400:
202:
218:
245:
Any sort of saturated molecule can be the starting point for generating isolobal fragments. The molecule's bonding and nonbonding molecular orbitals (MOs) should be filled and the antibonding MOs empty. With each consecutive generation of an isolobal fragment, electrons are removed from the bonding
246:
orbitals and a frontier orbital is created. The frontier orbitals are at a higher energy level than the bonding and nonbonding MOs. Each frontier orbital contains one electron. For example, consider Figure 5, which shows the production of frontier orbitals in tetrahedral and octahedral molecules.
197:
complex because Mo has obtained an additional electron making it d. To remedy this, Mo can be exchanged for Mn, which would form a neutral d complex in this case, as shown in Figure 3. This trend can continue until only one ligand is left coordinated to the metal center.
168:
starting point must be d. Removal of a ligand is analogous to the removal of hydrogen of methane in the previous example resulting in a frontier orbital, which points toward the removed ligand. Cleaving the bond between the metal center and one ligand results in a
136:
as the frontier orbital points in the direction of the missing hydrogen atom. Further removal of hydrogen results in the formation of a second frontier orbital. This process can be repeated until only one bond remains to the molecule's central atom.
250:
132:(MOs) are filled and all antibonding MOs are empty. For example, methane is a simple molecule from which to form a main group fragment. The removal of a hydrogen atom from methane generates a methyl radical. The molecule retains its
228:
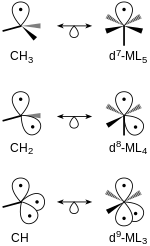
Isolobal fragments of tetrahedral and octahedral molecules can be related. Structures with the same number of frontier orbitals are isolobal to one another. For example, the methane with two hydrogen atoms removed,
410:
The analogy applies to other shapes besides tetrahedral and octahedral geometries. The derivations used in octahedral geometry are valid for most other geometries. The exception is square-planar because
272:
involved in bonding becomes a nonbonding singly occupied frontier orbital. The frontier orbital’s increased energy level is also shown in the figure. Similarly when starting with a metal complex such as
856:
208:
Production of a frontier orbital in an octahedral complex. Since the process is not charge producing, the metal center must change from d Mo to d Mn to retain the neutral charge.
90:
and structure. A graphic representation of isolobal structures, with the isolobal pairs connected through a double-headed arrow with half an orbital below, is found in Figure 1.
415:
typically abide by the 16-electron rule. Assuming ligands act as two-electron donors the metal center in square-planar molecules is d. To relate an octahedral fragment, ML
297:
The isolobal analogy can also be used with isoelectronic fragments having the same coordination number, which allows charged species to be considered. For example, Re(CO)
289:
The isolobal analogy has applications beyond simple octahedral complexes. It can be used with a variety of ligands, charged species and non-octahedral complexes.
112:. In his Nobel Prize lecture, Hoffmann stressed that the isolobal analogy is a useful, yet simple, model and thus is bound to fail in certain instances.
646:
689:
462:
704:
94:
312:
In a similar sense, the addition or removal of electrons from two isolobal fragments results in two new isolobal fragments. Since Re(CO)
79:
796:
75:
952:
831:
826:
791:
74:
of a lesser-known species from that of a better-known species if the two molecular fragments have similar frontier orbitals, the
487:
In reference 10 of his Nobel Prize acceptance speech, Hoffmann states that the term "isolobal" was introduced in reference 1e, "
841:
836:
821:
724:
846:
639:
141:
897:
902:
454:
181:
radical complex. In order to satisfy the zero-charge criteria the metal center must be changed. For example, a MoL
714:
120:
To begin to generate an isolobal fragment, the molecule needs to follow certain criteria. Molecules based around
786:
750:
655:
632:
149:
105:
31:
931:
816:
185:
complex is d and neutral. However, removing a ligand to form the first frontier orbital would result in a
153:
71:
851:
412:
806:
669:
926:
745:
719:
674:
740:
133:
129:
121:
59:
47:
525:
921:
892:
882:
811:
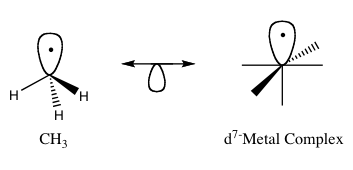
421:, where M has a d electron configuration to a square planar analogous fragment, the formula ML
887:
781:
684:
679:
502:
470:
431:
Further examples of the isolobal analogy in various shapes and forms are shown in figure 8.
87:
63:
43:
760:
755:
490:
269:
877:
776:
699:
450:
55:
946:
872:
109:
83:
51:
581:
70:
in them are similar – not identical, but similar." One can predict the bonding and
256:
Molecular orbital diagram depiction of frontier orbitals in methane and a basic ML
801:
529:
341:
Figure 7: Isolobal relationship between octahedral and square planar complexes.
399:
217:
93:
27:
Method of predicting the bonding properties of certain organometallic compounds
161:
125:
913:
709:
624:
474:
455:"Building Bridges Between Inorganic and Organic Chemistry (Nobel Lecture)"
201:
493:; Hoffmann, R. (1976). "Comparative bonding study of conical fragments".
67:
506:
17:
249:
157:
309:. Any 17-electron metal complex would be isolobal in this example.
526:"The Nobel Prize in Chemistry 1981: Kenichi Fukui, Roald Hoffmann"
398:
248:
216:
200:
92:
628:
237:
complex formed from an octahedral starting complex (Figure 4).
104:
For his work on the isolobal analogy, Hoffmann was awarded the
584:; Overton, T.; Rourke, J.; Weller, M.; Armstrong, F. (2006).
224:
Isolobal fragments of tetrahedral and octahedral geometries.
428:
where M has a d electron configuration should be followed.
857:
Arene complexes of univalent gallium, indium, and thallium
277:, the dsp hybrid orbitals are affected. Furthermore, the t
58:
described molecular fragments as isolobal "if the number,
213:
Relationship between tetrahedral and octahedral fragments
406:
Examples of non-basic shapes in the isolobal analogy.
911:
865:
769:
733:
662:
339:
614:Douglas, B.; McDaniel, D.; Alexander, J. (1994).
264:As seen above, when a fragment is formed from CH
62:properties, approximate energy and shape of the
42:) is a strategy used to relate the structure of
305:and therefore, and are also isolobal with CH
640:
8:
164:). Consequently, the metal center for the ML
82:(LUMO). Isolobal compounds are analogues to
741:Oxidative addition / reductive elimination
647:
633:
625:
616:Concepts and Models of Inorganic Chemistry
445:
443:
281:nonbonding metal orbitals are unaltered.
690:Polyhedral skeletal electron pair theory
86:compounds that share the same number of
54:properties of organometallic compounds.
50:molecular fragments in order to predict
439:
148:, can be created in a similar fashion.
552:Modern Approaches to Inorganic Bonding
599:Miessler, G. L.; Tarr, D. A. (2008).
100:Basic example of the isolobal analogy
7:
797:Transition metal fullerene complexes
80:lowest unoccupied molecular orbital
832:Transition metal carbyne complexes
827:Transition metal carbene complexes
792:Transition metal indenyl complexes
603:(3rd ed.). Pearson Education.
116:Construction of isolobal fragments
76:highest occupied molecular orbital
25:
842:Transition metal alkyne complexes
837:Transition metal alkene complexes
618:(3rd ed.). Wiley & Sons.
847:Transition-metal allyl complexes
156:, have no net charge, and their
128:when all bonding and nonbonding
822:Transition metal acyl complexes
160:should be two electron donors (
569:. Wiley-VCH. pp. 172–176.
108:in 1981, which he shared with
1:
152:should initially satisfy the
565:Gispert, Joan Ribas (2008).
38:(more formally known as the
898:Shell higher olefin process
705:Dewar–Chatt–Duncanson model
489:Elian, M.; Chen, M. M.-L.;
969:
787:Cyclopentadienyl complexes
751:β-hydride elimination
725:Metal–ligand multiple bond
150:Transition metal complexes
140:The isolobal fragments of
852:Transition metal carbides
550:Department of Chemistry.
285:Extensions of the analogy
953:Organometallic chemistry
656:Organometallic chemistry
336:Non-octahedral complexes
106:Nobel Prize in Chemistry
32:organometallic chemistry
817:Half sandwich compounds
413:square-planar complexes
293:Isoelectronic fragments
932:Bioinorganic chemistry
567:Coordination Chemistry
475:10.1002/anie.198207113
407:
261:
225:
209:
154:eighteen electron rule
101:
903:Ziegler–Natta process
807:Metal tetranorbornyls
554:. University of Hull.
463:Angew. Chem. Int. Ed.
402:
252:
233:is isolobal to a d ML
220:
204:
96:
912:Related branches of
670:Crystal field theory
320:, is isolobal with
241:MO theory dependence
142:octahedral complexes
927:Inorganic chemistry
746:Migratory insertion
720:Agostic interaction
675:Ligand field theory
601:Inorganic Chemistry
586:Inorganic Chemistry
507:10.1021/ic50159a034
342:
316:is isolobal with CH
301:is isolobal with CH
124:should satisfy the
122:main group elements
812:Sandwich compounds
770:Types of compounds
695:Isolobal principle
408:
340:
262:
226:
210:
134:molecular geometry
130:molecular orbitals
102:
66:and the number of
36:isolobal principle
940:
939:
922:Organic chemistry
893:Olefin metathesis
883:Grignard reaction
782:Grignard reagents
397:
396:
144:, such as type ML
88:valence electrons
64:frontier orbitals
16:(Redirected from
960:
888:Monsanto process
685:d electron count
680:18-electron rule
649:
642:
635:
626:
620:
619:
611:
605:
604:
596:
590:
589:
577:
571:
570:
562:
556:
555:
547:
541:
540:
538:
536:
522:
516:
513:concept is older
511:", but that the
510:
501:(5): 1148–1155.
491:Mingos, D. M. P.
485:
479:
478:
459:
447:
343:
331:
330:
329:
268:, one of the sp
196:
195:
194:
180:
179:
178:
40:isolobal analogy
21:
968:
967:
963:
962:
961:
959:
958:
957:
943:
942:
941:
936:
907:
861:
777:Gilman reagents
765:
761:Carbometalation
756:Transmetalation
729:
658:
653:
623:
613:
612:
608:
598:
597:
593:
580:Shriver, D.F.;
579:
578:
574:
564:
563:
559:
549:
548:
544:
534:
532:
524:
523:
519:
488:
486:
482:
469:(10): 711–724.
457:
449:
448:
441:
437:
427:
420:
393:
389:
383:
372:
364:
357:
353:
347:
338:
328:
325:
324:
323:
321:
319:
315:
308:
304:
300:
295:
287:
280:
276:
270:hybrid orbitals
267:
259:
243:
236:
232:
215:
193:
190:
189:
188:
186:
184:
177:
174:
173:
172:
170:
167:
147:
118:
78:(HOMO) and the
28:
23:
22:
15:
12:
11:
5:
966:
964:
956:
955:
945:
944:
938:
937:
935:
934:
929:
924:
918:
916:
909:
908:
906:
905:
900:
895:
890:
885:
880:
878:Cativa process
875:
869:
867:
863:
862:
860:
859:
854:
849:
844:
839:
834:
829:
824:
819:
814:
809:
804:
799:
794:
789:
784:
779:
773:
771:
767:
766:
764:
763:
758:
753:
748:
743:
737:
735:
731:
730:
728:
727:
722:
717:
712:
707:
702:
697:
692:
687:
682:
677:
672:
666:
664:
660:
659:
654:
652:
651:
644:
637:
629:
622:
621:
606:
591:
572:
557:
542:
530:nobelprize.org
517:
480:
438:
436:
433:
422:
416:
395:
394:
391:
387:
384:
381:
377:
376:
373:
370:
366:
365:
359:
354:
349:
337:
334:
326:
317:
313:
306:
302:
298:
294:
291:
286:
283:
278:
274:
265:
257:
242:
239:
234:
230:
214:
211:
191:
182:
175:
165:
145:
117:
114:
56:Roald Hoffmann
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
965:
954:
951:
950:
948:
933:
930:
928:
925:
923:
920:
919:
917:
915:
910:
904:
901:
899:
896:
894:
891:
889:
886:
884:
881:
879:
876:
874:
873:Carbonylation
871:
870:
868:
864:
858:
855:
853:
850:
848:
845:
843:
840:
838:
835:
833:
830:
828:
825:
823:
820:
818:
815:
813:
810:
808:
805:
803:
800:
798:
795:
793:
790:
788:
785:
783:
780:
778:
775:
774:
772:
768:
762:
759:
757:
754:
752:
749:
747:
744:
742:
739:
738:
736:
732:
726:
723:
721:
718:
716:
713:
711:
708:
706:
703:
701:
700:π backbonding
698:
696:
693:
691:
688:
686:
683:
681:
678:
676:
673:
671:
668:
667:
665:
661:
657:
650:
645:
643:
638:
636:
631:
630:
627:
617:
610:
607:
602:
595:
592:
587:
583:
576:
573:
568:
561:
558:
553:
546:
543:
531:
527:
521:
518:
514:
508:
504:
500:
496:
492:
484:
481:
476:
472:
468:
465:
464:
456:
452:
446:
444:
440:
434:
432:
429:
425:
419:
414:
405:
401:
385:
379:
378:
374:
368:
367:
362:
356:Square-planar
355:
352:
345:
344:
335:
333:
310:
292:
290:
284:
282:
271:
260:metal complex
255:
251:
247:
240:
238:
223:
219:
212:
207:
203:
199:
163:
159:
155:
151:
143:
138:
135:
131:
127:
123:
115:
113:
111:
110:Kenichi Fukui
107:
99:
95:
91:
89:
85:
84:isoelectronic
81:
77:
73:
69:
65:
61:
57:
53:
49:
45:
41:
37:
33:
19:
866:Applications
802:Metallocenes
694:
615:
609:
600:
594:
585:
575:
566:
560:
551:
545:
535:December 22,
533:. Retrieved
520:
512:
498:
494:
483:
466:
461:
451:Hoffmann, R.
430:
423:
417:
409:
403:
360:
350:
311:
296:
288:
263:
253:
244:
227:
221:
205:
139:
119:
103:
97:
39:
35:
29:
715:spin states
495:Inorg. Chem
162:Lewis bases
663:Principles
588:. Freeman.
582:Atkins, P.
435:References
346:Octahedral
126:octet rule
72:reactivity
914:chemistry
734:Reactions
710:Hapticity
404:Figure 8:
380:d: Os(CO)
369:d: Mo(CO)
254:Figure 5:
222:Figure 4:
206:Figure 3:
98:Figure 1:
68:electrons
48:inorganic
947:Category
453:(1982).
386:d: Ni(PR
60:symmetry
18:Isolobal
158:ligands
52:bonding
44:organic
34:, the
458:(PDF)
537:2010
375:d:
273:d-ML
46:and
503:doi
471:doi
187:MoL
30:In
949::
528:.
499:15
497:.
467:21
460:.
442:^
426:−2
363:−2
358:ML
348:ML
332:.
322:CH
279:2g
229:CH
171:ML
648:e
641:t
634:v
539:.
515:.
509:.
505::
477:.
473::
424:n
418:n
392:2
390:)
388:3
382:4
371:5
361:n
351:n
327:3
318:3
314:5
307:3
303:3
299:5
275:6
266:4
258:6
235:4
231:2
192:5
183:6
176:5
166:6
146:6
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.