other human
2645:HbE Gower 2
2631:HbE Gower 1
1724:(1): 9–12.
1200:Experientia
1022:shows that
916:crustaceans
855:homogeneous
843:kilodaltons
791:crustaceans
698:superfamily
670:crustaceans
666:cephalopods
620:vertebrates
606:molecule (O
576:Hemocyanins
442:Identifiers
289:Identifiers
210::110-373
197::110-373
191::110-373
53:Identifiers
18:Haemocyanin
3097:Categories
3053:Cytochrome
3011:Cytoglobin
2791:pathology:
2614:stages of
2529:Beta locus
2437:Hemoglobin
1144:References
1128:Hemoglobin
1018:Resonance
968:tyrosinase
960:tyrosinase
815:arthropods
730:N-terminal
706:hexamerins
662:Arthropoda
612:hemoglobin
527:structures
374:structures
138:structures
3081:see also
2994:Myoglobin
2897:Compounds
2768:HbF/Fetal
2698:HbF/Fetal
2624:Embryonic
2605:Tetramers
1834:Structure
1353:March 18,
1220:0014-4754
1133:Myoglobin
1037:OxyHc is
964:histidine
787:imidazole
783:histidine
767:porphyrin
765:atoms in
624:hemolymph
618:found in
485:PDOC00184
473:IPR005203
332:PDOC00184
320:IPR005204
253:C:136-393
96:PDOC00184
84:IPR000896
2953:Nonhuman
2445:Subunits
2411:Proteins
2320:22333134
2265:82961592
2230:14599624
2066:28103018
2014:17566671
1979:10916160
1941:12823556
1856:26602184
1703:22791630
1661:19791394
1617:11158377
1576:10051586
1484:10614298
1476:15199959
1441:26023927
1433:11916114
1331:22403673
1291:PLOS ONE
1272:10961996
1228:33290596
1181:24486681
1112:See also
1002:carapace
976:zymogens
851:hexamers
740:fat body
726:zymogens
718:dipteran
658:Mollusca
588:proteins
544:RCSB PDB
468:InterPro
391:RCSB PDB
315:InterPro
155:RCSB PDB
79:InterPro
2428:Globins
2374:PDBe-KB
2364:UniProt
2311:3306762
2140:4260701
2120:Bibcode
2057:5963733
1900:8561049
1814:1126935
1773:9187351
1738:7750550
1641:Bibcode
1544:Bibcode
1398:8015442
1322:3293826
1299:Bibcode
890:
769:rings (
733:peptide
716:, and (
586:) are
480:PROSITE
461:PF03723
327:PROSITE
308:PF03722
91:PROSITE
72:PF00372
46:octopus
3021:plant:
2986:human:
2480:pseudo
2369:P04253
2335:
2318:
2308:
2294:: 19.
2263:
2228:
2138:
2112:Nature
2064:
2054:
2012:
1977:
1939:
1898:
1888:
1854:
1812:
1771:
1736:
1701:
1659:
1615:
1574:
1564:
1482:
1474:
1439:
1431:
1396:
1329:
1319:
1270:
1226:
1218:
1179:
847:dimers
835:copper
775:copper
747:copper
678:, the
604:oxygen
600:copper
559:PDBsum
533:
523:
505:SUPFAM
447:Symbol
406:PDBsum
380:
370:
352:SUPFAM
294:Symbol
170:PDBsum
144:
134:
116:SUPFAM
58:Symbol
3046:Other
2978:Other
2809:Barts
2730:Adult
2691:Fetal
2261:S2CID
2136:S2CID
1679:(PDF)
1567:26728
1480:S2CID
1437:S2CID
1224:S2CID
1076:tumor
857:, or
628:color
501:SCOPe
492:SCOP2
348:SCOPe
339:SCOP2
112:SCOPe
103:SCOP2
2589:HBE1
2577:HBG2
2572:HBG1
2504:HBQ1
2475:HBA2
2470:HBA1
2415:heme
2362:for
2333:ISBN
2316:PMID
2226:PMID
2062:PMID
2010:PMID
1994:Gene
1975:PMID
1937:PMID
1896:PMID
1886:ISBN
1852:PMID
1810:PMID
1769:PMID
1734:PMID
1699:PMID
1657:PMID
1613:PMID
1572:PMID
1472:PMID
1429:PMID
1394:PMID
1355:2013
1327:PMID
1268:PMID
1216:ISSN
1177:PMID
970:and
887:4YD9
771:heme
763:iron
668:and
660:and
632:blue
552:PDBj
548:PDBe
531:ECOD
521:Pfam
497:1lla
456:Pfam
399:PDBj
395:PDBe
378:ECOD
368:Pfam
344:1lla
303:Pfam
250:1hc2
244:1hc5
237:1hc3
231:1hc4
225:1hc6
219:1hcy
213:1hc1
207:1ll1
200:1lla
194:1nol
188:1oxy
163:PDBj
159:PDBe
142:ECOD
132:Pfam
108:1lla
67:Pfam
2875:HbO
2861:HbE
2847:HbC
2833:HbS
2819:HbD
2799:HbH
2751:HbA
2737:HbA
2712:HbA
2560:HBD
2548:HBB
2531:on
2516:HBM
2492:HBZ
2360:PDB
2306:PMC
2296:doi
2253:doi
2249:142
2218:doi
2214:186
2191:doi
2187:176
2164:doi
2160:121
2128:doi
2116:309
2093:doi
2089:114
2052:PMC
2044:doi
2040:117
2002:doi
1998:398
1967:doi
1927:doi
1923:270
1878:doi
1842:doi
1800:doi
1796:250
1761:doi
1757:408
1726:doi
1722:364
1691:doi
1687:317
1649:doi
1603:doi
1562:PMC
1552:doi
1511:doi
1464:doi
1460:198
1421:doi
1417:172
1384:doi
1317:PMC
1307:doi
1258:doi
1254:275
1208:doi
1169:doi
1039:EPR
883:PDB
849:or
712:or
648:by
539:PDB
386:PDB
182:PDB
150:PDB
3099::
2877:(α
2863:(α
2849:(α
2835:(α
2821:(α
2811:(γ
2801:(β
2770:(α
2756:(α
2739:(α
2714:(α
2700:(α
2675:(ζ
2661:(ζ
2647:(α
2633:(ζ
2533:11
2455:16
2366::
2314:.
2304:.
2292:12
2290:.
2286:.
2259:.
2247:.
2224:.
2212:.
2185:.
2158:.
2134:.
2126:.
2114:.
2087:.
2074:^
2060:.
2050:.
2038:.
2034:.
2022:^
2008:.
1996:.
1973:.
1963:25
1961:.
1949:^
1935:.
1921:.
1917:.
1894:.
1884:.
1864:^
1850:.
1838:23
1836:.
1832:.
1808:.
1794:.
1790:.
1767:.
1755:.
1732:.
1720:.
1697:.
1685:.
1681:.
1655:.
1647:.
1635:.
1611:.
1599:18
1597:.
1593:.
1570:.
1560:.
1550:.
1540:96
1538:.
1534:.
1507:39
1505:.
1501:.
1478:.
1470:.
1458:.
1435:.
1427:.
1415:.
1392:.
1380:11
1378:.
1374:.
1341:.
1325:.
1315:.
1305:.
1293:.
1289:.
1266:.
1252:.
1248:.
1236:^
1222:.
1214:.
1204:48
1202:.
1198:.
1175:.
1165:45
1163:.
1151:^
885::
830:.
828:pH
735:.
708:,
704:,
584:Hc
550:;
546:;
529:/
503:/
499:/
397:;
393:;
376:/
350:/
346:/
161:;
157:;
140:/
114:/
110:/
2916:/
2885:)
2883:2
2881:β
2879:2
2871:)
2869:2
2867:β
2865:2
2857:)
2855:2
2853:β
2851:2
2843:)
2841:2
2839:β
2837:2
2829:)
2827:2
2825:β
2823:2
2815:)
2813:4
2805:)
2803:4
2778:)
2776:2
2774:γ
2772:2
2764:)
2762:2
2760:δ
2758:2
2753:2
2747:)
2745:2
2743:β
2741:2
2722:)
2720:2
2718:β
2716:2
2708:)
2706:2
2704:γ
2702:2
2683:)
2681:2
2679:β
2677:2
2669:)
2667:2
2665:γ
2663:2
2655:)
2653:2
2651:ε
2649:2
2641:)
2639:2
2637:ε
2635:2
2584:ε
2567:γ
2555:δ
2543:β
2535::
2511:μ
2499:θ
2487:ζ
2465:α
2457::
2421:)
2417:(
2403:e
2396:t
2389:v
2376:.
2341:.
2322:.
2298::
2267:.
2255::
2232:.
2220::
2197:.
2193::
2170:.
2166::
2142:.
2130::
2122::
2099:.
2095::
2068:.
2046::
2016:.
2004::
1981:.
1969::
1943:.
1929::
1902:.
1880::
1858:.
1844::
1816:.
1802::
1775:.
1763::
1740:.
1728::
1705:.
1693::
1663:.
1651::
1643::
1637:7
1619:.
1605::
1578:.
1554::
1546::
1519:.
1513::
1486:.
1466::
1443:.
1423::
1400:.
1386::
1357:.
1333:.
1309::
1301::
1295:7
1274:.
1260::
1230:.
1210::
1183:.
1171::
1029:2
1024:O
952:2
948:2
946:O
936:2
878:2
876:O
874:2
839:2
608:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.