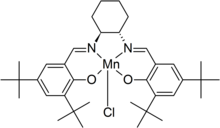589:
33:
625:
641:
138:
24:
362:
607:
stabilize a radical, making a radical intermediate more unlikely. In this case, a concerted mechanism in which the bond to the oxygen is simultaneously broken with metal-center while it is formed with the substrate is probable. However, more recent studies have indicated a radical intermediate is possible, challenging the assumption that non-conjugated alkenes undergo concerted mechanisms.
656:. The low temperature of the reaction favors only one pathway, the cis pathway, while m-CPBA is used because of water's high freezing point. Little success has occurred with the epoxidation of trans alkenes by manganese compounds but other salen coordination compounds, such as oxochromium complexes, can be used.
636:
bond from the side at a perpendicular orientation in relation to the catalyst in order to allow favorable orbital overlap. This mechanism, which was originally proposed by John Groves to explain porphyrin-catalyzed epoxidation reactions, is commonly referred to as a "side-on perpendicular approach".
533:
Enantiomerically pure epoxides are desirable as building blocks for complex molecules with specific chirality. Biologically active compounds can exhibit radically different activity based on differences in chirality and therefore the ability to obtain desired stereocenters in a molecule is of great
648:
The ease with which
Jacobsen's catalyst selectively epoxidizes cis-alkenes has been difficult to replicate with terminal and trans-alkenes. Structural changes to the ligand and adaptations to the protocol for the epoxidation reaction, however, have led to some successes in these areas. For example,
606:
alkenes (i.e. those in which there are multiple double bonds on alternating carbons) most effectively, the generally accepted mechanism is based on a radical intermediate which is stabilized due to the conjugated nature of the substrate. For non-conjugated alkenes, the substrate is far less able to
95:
637:
The approach is over the diamine bridge, where the steric bulk of the tert-butyl groups on the periphery of the ligand do not interfere with the alkene's approach (see below). However, as is the case with the overall mechanism, the pathway of alkene approach is also debated.
534:
importance to the pharmaceutical industry. Jacobsen's catalyst and other asymmetric catalysts are particularly useful in this field; for example, Jacobsen's catalyst was used to synthesize phenylisoserine, a side chain to the famous anti-cancer drug
230:
InChI=1S/C36H54N2O2.ClH.Mn/c1-33(2,3)25-17-23(31(39)27(19-25)35(7,8)9)21-37-29-15-13-14-16-30(29)38-22-24-18-26(34(4,5)6)20-28(32(24)40)36(10,11)12;;/h17-22,29-30,39-40H,13-16H2,1-12H3;1H;/q;;+3/p-3/b37-21+,38-22+;;/t29-,30-;;/m0../s1
370:
342:
579:(see intermediate formed in the reaction scheme below). Reaction with manganese(II) acetate in the presence of air gives the manganese(III) complex, which may be isolated as the chloro derivative after the addition of
220:
InChI=1S/C36H54N2O2.ClH.Mn/c1-33(2,3)25-17-23(31(39)27(19-25)35(7,8)9)21-37-29-15-13-14-16-30(29)38-22-24-18-26(34(4,5)6)20-28(32(24)40)36(10,11)12;;/h17-22,29-30,39-40H,13-16H2,1-12H3;1H;/q;;+3/p-3/t29-,30-;;/m1../s1
566:
Both enantiomers of
Jacobsen's catalyst are commercially available. Jacobsen's catalyst can be prepared by separating 1,2-diaminocyclohexane into its component enantiomers and then reacting the appropriate
649:
derivatives of
Jacobsen's catalyst with small structural changes to the salen backbone have been used in conjunction with low temperatures and the oxidant m-chloroperbenzoic acid (m-CPBA) to epoxidize the
640:
583:. Shown below is the preparation of the (R,R)-enantiomer. The synthesis has been adapted for undergraduate level chemistry courses in order to stress the importance of enantiomerically pure compounds.
526:
alkenes into epoxides. Before its development, catalysts for the asymmetric epoxidation of alkenes required the substrate to have a directing functional group, such as an alcohol as seen in the
436:
1042:
Daly, AM; Renehan, MF; Gilheany, DG (2001). "High
Enantioselectivities in an (E)-Alkene Epoxidation by Catalytically Active Chromium Salen Complexes. Insight into the Catalytic Cycle".
624:
550:, tetradentate, meaning it binds to the central manganese metal through four bonds, one to each oxygen and nitrogen atom of the salen backbone. Its chirality is conferred from the
632:
After the addition of the oxidant to the system, O=Mn(V) is generally accepted to be the active oxidant species formed (step A). The substrate is thought to approach the metal-
1147:
Getzler, YDYL; Mahadevan, V; Lobkovsky, EB; Coates GW (2002). "Synthesis of beta-lactones: A highly active and selective catalyst for epoxide carbonylation".
243:
761:; Zhang, Wei; Muci, Alexander R.; Ecker, James R.; Deng, Li (1991). "Highly enantioselective epoxidation catalysts derived from 1,2-diaminocyclohexane".
432:
763:
726:
572:
891:"(R,R)-N,N'-Bis(3,5-Di-tert-Butylsalicylidene)-1,2-Cyclohexanediamino Manganese(III) Chloride, A Highly Enantioselective Epoxidation Catalyst"
808:
621:(NaClO), a cheaper alternative, works as well. While other oxidants subsequently have been used, bleach continues to be the most common.
664:
The ligand structure of
Jacobsen's catalyst is easily modified for use over a wide range of reactions, such as epoxide-ring openings,
1188:
211:
915:
Hanson, J (2001). "Synthesis and Use of
Jacobsen's Catalyst: Enantioselective Epoxidation in the Introductory Organic Laboratory".
862:
Deng, L; Jacobsen, EN (1992). "A Practical, Highly
Enantioselective Synthesis of the Taxol Side Chain via Asymmetric Catalysis".
1198:
1015:
Palucki, M; Pospisil, PJ; Zhang, W; Jacobsen, EN (1994). "Highly
Enantioselective, Low-Temperature Epoxidation of Styrene".
1203:
800:
424:
483:
988:
Groves, JT; Nemo, TE (1983). "Epoxidation
Reactions Catalyzed by Iron Porphyrins - Oxygen Transfer from Iodsylbenzene".
406:
188:
498:
is the common name for N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride, a
460:
361:
32:
530:. This compound has two enantiomers, which give the appropriate epoxide product from the alkene starting material.
827:
Caputo, CA; Jones, ND (2005). "Developments in
Asymmetric Catalysis by Chiral Chelating Nitrogen-Donor Ligands".
724:(March 1990). "Enantioselective epoxidation of unfunctionalized olefins catalyzed by salen manganese complexes".
145:
794:
588:
452:
448:
758:
721:
668:, and conjugate additions. For example, an analogous catalyst with an aluminum center has been used for the
440:
133:
644:
One proposed substrate approach pathway - Note: Substrates are perpendicular to the plane of the catalyst.
665:
527:
499:
384:
354:
45:
953:
McGarrigle, EM; Gilheany, DG (2005). "Chromium- and Manganese-salen Promoted Epoxidation of Alkenes".
695:
685:
551:
515:
511:
61:
1208:
1193:
412:
420:
1164:
1129:
1094:
1059:
970:
895:
844:
804:
603:
115:
23:
1156:
1121:
1086:
1051:
1024:
997:
962:
924:
871:
836:
772:
735:
580:
519:
266:
71:
444:
197:
49:
N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride
650:
618:
428:
137:
611:
602:
In general, two mechanisms have been suggested. Because Jacobsen's catalyst epoxidizes
477:
1182:
669:
327:
177:
126:
468:
700:
633:
547:
507:
416:
890:
690:
576:
398:
251:
CC(C)(C)c1cc2/C=5/4CCCC4/6=C/c3cc(cc(c3O56(Cl)Oc2c(c1)C(C)(C)C)C(C)(C)C)C(C)(C)C
1077:
Jacobsen, EN (2000). "Asymmetric Catalysis of Epoxide Ring-Opening Reactions".
456:
555:
306:
106:
394:
1125:
628:
A simplified view of the catalytic cycle associated with Jacobsen's catalyst
523:
503:
1168:
1133:
1098:
1063:
974:
848:
610:
In the original catalytic reaction, iodosylarenes (PhIO) were used as the
673:
568:
1028:
1001:
875:
776:
739:
928:
653:
614:
164:
146:
1160:
1090:
1055:
966:
390:
840:
476:
Except where otherwise noted, data are given for materials in their
535:
94:
84:
558:
substituents, which amplify the asymmetry around the Mn center.
464:
1112:
Yoon, TP; Jacobsen, EN (2003). "Privileged Chiral Catalysts".
639:
623:
587:
332:
330 to 332 °C (626 to 630 °F; 603 to 605 K)
796:
The Organometallic Chemistry of the Transition Metals
176:
70:
538:, in a four-step synthesis as early as 1992.
8:
720:Zhang, W.; Loebach, J. L.; Wilson, S. R.;
136:
114:
15:
196:
948:
946:
944:
942:
940:
938:
764:Journal of the American Chemical Society
727:Journal of the American Chemical Society
712:
573:3,5-di-tert-butyl-2-hydroxybenzaldehyde
518:, which is renowned for its ability to
248:
216:
788:
786:
554:. The aryl groups are decorated with
127:
822:
820:
753:
751:
749:
223:Key: LJVAWOSDJSQANR-SEILFYAJSA-K
7:
672:of epoxides in order to obtain beta-
617:, but soon after it was found that
167:
546:Jacobsen's catalyst consists of a
14:
889:Larrow, JF; Jacobsen, EN (1998).
287:
360:
290:
284:
278:
31:
22:
480:(at 25 °C , 100 kPa).
542:Structure and basic properties
296:
272:
1:
793:Robert H. Crabtree (2005).
1225:
474:
341:
336:
259:
239:
207:
54:
44:
39:
30:
21:
1189:Manganese(III) compounds
552:diamine-derived backbone
407:Precautionary statements
17:Jacobsen's catalyst
1126:10.1126/science.1083622
645:
629:
592:
1199:Metal salen complexes
666:Diels-Alder reactions
643:
627:
591:
528:Sharpless epoxidation
500:coordination compound
1204:Tert-butyl compounds
696:Jacobsen epoxidation
686:Asymmetric catalysis
516:Jacobsen epoxidation
1120:(5613): 1691–1693.
1029:10.1021/ja00099a062
1002:10.1021/ja00356a015
876:10.1021/jo00041a054
829:Dalton Transactions
777:10.1021/ja00018a068
740:10.1021/ja00163a052
512:asymmetric catalyst
510:. It is used as an
496:Jacobsen's catalyst
314: g·mol
18:
929:10.1021/ed078p1266
799:. Wiley. pp.
646:
630:
598:Reaction mechanism
593:
520:enantioselectively
484:Infobox references
16:
1161:10.1021/ja017434u
1091:10.1021/ar960061v
1056:10.1021/ol0069406
1023:(20): 9333–9334.
996:(18): 5786–5791.
967:10.1021/cr0306945
896:Organic Syntheses
870:(15): 4320–4323.
835:(41): 1563–1602.
810:978-0-471-66256-3
759:Jacobsen, Eric N.
508:salen-type ligand
492:Chemical compound
490:
489:
385:Hazard statements
96:Interactive image
1216:
1173:
1172:
1155:(7): 1174–1175.
1149:J. Am. Chem. Soc
1144:
1138:
1137:
1109:
1103:
1102:
1074:
1068:
1067:
1039:
1033:
1032:
1017:J. Am. Chem. Soc
1012:
1006:
1005:
990:J. Am. Chem. Soc
985:
979:
978:
961:(5): 1563–1602.
950:
933:
932:
912:
906:
904:
886:
880:
879:
859:
853:
852:
841:10.1039/b709283k
824:
815:
814:
790:
781:
780:
755:
744:
743:
734:(7): 2801–2803.
717:
581:lithium chloride
470:
466:
462:
458:
454:
450:
446:
442:
438:
434:
430:
426:
422:
418:
414:
400:
396:
392:
364:
313:
298:
292:
289:
286:
280:
274:
267:Chemical formula
200:
180:
169:
148:
140:
129:
118:
98:
74:
35:
26:
19:
1224:
1223:
1219:
1218:
1217:
1215:
1214:
1213:
1179:
1178:
1177:
1176:
1146:
1145:
1141:
1111:
1110:
1106:
1076:
1075:
1071:
1041:
1040:
1036:
1014:
1013:
1009:
987:
986:
982:
952:
951:
936:
914:
913:
909:
888:
887:
883:
861:
860:
856:
826:
825:
818:
811:
792:
791:
784:
757:
756:
747:
722:Jacobsen, E. N.
719:
718:
714:
709:
682:
662:
651:terminal alkene
619:chlorine bleach
600:
564:
544:
493:
486:
481:
409:
387:
373:
357:
311:
301:
295:
283:
277:
269:
255:
252:
247:
246:
235:
232:
231:
225:
224:
221:
215:
214:
203:
183:
170:
158:
121:
101:
88:
77:
64:
50:
12:
11:
5:
1222:
1220:
1212:
1211:
1206:
1201:
1196:
1191:
1181:
1180:
1175:
1174:
1139:
1104:
1085:(6): 421–431.
1079:Acc. Chem. Res
1069:
1050:(5): 663–666.
1034:
1007:
980:
934:
907:
881:
854:
816:
809:
782:
745:
711:
710:
708:
705:
704:
703:
698:
693:
688:
681:
678:
661:
658:
612:stoichiometric
599:
596:
595:
594:
563:
560:
543:
540:
491:
488:
487:
482:
478:standard state
475:
472:
471:
437:P305+P351+P338
410:
405:
402:
401:
388:
383:
380:
379:
374:
369:
366:
365:
358:
353:
350:
349:
339:
338:
334:
333:
330:
324:
323:
320:
316:
315:
309:
303:
302:
299:
293:
281:
275:
270:
265:
262:
261:
257:
256:
254:
253:
250:
242:
241:
240:
237:
236:
234:
233:
229:
228:
226:
222:
219:
218:
210:
209:
208:
205:
204:
202:
201:
193:
191:
185:
184:
182:
181:
173:
171:
163:
160:
159:
157:
156:
152:
150:
142:
141:
131:
123:
122:
120:
119:
111:
109:
103:
102:
100:
99:
91:
89:
82:
79:
78:
76:
75:
67:
65:
60:
57:
56:
52:
51:
48:
42:
41:
37:
36:
28:
27:
13:
10:
9:
6:
4:
3:
2:
1221:
1210:
1207:
1205:
1202:
1200:
1197:
1195:
1192:
1190:
1187:
1186:
1184:
1170:
1166:
1162:
1158:
1154:
1150:
1143:
1140:
1135:
1131:
1127:
1123:
1119:
1115:
1108:
1105:
1100:
1096:
1092:
1088:
1084:
1080:
1073:
1070:
1065:
1061:
1057:
1053:
1049:
1045:
1038:
1035:
1030:
1026:
1022:
1018:
1011:
1008:
1003:
999:
995:
991:
984:
981:
976:
972:
968:
964:
960:
956:
949:
947:
945:
943:
941:
939:
935:
930:
926:
922:
918:
917:J. Chem. Educ
911:
908:
902:
898:
897:
892:
885:
882:
877:
873:
869:
865:
858:
855:
850:
846:
842:
838:
834:
830:
823:
821:
817:
812:
806:
802:
798:
797:
789:
787:
783:
778:
774:
770:
766:
765:
760:
754:
752:
750:
746:
741:
737:
733:
729:
728:
723:
716:
713:
706:
702:
699:
697:
694:
692:
689:
687:
684:
683:
679:
677:
675:
671:
670:carbonylation
667:
659:
657:
655:
652:
642:
638:
635:
626:
622:
620:
616:
613:
608:
605:
597:
590:
586:
585:
584:
582:
578:
574:
570:
561:
559:
557:
553:
549:
541:
539:
537:
531:
529:
525:
521:
517:
513:
509:
505:
501:
497:
485:
479:
473:
411:
408:
404:
403:
389:
386:
382:
381:
378:
375:
372:
368:
367:
363:
359:
356:
352:
351:
347:
345:
340:
335:
331:
329:
328:Melting point
326:
325:
321:
318:
317:
310:
308:
305:
304:
271:
268:
264:
263:
258:
249:
245:
238:
227:
217:
213:
206:
199:
195:
194:
192:
190:
187:
186:
179:
175:
174:
172:
166:
162:
161:
154:
153:
151:
149:
144:
143:
139:
135:
132:
130:
128:ECHA InfoCard
125:
124:
117:
113:
112:
110:
108:
105:
104:
97:
93:
92:
90:
86:
81:
80:
73:
69:
68:
66:
63:
59:
58:
53:
47:
43:
38:
34:
29:
25:
20:
1152:
1148:
1142:
1117:
1113:
1107:
1082:
1078:
1072:
1047:
1043:
1037:
1020:
1016:
1010:
993:
989:
983:
958:
954:
920:
916:
910:
900:
894:
884:
867:
864:J. Org. Chem
863:
857:
832:
828:
795:
771:(18): 7063.
768:
762:
731:
725:
715:
701:Salen ligand
663:
647:
631:
609:
601:
565:
548:salen ligand
545:
532:
495:
494:
376:
343:
322:brown solid
55:Identifiers
923:(9): 1266.
691:Enantiomers
577:Schiff base
562:Preparation
371:Signal word
319:Appearance
260:Properties
134:100.108.565
72:138124-32-0
1183:Categories
707:References
660:Variations
604:conjugated
575:to form a
556:tert-butyl
522:transform
355:Pictograms
307:Molar mass
198:WPP775Y8PO
107:ChemSpider
83:3D model (
62:CAS Number
46:IUPAC name
1209:Catalysts
1194:Catalysis
1044:Org. Lett
955:Chem. Rev
524:prochiral
504:manganese
461:P403+P233
453:P337+P313
449:P332+P313
433:P304+P340
429:P302+P352
346:labelling
155:604-063-0
147:EC Number
1169:11841278
1134:12637734
1099:10891060
1064:11259031
975:15884784
849:17940641
680:See also
674:lactones
569:tartrate
337:Hazards
178:73602790
116:21171274
1114:Science
654:styrene
615:oxidant
514:in the
377:Warning
165:PubChem
1167:
1132:
1097:
1062:
973:
847:
807:
803:–408.
506:and a
312:635.21
244:SMILES
40:Names
571:with
536:Taxol
212:InChI
85:JSmol
1165:PMID
1130:PMID
1095:PMID
1060:PMID
971:PMID
845:PMID
805:ISBN
469:P501
465:P405
457:P362
445:P321
441:P312
425:P280
421:P271
417:P264
413:P261
399:H335
395:H319
391:H315
189:UNII
1157:doi
1153:124
1122:doi
1118:299
1087:doi
1052:doi
1025:doi
1021:116
998:doi
994:105
963:doi
959:105
925:doi
903:: 1
872:doi
837:doi
801:405
773:doi
769:113
736:doi
732:112
634:oxo
502:of
344:GHS
168:CID
1185::
1163:.
1151:.
1128:.
1116:.
1093:.
1083:33
1081:.
1058:.
1046:.
1019:.
992:.
969:.
957:.
937:^
921:78
919:.
901:75
899:.
893:.
868:57
866:.
843:.
833:41
831:.
819:^
785:^
767:.
748:^
730:.
676:.
467:,
463:,
459:,
455:,
451:,
447:,
443:,
439:,
435:,
431:,
427:,
423:,
419:,
415:,
397:,
393:,
348::
288:Mn
285:Cl
282:52
276:36
1171:.
1159::
1136:.
1124::
1101:.
1089::
1066:.
1054::
1048:3
1031:.
1027::
1004:.
1000::
977:.
965::
931:.
927::
905:.
878:.
874::
851:.
839::
813:.
779:.
775::
742:.
738::
300:2
297:O
294:2
291:N
279:H
273:C
87:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.