426:
with a higher acceleration potential (several 1000 V) in order to minimize the energy distribution of the ion beam. Modern instruments operate at 6-10kV. The radius of deflection of an ion within a magnetic field depends on the kinetic energy and the mass/charge ratio of the ion (strictly, the magnet is a momentum analyzer not just a mass analyzer). Because of the large energy distribution, ions with similar mass/charge ratio can have very different kinetic energies and will thus experience different deflection for the same magnetic field. In practical terms one would see that ions with the same mass/charge ratio focus at different points in space. However, in a mass-spectrometer one wants ions with the same mass/charge ratio to focus at the same point, e.g. where the detector is located. In order to overcome these limitations, commercial MC-ICP-MS are double-focusing instruments. In a double-focusing mass-spectrometer ions are focused due to kinetic energy by the ESA (electro-static-analyzer) and kinetic energy + mass/charge (momentum) in the magnetic field. Magnet and ESA are carefully chosen to match the energy focusing properties of one another and are arranged so that the direction of energy focusing is in opposite directions. To simplify, two components have an energy focus term, when arranged properly, the energy term cancels out and ions with the same mass/charge ratio focus at the same point in space. It is important to note, double-focusing does not reduce the kinetic energy distribution and different kinetic energies are not filtered or homogenized. Double-focusing works for single as well as multi-collector instruments. In single collector instruments ESA and magnet can be arranged in either forward geometry (first ESA then magnet) or reversed geometry (magnet first then ESA), as only point-to-point focusing is required. In multi-collector instruments, only forward geometry (ESA then magnet) is possible due to the array of detectors and the requirements of a focal plane rather than a focal point.
255:) by means of a system of valves, so that a number of comparison measurements are made of both gases. In continuous flow IRMS, sample preparation occurs immediately before introduction to the IRMS, and the purified gas produced from the sample is measured just once. The standard gas may be measured before and after the sample or after a series of sample measurements. While continuous-flow IRMS instruments can achieve higher sample throughput and are more convenient to use than dual inlet instruments, the yielded data is of approximately 10-fold lower precision.
185:(heavy hydrogen) is almost double the mass of the common hydrogen isotope. Water molecules containing the common hydrogen isotope (and the common oxygen isotope, mass 16) have a mass of 18. Water incorporating a deuterium atom has a mass of 19, over 5% heavier. The energy to vaporise the heavy water molecule is higher than that to vaporize the normal water so isotope fractionation occurs during the process of evaporation. Thus a sample of sea water will exhibit a quite detectable isotopic-ratio difference when compared to Antarctic snowfall.
38:
442:
114:
353:
1418:
216:
1442:
1430:
504:(AMS) can be used. For example, the decay rate of the radioisotope C is widely used to date organic materials, but this approach was once limited to relatively large samples no more than a few thousand years old. AMS extended the range of C dating to about 60,000 years BP, and is about 10 times more sensitive than conventional IRMS.
379:
SIMS is a common method used in U-Pb analysis, as the primary ion beam is used to bombard the surface of a single zircon grain in order to yield a secondary beam of Pb ions. The Pb ions are analyzed using a double focusing mass spectrometer that comprises both an electrostatic and magnetic analyzer.
425:
The Ar-ICP produces an ion-beam with a large inherent kinetic energy distribution, which makes the design of the mass-spectrometer somewhat more complex than it is the case for conventional TIMS instruments. First, different from
Quadrupole ICP-MS systems, magnetic sector instruments have to operate
507:
AMS works by accelerating negative ions through a large (mega-volt) potential, followed by charge exchange and acceleration back to ground. During charge exchange, interfering species can be effectively removed. In addition, the high energy of the beam allows the use of energy-loss detectors, that
263:
A static gas mass spectrometer is one in which a gaseous sample for analysis is fed into the source of the instrument and then left in the source without further supply or pumping throughout the analysis. This method can be used for 'stable isotope' analysis of light gases (as above), but it is
145:
design that this type of instrument is often called the 'Nier type'. In the most general terms the instrument operates by ionizing the sample of interest, accelerating it over a potential in the kilo-volt range, and separating the resulting stream of ions according to their mass-to-charge ratio
528:
and water by combustion. The gas stream finally enters a capillary, is dried, ionized, and analyzed. This process allows a mixture of compounds to be purified and analyzed continuously, which can decrease the analysis time by a factor of four. Moving wire IRMS is quite sensitive, and samples
392:) can be used. A SHRIMP is a double-focusing mass spectrometer that allows for a large spatial separation between different ion masses based on its relatively large size. For U-Pb analysis, the SHRIMP allows for the separation of Pb from other interfering molecular ions, such as HfO
153:
Many radiogenic isotope measurements are made by ionization of a solid source, whereas stable isotope measurements of light elements (e.g. H, C, O) are usually made in an instrument with a gas source. In a "multicollector" instrument, the ion collector typically has an array of
817:
Williams, I.S. (1998), "U-Th-Pb geochronology by ion microprobe", In: McKibben, M.A.; Shanks III, W.C.; Ridley, W.I.; (Editors), "Applications of microanalytical techniques to understanding mineralizing processes", Reviews in
Economic Geology Special Publication 7:
319:
When these isotope ratios are measured by TIMS, mass-dependent fractionation occurs as species are emitted by the hot filament. Fractionation occurs due to the excitation of the sample and therefore must be corrected for accurate measurement of the isotope ratio.
323:
There are several advantages of the TIMS method. It has a simple design, is less expensive than other mass spectrometers, and produces stable ion emissions. It requires a stable power supply, and is suitable for species with a low ionization potential, such as
133:
type in this field of research for two reasons. First, it can be set up for multiple-collector analysis, and second, it gives high-quality 'peak shapes'. Both of these considerations are important for isotope-ratio analysis at very high precision and accuracy.
335:
The disadvantages of this method stem from the maximum temperature achieved in thermal ionization. The hot filament reaches a temperature of less than 2500°C, leading to the inability to create atomic ions of species with a high ionization potential, such as
421:
has normally been determined by TIMS. However, some systems (e.g. Hf-W and Lu-Hf) are difficult or impossible to analyse by TIMS, due to the high ionization potential of the elements involved. Therefore, these methods can now be analysed using MC-ICP-MS.
376:(SIMS). This type of ion-microprobe analysis normally works by focusing a primary (oxygen) ion beam on a sample in order to generate a series of secondary positive ions that can be focused and measured based on their mass/charge ratios.
227:
It is critical that the sample be processed before entering the mass spectrometer so that only a single chemical species enters at a given time. Generally, samples are combusted or pyrolyzed and the desired gas species (usually hydrogen
409:
analysis uses a quadrupole analyser, which only allows single-collector analysis. Due to the inherent instability of the plasma, this limits the precision of ICP-MS with a quadrupole analyzer to around 1%, which is insufficient for most
383:
A major issue that arises in SIMS analysis is the generation of isobaric interference between sputtered molecular ions and the ions of interest. This issue occurs with U–Pb dating as Pb ions have essentially the same mass as
358:
192:, an accurate determination of the isotopic make up of the sample is obtained. For example, carbon isotope ratios are measured relative to the international standard for C. The C standard is produced from a fossil
836:
Hinton, R.W. and Long, J.V.P. (1979). High-resolution ion microprobe measurement of lead isotopes: variations within single zircons from Lac Seul, Northwestern
Ontario. Earth Planet. Sci. lett. 45, 309-325.,
404:
An MC-ICP-MS instrument is a multiple collector mass spectrometer with a plasma source. MC-ICP-MS was developed to improve the precision achievable by ICP-MS during isotope-ratio measurements. Conventional
124:
The isotope-ratio mass spectrometer (IRMS) allows the precise measurement of mixtures of naturally occurring isotopes. Most instruments used for precise determination of isotope ratios are of the magnetic
524:. The solution (or outflow from the chromatography) is dried onto a nickel or stainless steel wire. After the residue is deposited on the wire, it enters a furnace where the sample is converted to CO
188:
Samples must be introduced to the mass spectrometer as pure gases, achieved through combustion, gas chromatographic feeds, or chemical trapping. By comparing the detected isotopic ratios to a measured
360:
359:
1162:
251:
The two most common types of IRMS instruments are continuous flow and dual inlet. In dual inlet IRMS, purified gas obtained from a sample is alternated rapidly with a standard gas (of
356:
357:
380:
This assembly allows the secondary ions to be focused based on their kinetic energy and mass-charge ratio in order to be accurately collected using a series of
Faraday cups.
344:(Hf-W). Though the TIMS method can create molecular ions instead in this case, species with high ionization potential can be analyzed more effectively with MC-ICP-MS.
1345:
1340:
1092:
252:
209:
205:
189:
620:
Stellaard F, Elzinga H (2005). "Analytical techniques in biomedical stable isotope applications: (isotope ratio) mass spectrometry or infrared spectrometry?".
1197:
1147:
1335:
1157:
1008:
849:
Caimi, R. J.; Brenna, J. T. (1996). "Direct analysis of carbon isotope variability in albumins by liquid flow-injection isotope ratio mass spectrometry".
1172:
1446:
1363:
1253:
1330:
1072:
1398:
508:
can distinguish between species with the same mass/charge ratio. Together, these processes allow the analysis of extreme isotope ratios above 10.
1388:
1152:
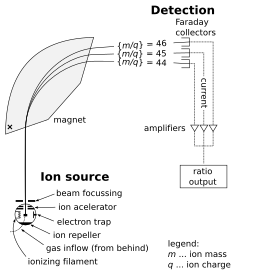
146:(m/z). Beams with lighter ions bend at a smaller radius than beams with heavier ions. The current of each ion beam is then measured using a '
1077:
968:
937:
1082:
297:
1315:
1112:
1102:
1054:
577:
Paul D, Skrzypek G, Fórizs I (2007). "Normalization of measured stable isotopic compositions to isotope reference scales - a review".
1393:
1355:
1177:
783:
1378:
1233:
1142:
1107:
1182:
1001:
373:
101:
isotope analysis involves measuring the abundances of decay-products of natural radioactivity, and is used in most long-lived
1383:
1368:
174:, causing measurable effects on the isotopic composition of samples, characteristic of their biological or physical history.
313:
301:
372:
An alternative approach used to measure the relative abundance of radiogenic isotopes when working with a solid surface is
1373:
1320:
501:
1294:
1403:
1097:
542:
1434:
1279:
659:
166:
Measurement of natural variations in the abundances of stable isotopes of the same element is normally referred to as
1473:
1422:
1049:
994:
762:
Brenna JT, Corso TN, Tobias HJ, Caimi RJ (1997). "High-precision continuous-flow isotope ratio mass spectrometry".
665:
885:
888:(2005). "Moving-wire device for carbon isotopic analyses of nanogram quantities of nonvolatile organic carbon".
1325:
1310:
1238:
1223:
1028:
130:
305:
1122:
277:
208:(Vienna Pee Dee Belemnite) and has C:C ratio of 0.0112372. Oxygen isotope ratios are measured relative the
1192:
698:
94:
37:
1468:
1127:
521:
171:
170:
analysis. This field is of interest because the differences in mass between different isotopes leads to
89:
This technique has two different applications in the earth and environmental sciences. The analysis of '
441:
309:
727:
Meier-Augenstein, W. (1999). "Applied gas chromatography coupled to isotope ratio mass spectrometry".
771:
712:
C. B. Bouthitt; K. Garnett. "The
Evolution of the Multicollector in Isotope Ratio Mass Spectromety".
586:
492:
273:
90:
1274:
1243:
1117:
1087:
1202:
418:
293:
289:
269:
102:
43:
361:
17:
1017:
974:
964:
943:
933:
905:
866:
787:
744:
637:
602:
557:
463:
142:
126:
113:
79:
63:
1187:
1167:
1132:
897:
858:
779:
736:
629:
594:
473:
197:
775:
590:
1137:
241:
220:
201:
167:
93:' is normally concerned with measuring isotopic variations arising from mass-dependent
740:
1462:
1284:
1044:
862:
827:
Dickin, A. P. (2005). Radiogenic
Isotope Geology 2nd ed. Cambridge University Press.
82:, in which mass spectrometric methods are used to measure the relative abundance of
1248:
388:. In order to overcome this problem, a sensitive high-resolution ion microprobe (
1289:
1228:
1207:
530:
477:
265:
155:
147:
138:
928:
Goetz, A.; Platzner, I. T. (Itzhak Thomas); Habfast, K.; Walder, A. J. (1997).
215:
1064:
633:
552:
411:
98:
978:
960:
947:
517:
325:
248:)) is purified by means of traps, filters, catalysts and/or chromatography.
193:
182:
909:
870:
748:
669:
641:
606:
791:
368:
instrument illustrating the ion beam path. After Figure 4, Williams, 1998.
42:
Magnetic sector mass spectrometer used in isotope ratio analysis, through
1258:
547:
341:
296:
of a solid sample loaded into the source of the mass spectrometer (hence
178:
446:
Accelerator mass spectrometer at
Lawrence Livermore National Laboratory
83:
901:
784:
10.1002/(SICI)1098-2787(1997)16:5<227::AID-MAS1>3.0.CO;2-J
598:
406:
389:
365:
337:
986:
351:
214:
112:
520:
ratios of compounds in a solution, such as after purification by
329:
158:, which allows the simultaneous detection of multiple isotopes.
117:
Schematic of an isotope-ratio mass spectrometer for measuring CO
990:
400:
Multiple collector inductively coupled plasma mass spectrometry
1033:
808:
2nd ed. (Cambridge: Cambridge
University Press), pp. 21-22.
200:, which is a limestone formed in the Cretaceous period in
27:
Usage of mass spectrometry to measure remaining isotopes
1354:
1303:
1267:
1216:
1063:
653:
651:
488:
483:
469:
459:
451:
59:
51:
533:of carbon can yield precise (within 1‰) results.
955:Yamasaki, Shin-ichi; Boutton, Thomas W. (1996).
500:For isotopes occurring at extremely low levels,
219:Isotope-ratio mass spectrometer used to measure
355:
129:type. This type of analyzer is superior to the
844:
842:
264:particularly used in the isotopic analysis of
1002:
212:, V-SMOW (Vienna Standard Mean Ocean Water).
8:
622:Isotopes in Environmental and Health Studies
434:
30:
288:Several of the isotope systems involved in
1009:
995:
987:
440:
36:
714:Proceedings of the 18th AMZSMS Conference
516:Moving wire IRMS is useful for analyzing
569:
137:The sector-type instrument designed by
97:in natural systems. On the other hand,
930:Modern isotope ratio mass spectrometry
433:
204:, U.S.A. The fossil is referred to as
29:
7:
1429:
692:Encyclopaedia of Analytical Science
298:thermal ionization mass spectrometry
284:Thermal ionization mass spectrometry
223:ratios, with gas bench in foreground
1441:
694:Encyclopaedia of Analytical Science
25:
1440:
1428:
1417:
1416:
374:secondary-ion mass spectrometry
348:Secondary-ion mass spectrometry
300:, TIMS). These methods include
72:Isotope-ratio mass spectrometry
31:Isotope-ratio mass spectrometry
18:Isotope ratio mass spectrometry
529:containing as little as 1 nano
1:
884:Sessions, A.L.; Sylva, S.P.;
741:10.1016/S0021-9673(98)01057-7
502:accelerator mass spectrometry
435:Accelerator mass spectrometry
430:Accelerator mass spectrometry
280:and helium isotope analysis.
863:10.1016/1044-0305(96)00010-4
543:Bainbridge mass spectrometer
259:Static gas mass spectrometry
162:Gas source mass spectrometry
1280:Microchannel plate detector
579:Rapid Commun. Mass Spectrom
417:Isotope-ratio analysis for
177:As a specific example, the
1490:
957:Mass spectrometry of soils
806:Radiogenic Isotope Geology
690:Townsend, A., ed. (1995).
666:Cambridge University Press
661:Radiogenic Isotope Geology
268:(rare or inert gases) for
253:known isotopic composition
150:' or multiplier detector.
1412:
1024:
851:J. Am. Soc. Mass Spectrom
764:Mass Spectrometry Reviews
634:10.1080/10256010500384333
439:
314:samarium–neodymium dating
302:rubidium–strontium dating
276:. Important examples are
78:) is a specialization of
35:
1295:Langmuir–Taylor detector
364:Schematic diagram of a
141:was such an advance in
1239:Quadrupole mass filter
699:Academic Press Limited
369:
224:
121:
95:isotopic fractionation
658:Dickin, A.P. (2005).
522:liquid chromatography
363:
292:depend on IRMS using
236:), carbon dioxide (CO
218:
172:isotope fractionation
116:
932:. London: J. Wiley.
890:Analytical Chemistry
804:Dickin, A.P., 2005.
493:Particle accelerator
274:isotope geochemistry
1275:Electron multiplier
1244:Quadrupole ion trap
776:1997MSRv...16..227B
591:2007RCMS...21.3006P
436:
306:uranium–lead dating
86:in a given sample.
32:
419:radiometric dating
370:
294:thermal ionization
290:radiometric dating
278:argon–argon dating
270:radiometric dating
225:
122:
103:radiometric dating
44:thermal ionization
1474:Mass spectrometry
1456:
1455:
1018:Mass spectrometry
970:978-0-8247-9699-0
939:978-0-471-97416-1
902:10.1021/ac051251z
896:(20): 6519–6527.
558:Table of nuclides
498:
497:
474:Organic molecules
464:Mass spectrometry
414:isotope systems.
143:mass spectrometer
80:mass spectrometry
69:
68:
64:mass spectrometry
16:(Redirected from
1481:
1444:
1443:
1432:
1431:
1420:
1419:
1011:
1004:
997:
988:
982:
951:
914:
913:
881:
875:
874:
846:
837:
834:
828:
825:
819:
815:
809:
802:
796:
795:
759:
753:
752:
735:(1–2): 351–371.
729:J. Chromatogr. A
724:
718:
717:
709:
703:
702:
687:
681:
680:
678:
677:
668:. Archived from
655:
646:
645:
617:
611:
610:
599:10.1002/rcm.3185
574:
512:Moving wire IRMS
484:Other techniques
444:
437:
354:
310:lead–lead dating
198:Peedee Formation
40:
33:
21:
1489:
1488:
1484:
1483:
1482:
1480:
1479:
1478:
1459:
1458:
1457:
1452:
1408:
1350:
1299:
1263:
1212:
1059:
1020:
1015:
985:
971:
954:
940:
927:
923:
918:
917:
883:
882:
878:
848:
847:
840:
835:
831:
826:
822:
816:
812:
803:
799:
761:
760:
756:
726:
725:
721:
711:
710:
706:
689:
688:
684:
675:
673:
657:
656:
649:
619:
618:
614:
585:(18): 3006–14.
576:
575:
571:
566:
539:
527:
514:
476:
447:
432:
402:
395:
387:
362:
352:
350:
286:
261:
247:
239:
235:
231:
164:
120:
111:
91:stable isotopes
47:
28:
23:
22:
15:
12:
11:
5:
1487:
1485:
1477:
1476:
1471:
1461:
1460:
1454:
1453:
1451:
1450:
1438:
1426:
1413:
1410:
1409:
1407:
1406:
1401:
1396:
1391:
1386:
1381:
1376:
1371:
1366:
1360:
1358:
1352:
1351:
1349:
1348:
1343:
1338:
1333:
1328:
1323:
1318:
1313:
1307:
1305:
1304:MS combination
1301:
1300:
1298:
1297:
1292:
1287:
1282:
1277:
1271:
1269:
1265:
1264:
1262:
1261:
1256:
1251:
1246:
1241:
1236:
1234:Time-of-flight
1231:
1226:
1220:
1218:
1214:
1213:
1211:
1210:
1205:
1200:
1195:
1190:
1185:
1180:
1175:
1170:
1165:
1160:
1155:
1150:
1145:
1140:
1135:
1130:
1125:
1120:
1115:
1110:
1105:
1100:
1095:
1090:
1085:
1080:
1075:
1069:
1067:
1061:
1060:
1058:
1057:
1052:
1047:
1042:
1031:
1025:
1022:
1021:
1016:
1014:
1013:
1006:
999:
991:
984:
983:
969:
952:
938:
924:
922:
919:
916:
915:
876:
857:(6): 605–610.
838:
829:
820:
810:
797:
754:
719:
704:
682:
647:
612:
568:
567:
565:
562:
561:
560:
555:
550:
545:
538:
535:
525:
513:
510:
496:
495:
490:
486:
485:
481:
480:
471:
467:
466:
461:
460:Classification
457:
456:
453:
449:
448:
445:
431:
428:
401:
398:
393:
385:
349:
346:
285:
282:
260:
257:
245:
242:sulfur dioxide
237:
233:
232:), nitrogen (N
229:
221:stable isotope
202:South Carolina
168:stable isotope
163:
160:
118:
110:
107:
67:
66:
61:
60:Classification
57:
56:
53:
49:
48:
41:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1486:
1475:
1472:
1470:
1467:
1466:
1464:
1449:
1448:
1439:
1437:
1436:
1427:
1425:
1424:
1415:
1414:
1411:
1405:
1402:
1400:
1397:
1395:
1392:
1390:
1387:
1385:
1382:
1380:
1377:
1375:
1372:
1370:
1367:
1365:
1362:
1361:
1359:
1357:
1356:Fragmentation
1353:
1347:
1344:
1342:
1339:
1337:
1334:
1332:
1329:
1327:
1324:
1322:
1319:
1317:
1314:
1312:
1309:
1308:
1306:
1302:
1296:
1293:
1291:
1288:
1286:
1285:Daly detector
1283:
1281:
1278:
1276:
1273:
1272:
1270:
1266:
1260:
1257:
1255:
1252:
1250:
1247:
1245:
1242:
1240:
1237:
1235:
1232:
1230:
1227:
1225:
1222:
1221:
1219:
1217:Mass analyzer
1215:
1209:
1206:
1204:
1201:
1199:
1196:
1194:
1191:
1189:
1186:
1184:
1181:
1179:
1176:
1174:
1171:
1169:
1166:
1164:
1161:
1159:
1156:
1154:
1151:
1149:
1146:
1144:
1141:
1139:
1136:
1134:
1131:
1129:
1126:
1124:
1121:
1119:
1116:
1114:
1111:
1109:
1106:
1104:
1101:
1099:
1096:
1094:
1091:
1089:
1086:
1084:
1081:
1079:
1076:
1074:
1071:
1070:
1068:
1066:
1062:
1056:
1053:
1051:
1048:
1046:
1045:Mass spectrum
1043:
1041:
1040:
1036:
1032:
1030:
1027:
1026:
1023:
1019:
1012:
1007:
1005:
1000:
998:
993:
992:
989:
980:
976:
972:
966:
962:
958:
953:
949:
945:
941:
935:
931:
926:
925:
920:
911:
907:
903:
899:
895:
891:
887:
880:
877:
872:
868:
864:
860:
856:
852:
845:
843:
839:
833:
830:
824:
821:
814:
811:
807:
801:
798:
793:
789:
785:
781:
777:
773:
770:(5): 227–58.
769:
765:
758:
755:
750:
746:
742:
738:
734:
730:
723:
720:
715:
708:
705:
700:
696:
693:
686:
683:
672:on 2014-03-27
671:
667:
663:
662:
654:
652:
648:
643:
639:
635:
631:
628:(4): 345–61.
627:
623:
616:
613:
608:
604:
600:
596:
592:
588:
584:
580:
573:
570:
563:
559:
556:
554:
551:
549:
546:
544:
541:
540:
536:
534:
532:
523:
519:
511:
509:
505:
503:
494:
491:
487:
482:
479:
475:
472:
468:
465:
462:
458:
454:
450:
443:
438:
429:
427:
423:
420:
415:
413:
408:
399:
397:
391:
381:
377:
375:
367:
347:
345:
343:
339:
333:
331:
327:
321:
317:
315:
311:
307:
303:
299:
295:
291:
283:
281:
279:
275:
271:
267:
258:
256:
254:
249:
243:
222:
217:
213:
211:
207:
203:
199:
196:found in the
195:
191:
186:
184:
180:
175:
173:
169:
161:
159:
157:
151:
149:
144:
140:
135:
132:
128:
115:
108:
106:
104:
100:
96:
92:
87:
85:
81:
77:
73:
65:
62:
58:
54:
50:
45:
39:
34:
19:
1469:Geochemistry
1445:
1433:
1421:
1249:Penning trap
1038:
1034:
959:. New York:
956:
929:
921:Bibliography
893:
889:
879:
854:
850:
832:
823:
813:
805:
800:
767:
763:
757:
732:
728:
722:
713:
707:
695:
691:
685:
674:. Retrieved
670:the original
660:
625:
621:
615:
582:
578:
572:
515:
506:
499:
478:Biomolecules
424:
416:
403:
382:
378:
371:
334:
322:
318:
287:
262:
250:
226:
187:
176:
165:
156:Faraday cups
152:
136:
123:
109:Introduction
88:
75:
71:
70:
1447:WikiProject
1290:Faraday cup
1229:Wien filter
1050:MS software
886:Hayes, J.M.
266:noble gases
148:Faraday cup
139:Alfred Nier
1463:Categories
1065:Ion source
697:. London:
676:2008-10-09
564:References
553:Isotopomer
412:radiogenic
340:(Os), and
328:(Sr), and
131:quadrupole
99:radiogenic
1326:Hybrid MS
961:M. Dekker
716:: THO–07.
518:carbon-13
326:strontium
194:belemnite
183:deuterium
105:methods.
1423:Category
1268:Detector
1259:Orbitrap
1055:Acronyms
979:34473560
948:36461690
910:16223235
871:24203433
749:10377971
642:16543190
607:17705258
548:Isoscape
537:See also
470:Analytes
342:tungsten
210:standard
190:standard
181:isotope
179:hydrogen
84:isotopes
1435:Commons
1163:MALDESI
792:9538528
772:Bibcode
587:Bibcode
489:Related
452:Acronym
52:Acronym
1341:IMS/MS
1254:FT-ICR
1224:Sector
977:
967:
946:
936:
908:
869:
790:
747:
640:
605:
407:ICP-MS
390:SHRIMP
366:SHRIMP
338:osmium
332:(Pb).
240:), or
127:sector
1394:IRMPD
1346:CE-MS
1336:LC/MS
1331:GC/MS
1311:MS/MS
1198:SELDI
1158:MALDI
1153:LAESI
1093:DAPPI
1399:NETD
1364:BIRD
1183:SIMS
1178:SESI
1113:EESI
1108:DIOS
1103:DESI
1098:DART
1083:APPI
1078:APLI
1073:APCI
1029:Mass
975:OCLC
965:ISBN
944:OCLC
934:ISBN
906:PMID
867:PMID
818:1–35
788:PMID
745:PMID
638:PMID
603:PMID
531:mole
330:lead
312:and
206:VPDB
76:IRMS
55:IRMS
1404:SID
1389:HCD
1384:ETD
1379:EDD
1374:ECD
1369:CID
1321:AMS
1316:QqQ
1193:SSI
1173:PTR
1168:MIP
1148:ICP
1128:FAB
1123:ESI
898:doi
859:doi
780:doi
737:doi
733:842
630:doi
595:doi
455:AMS
384:HfO
272:or
244:(SO
1465::
1208:TS
1203:TI
1188:SS
1143:IA
1138:GD
1133:FD
1118:EI
1088:CI
973:.
963:.
942:.
904:.
894:77
892:.
865:.
853:.
841:^
786:.
778:.
768:16
766:.
743:.
731:.
664:.
650:^
636:.
626:41
624:.
601:.
593:.
583:21
581:.
396:.
316:.
308:,
304:,
228:(H
1039:z
1037:/
1035:m
1010:e
1003:t
996:v
981:.
950:.
912:.
900::
873:.
861::
855:7
794:.
782::
774::
751:.
739::
701:.
679:.
644:.
632::
609:.
597::
589::
526:2
394:2
386:2
246:2
238:2
234:2
230:2
119:2
74:(
46:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.