513:
InChI=1S/C65H90N14O19S2.Lu/c1-38(80)56-64(96)73-51(63(95)75-57(39(2)81)65(97)98)37-100-99-36-50(72-59(91)47(28-40-10-4-3-5-11-40)68-52(83)32-76-20-22-77(33-53(84)85)24-26-79(35-55(88)89)27-25-78(23-21-76)34-54(86)87)62(94)70-48(29-41-15-17-43(82)18-16-41)60(92)71-49(30-42-31-67-45-13-7-6-12-44(42)45)61(93)69-46(58(90)74-56)14-8-9-19-66;/h3-7,10-13,15-18,31,38-39,46-51,56-57,67,80-82H,8-9,14,19-30,32-37,66H2,1-2H3,(H,68,83)(H,69,93)(H,70,94)(H,71,92)(H,72,91)(H,73,96)(H,74,90)(H,75,95)(H,84,85)(H,86,87)(H,88,89)(H,97,98);/q;+3/p-3/t38-,39-,46+,47-,48+,49-,50+,51+,56+,57+;/m1./s1/i;1+2
687:. All participants received Lu dotatate with octreotide. Participants and health care providers knew which treatment was given. The benefit of Lu dotatate was evaluated by measuring if and how much the tumor size changed during treatment (the overall response rate). Complete or partial tumor shrinkage was reported in 16 percent of a subset of 360 participants with GEP-NETs who were evaluated for response by the FDA. Participants initially enrolled in the study received Lu dotatate as part of an expanded access program.
385:
48:
471:
710:
SSTR-positive gastroenteropancreatic neuroendocrine tumors or pheochromocytoma/paraganglioma. Approval was also based on the extrapolation of efficacy outcomes observed in NETTER-1 (NCT01578239), a randomized, multicenter, open-label, active-controlled trial in 229 participants with locally advanced/inoperable or metastatic SSTR-positive midgut carcinoid tumors, which supported the original approval of lutetium Lu 177 dotatate in adults.
1802:
1403:
1346:
1012:
963:
705:
In April 2024, the FDA approved Lu dotatate for the treatment of children aged 12 years and older with somatostatin receptor-positive (SSTR)-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs), including foregut, midgut, and hindgut neuroendocrine tumors. It was approved for adults in
678:
Participants were randomly assigned to receive either Lu dotatate with long-acting octreotide or long-acting octreotide, at a higher dose, alone. Lu dotatate was injected through the vein and long-acting octreotide was injected in the muscle. Both, participants and health care providers knew which
713:
Safety was evaluated in nine pediatric participants in NETTER-P, including four participants with gastroenteropancreatic neuroendocrine tumors. The major outcome measures were absorbed radiation doses in target organs and incidence of adverse reactions after the first treatment cycle. Additional
675:(FDA) approved Lu dotatate based primarily on evidence from one clinical trial, NETTER-1 of 229 participants with somatostatin-receptor positive midgut GEP-NETs. Enrolled participants had tumors which could not be surgically removed and were worsening while receiving treatment with octreotide.
709:
Approval for children aged 12 years and older was based on pharmacokinetic, dosimetry, and safety data from NETTER-P (NCT04711135), an ongoing, international, multi-center, open-label, single-arm study of lutetium Lu 177 dotatate in adolescents with locally advanced/inoperable or metastatic
250:(Lu)lutetium(3+) 2--C-hydroxycarbonimidoyl}-6,9,12,15,18-pentahydroxy-7--13--16--1,2-dithia-5,8,11,14,17-pentaazacycloicosa-5,8,11,14,17-pentaen-19-yl]-C-hydroxycarbonimidoyl}-2-phenylethyl]-C-hydroxycarbonimidoyl}methyl)-7,10-bis(carboxymethyl)-1,4,7,10-tetraazacyclododecan-1-yl]acetate
656:
approved lutetium (Lu) oxodotreotide (brand name
Lutathera) "for the treatment of unresectable or metastatic, progressive, well differentiated (G1 and G2), somatostatin receptor positive gastroenteropancreatic neuroendocrine tumours (GEP-NETs) in adults" in September 2017.
615:
In the EU, lutetium (Lu) oxodotreotide is indicated for the treatment of unresectable or metastatic, progressive, well differentiated (G1 and G2), somatostatin receptor positive gastroenteropancreatic neuroendocrine tumours (GEP-NETs) in adults.
1388:
682:
The FDA considered additional data from a second study based on data from 1,214 participants with somatostatin receptor-positive tumors, including GEP-NETS, who received Lu dotatate at a single site in the
Netherlands,
1428:
1270:
1176:
679:
treatment was given. The benefit of Lu dotatate was evaluated by measuring the length of time that tumors did not grow after treatment and compared it to the control group (progression free survival).
997:
706:
2018. This is the first FDA approval of a radioactive drug, or radiopharmaceutical, for children aged twelve years of age and older with SSTR-positive gastroenteropancreatic neuroendocrine tumors.
714:
outcome measures included short-term adverse reactions following treatment with lutetium Lu 177 dotatate. The adverse reaction profile observed in NETTER-P was similar to that observed in adults.
1468:
948:
1380:
493:
CC(C1C(=O)NC(CSSCC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N1)CCCCN)CC2=CNC3=CC=CC=C32)CC4=CC=C(C=C4)O)NC(=O)C(CC5=CC=CC=C5)NC(=O)CN6CCN(CCN(CCN(CC6)CC(=O))CC(=O))CC(=O))C(=O)NC(C(C)O)C(=O)O)O.
612:
for the treatment of somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs), including foregut, midgut, and hindgut neuroendocrine tumors in adults.
741:
146:
1420:
1278:
808:
1168:
1331:
1461:
70:
485:
985:
1454:
936:
1242:
563:
1248:
846:
1209:
731:
699:
589:
emitted by Y, which deliver the therapeutic effect, may make it more suitable for large tumors with Lu reserved for smaller volumes
532:
505:
771:
800:
1083:"The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours"
242:
176:
88:
664:(GEP-NETs), including foregut, midgut and hindgut neuroendocrine tumors in adults, in January 2018. This was the first time a
1842:
1792:
1319:
1325:
1139:
991:
942:
672:
593:
324:
1145:
1827:
1505:
364:
107:
1822:
1203:
840:
597:
1031:"Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs)"
118:
1271:"European approval of lutetium oxodotreotide for gastroenteropancreatic neuroendocrine (GEP-NET) tumours"
1837:
571:
644:, starting before the radioactive administration and normally continuing for several hours afterwards.
1832:
1569:
661:
380:
1556:
665:
653:
641:
259:
47:
80:
632:
are particularly at risk as they help to remove Lu dotatate from the body. To protect them, an
1478:
1238:
1232:
1112:
1060:
901:
835:
212:
199:
60:
1198:
1081:
Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D, O'Dorisio MS, et al. (May 2013).
1102:
1094:
1050:
1042:
891:
881:
552:
401:
268:
333:
1806:
1614:
1492:
691:
637:
384:
1446:
764:"Lutathera 370 MBq/mL solution for infusion - Summary of Product Characteristics (SmPC)"
1722:
1668:
1602:
1107:
1082:
1055:
1030:
896:
869:
625:
1029:
Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, et al. (January 2012).
763:
1816:
1747:
1643:
1635:
1407:
1350:
1016:
967:
870:"Somatostatin receptor-based molecular imaging and therapy for neuroendocrine tumors"
736:
586:
293:
1780:
1767:
1762:
1742:
1712:
1678:
1663:
1648:
1620:
1593:
1516:
1512:
1500:
1482:
548:
159:
154:
138:
660:
Lu dotatate was approved in the United States for the treatment of SSTR positive
1757:
1717:
1688:
1625:
1581:
695:
125:
1046:
1772:
1752:
1737:
1727:
1673:
1658:
1653:
1610:
1576:
1564:
1541:
1536:
1527:
1098:
684:
633:
578:
443:
132:
27:
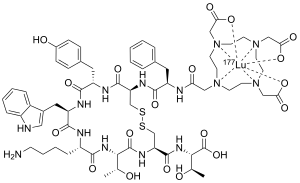
Chelate of Lu-177 with dotatate, a peptide derivative bound to a DOTA molecule
628:
it emits, however this can also be harmful to healthy tissue and organs. The
1130:
609:
74:
1116:
1064:
905:
886:
1732:
1693:
1231:
Volterrani D, Erba PA, Carrió I, Strauss HW, Mariani G (10 August 2019).
559:
555:
544:
304:
102:
17:
313:
1381:"FDA approves lutetium Lu 177 dotatate for pediatric patients 12 years"
582:
279:
668:
had been approved for the treatment of GEP-NETs in the United States.
629:
567:
1169:"Significance of Amino Acid Solution With Lutetium Lu 177 Dotatate"
868:
Wang L, Tang K, Zhang Q, Li H, Wen Z, Zhang H, et al. (2013).
353:
470:
461:
1421:"Novartis Gets FDA Approval for Lutathera in Pediatric Treatment"
1406:
This article incorporates text from this source, which is in the
1349:
This article incorporates text from this source, which is in the
1015:
This article incorporates text from this source, which is in the
986:"FDA approves lutetium Lu 177 dotatate for treatment of GEP-NETS"
966:
This article incorporates text from this source, which is in the
1553:
1234:
Nuclear
Medicine Textbook: Methodology and Clinical Applications
937:"FDA approves new treatment for certain digestive tract cancers"
344:
218:
193:
1450:
206:
97:
369:
698:
designations. The FDA granted the approval of
Lutathera to
1087:
European
Journal of Nuclear Medicine and Molecular Imaging
36:
185:
624:
The therapeutic effect of Lu derives from the ionizing
1790:
795:
793:
791:
789:
1634:
1601:
1592:
1552:
1526:
1490:
459:
442:
400:
395:
363:
343:
323:
303:
278:
258:
233:
175:
170:
145:
131:
117:
87:
69:
59:
54:
1076:
1074:
830:
828:
826:
690:The FDA granted the application for Lu dotatate
292:
1375:
1373:
1371:
1369:
1367:
1365:
1363:
1361:
1359:
1314:
1312:
1310:
1308:
1306:
1304:
1302:
1300:
1298:
1296:
801:"Lutathera- lutetium lu 177 dotatate injection"
732:"Summary Basis of Decision (SBD) for Lutathera"
566:. Specifically, it is used in the treatment of
267:
1462:
8:
662:gastroenteropancreatic neuroendocrine tumors
574:. It is a radiolabeled somatostatin analog.
106:
32:
226:In general: ℞ (Prescription only)
1598:
1469:
1455:
1447:
980:
978:
976:
383:
1106:
1054:
895:
885:
332:
931:
929:
927:
925:
923:
921:
919:
917:
915:
1797:
723:
510:
490:
379:
312:
247:
1148:from the original on 17 September 2020
31:
1781:Diagnostic radiopharmaceuticals (V09)
1334:from the original on 11 December 2019
1000:from the original on 11 December 2019
951:from the original on 11 December 2019
849:from the original on 11 December 2019
811:from the original on 16 November 2020
564:peptide receptor radionuclide therapy
79:
7:
1251:from the original on 10 January 2023
1179:from the original on 17 October 2021
596:(FDA) considers Lu dotatate to be a
577:Alternatives to Lu-dotatate include
158:
352:
283:
1431:from the original on 23 April 2024
1391:from the original on 25 April 2024
1320:"Drug Trials Snapshots: Lutathera"
996:(Press release). 26 January 2018.
947:(Press release). 26 January 2018.
25:
1385:U.S. Food and Drug Administration
1212:from the original on 23 July 2020
700:Advanced Accelerator Applications
1800:
1401:
1344:
1277:. 3 October 2017. Archived from
1010:
961:
774:from the original on 9 July 2021
744:from the original on 31 May 2022
421:
418:
412:
46:
1131:New Drug Therapy Approvals 2018
518:Key:MXDPZUIOZWKRAA-PRDSJKGBSA-K
1167:Thompson L (7 February 2019).
433:
427:
406:
1:
874:BioMed Research International
1326:Food and Drug Administration
1140:Food and Drug Administration
992:Food and Drug Administration
943:Food and Drug Administration
673:Food and Drug Administration
594:Food and Drug Administration
529:Lutetium (Lu) oxodotreotide
189:: Rx-only / Schedule C
41:lutetium (Lu) oxodotreotide
1859:
1047:10.1136/gutjnl-2011-300831
640:) is administered by slow
608:In the US, Lu dotatate is
585:. The longer range of the
396:Chemical and physical data
1706:
1237:. Springer. p. 782.
1204:European Medicines Agency
1099:10.1007/s00259-012-2330-6
841:European Medicines Agency
598:first-in-class medication
501:
481:
238:
45:
1144:(Report). January 2019.
1173:Oncology Nurse Advisor
572:somatostatin receptors
33:Lutetium (Lu) dotatate
845:. 17 September 2018.
1843:Radiopharmaceuticals
1570:Ibritumomab tiuxetan
1479:radiopharmaceuticals
1330:. 20 February 2018.
887:10.1155/2013/102819
740:. 23 October 2014.
666:radiopharmaceutical
654:European Commission
202:(Prescription only)
42:
1828:Lutetium complexes
1517:Strontium chloride
1788:
1787:
1702:
1701:
1427:. 23 April 2024.
1387:. 23 April 2024.
1244:978-3-319-95564-3
526:
525:
472:Interactive image
365:CompTox Dashboard
222:
210:
197:
188:
100:
16:(Redirected from
1850:
1823:Chelating agents
1805:
1804:
1803:
1796:
1599:
1471:
1464:
1457:
1448:
1441:
1440:
1438:
1436:
1417:
1411:
1405:
1404:
1400:
1398:
1396:
1377:
1354:
1348:
1347:
1343:
1341:
1339:
1316:
1291:
1290:
1288:
1286:
1267:
1261:
1260:
1258:
1256:
1228:
1222:
1221:
1219:
1217:
1195:
1189:
1188:
1186:
1184:
1164:
1158:
1157:
1155:
1153:
1135:
1127:
1121:
1120:
1110:
1078:
1069:
1068:
1058:
1026:
1020:
1014:
1013:
1009:
1007:
1005:
982:
971:
965:
964:
960:
958:
956:
933:
910:
909:
899:
889:
865:
859:
858:
856:
854:
836:"Lutathera EPAR"
832:
821:
820:
818:
816:
797:
784:
783:
781:
779:
760:
754:
753:
751:
749:
728:
545:chelated complex
474:
454:
452:
435:
429:
423:
420:
414:
408:
388:
387:
373:
371:
356:
336:
316:
296:
286:
285:
271:
220:
217:
208:
205:
195:
192:
187:
184:
162:
110:
99:
96:
83:
50:
43:
40:
38:
21:
1858:
1857:
1853:
1852:
1851:
1849:
1848:
1847:
1813:
1812:
1811:
1801:
1799:
1791:
1789:
1784:
1783:
1777:
1708:Isotopes used:
1698:
1630:
1615:Radium chloride
1588:
1548:
1522:
1486:
1475:
1445:
1444:
1434:
1432:
1419:
1418:
1414:
1402:
1394:
1392:
1379:
1378:
1357:
1345:
1337:
1335:
1318:
1317:
1294:
1284:
1282:
1281:on 3 April 2018
1269:
1268:
1264:
1254:
1252:
1245:
1230:
1229:
1225:
1215:
1213:
1208:. 24 May 2019.
1199:"LysaKare EPAR"
1197:
1196:
1192:
1182:
1180:
1166:
1165:
1161:
1151:
1149:
1133:
1129:
1128:
1124:
1080:
1079:
1072:
1028:
1027:
1023:
1011:
1003:
1001:
984:
983:
974:
962:
954:
952:
935:
934:
913:
867:
866:
862:
852:
850:
834:
833:
824:
814:
812:
799:
798:
787:
777:
775:
762:
761:
757:
747:
745:
730:
729:
725:
720:
692:priority review
650:
638:arginine/lysine
622:
620:Adverse effects
606:
522:
519:
514:
509:
508:
497:
494:
489:
488:
477:
450:
448:
438:
432:
426:
417:
411:
391:
367:
359:
339:
319:
299:
282:
274:
254:
251:
246:
245:
229:
166:
120:
113:
35:
34:
28:
23:
22:
15:
12:
11:
5:
1856:
1854:
1846:
1845:
1840:
1835:
1830:
1825:
1815:
1814:
1810:
1809:
1786:
1785:
1778:
1776:
1775:
1770:
1765:
1760:
1755:
1750:
1745:
1740:
1735:
1730:
1725:
1723:dysprosium-165
1720:
1715:
1709:
1707:
1704:
1703:
1700:
1699:
1697:
1696:
1691:
1686:
1676:
1671:
1666:
1661:
1656:
1651:
1646:
1640:
1638:
1632:
1631:
1629:
1628:
1623:
1618:
1607:
1605:
1603:alpha emitters
1596:
1590:
1589:
1587:
1586:
1585:
1584:
1574:
1573:
1572:
1561:
1559:
1550:
1549:
1547:
1546:
1545:
1544:
1533:
1531:
1524:
1523:
1521:
1520:
1510:
1509:
1508:
1497:
1495:
1488:
1487:
1476:
1474:
1473:
1466:
1459:
1451:
1443:
1442:
1425:MarketScreener
1412:
1355:
1292:
1262:
1243:
1223:
1190:
1159:
1122:
1070:
1021:
972:
911:
860:
822:
807:. 4 May 2020.
785:
755:
722:
721:
719:
716:
649:
646:
626:beta radiation
621:
618:
605:
602:
587:beta particles
570:which express
524:
523:
521:
520:
517:
515:
512:
504:
503:
502:
499:
498:
496:
495:
492:
484:
483:
482:
479:
478:
476:
475:
467:
465:
457:
456:
446:
440:
439:
436:
430:
424:
415:
409:
404:
398:
397:
393:
392:
390:
389:
381:DTXSID20195927
376:
374:
361:
360:
358:
357:
349:
347:
341:
340:
338:
337:
329:
327:
321:
320:
318:
317:
309:
307:
301:
300:
298:
297:
289:
287:
276:
275:
273:
272:
264:
262:
256:
255:
253:
252:
249:
241:
240:
239:
236:
235:
231:
230:
228:
227:
224:
215:
203:
190:
181:
179:
173:
172:
168:
167:
165:
164:
151:
149:
143:
142:
135:
129:
128:
123:
121:administration
115:
114:
112:
111:
93:
91:
85:
84:
77:
67:
66:
63:
57:
56:
52:
51:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1855:
1844:
1841:
1839:
1836:
1834:
1831:
1829:
1826:
1824:
1821:
1820:
1818:
1808:
1798:
1794:
1782:
1774:
1771:
1769:
1766:
1764:
1761:
1759:
1756:
1754:
1751:
1749:
1748:phosphorus-32
1746:
1744:
1741:
1739:
1736:
1734:
1731:
1729:
1726:
1724:
1721:
1719:
1716:
1714:
1711:
1710:
1705:
1695:
1692:
1690:
1687:
1684:
1683:Oxodotreotide
1680:
1677:
1675:
1672:
1670:
1667:
1665:
1662:
1660:
1657:
1655:
1652:
1650:
1647:
1645:
1642:
1641:
1639:
1637:
1636:beta emitters
1633:
1627:
1624:
1622:
1619:
1616:
1612:
1609:
1608:
1606:
1604:
1600:
1597:
1595:
1594:Radionuclides
1591:
1583:
1580:
1579:
1578:
1575:
1571:
1568:
1567:
1566:
1563:
1562:
1560:
1558:
1555:
1551:
1543:
1540:
1539:
1538:
1535:
1534:
1532:
1529:
1525:
1518:
1514:
1511:
1507:
1504:
1503:
1502:
1499:
1498:
1496:
1494:
1489:
1484:
1480:
1472:
1467:
1465:
1460:
1458:
1453:
1452:
1449:
1430:
1426:
1422:
1416:
1413:
1409:
1408:public domain
1390:
1386:
1382:
1376:
1374:
1372:
1370:
1368:
1366:
1364:
1362:
1360:
1356:
1352:
1351:public domain
1333:
1329:
1327:
1321:
1315:
1313:
1311:
1309:
1307:
1305:
1303:
1301:
1299:
1297:
1293:
1280:
1276:
1272:
1266:
1263:
1250:
1246:
1240:
1236:
1235:
1227:
1224:
1211:
1207:
1205:
1200:
1194:
1191:
1178:
1174:
1170:
1163:
1160:
1147:
1143:
1141:
1132:
1126:
1123:
1118:
1114:
1109:
1104:
1100:
1096:
1093:(5): 800–16.
1092:
1088:
1084:
1077:
1075:
1071:
1066:
1062:
1057:
1052:
1048:
1044:
1040:
1036:
1032:
1025:
1022:
1018:
1017:public domain
999:
995:
993:
987:
981:
979:
977:
973:
969:
968:public domain
950:
946:
944:
938:
932:
930:
928:
926:
924:
922:
920:
918:
916:
912:
907:
903:
898:
893:
888:
883:
879:
875:
871:
864:
861:
848:
844:
842:
837:
831:
829:
827:
823:
810:
806:
802:
796:
794:
792:
790:
786:
773:
769:
765:
759:
756:
743:
739:
738:
737:Health Canada
733:
727:
724:
717:
715:
711:
707:
703:
701:
697:
693:
688:
686:
680:
676:
674:
669:
667:
663:
658:
655:
647:
645:
643:
639:
635:
631:
627:
619:
617:
613:
611:
603:
601:
599:
595:
590:
588:
584:
580:
575:
573:
569:
565:
561:
557:
554:
550:
546:
542:
539:, brand name
538:
534:
530:
516:
511:
507:
500:
491:
487:
480:
473:
469:
468:
466:
463:
458:
447:
445:
441:
405:
403:
399:
394:
386:
382:
378:
377:
375:
366:
362:
355:
351:
350:
348:
346:
342:
335:
331:
330:
328:
326:
322:
315:
311:
310:
308:
306:
302:
295:
291:
290:
288:
281:
277:
270:
266:
265:
263:
261:
257:
248:
244:
237:
232:
225:
223: Rx-only
216:
214:
204:
201:
191:
183:
182:
180:
178:
174:
169:
161:
156:
153:
152:
150:
148:
144:
140:
137:Radiolabeled
136:
134:
130:
127:
124:
122:
116:
109:
104:
95:
94:
92:
90:
86:
82:
78:
76:
72:
68:
64:
62:
58:
55:Clinical data
53:
49:
44:
30:
19:
1838:Orphan drugs
1768:strontium-89
1763:samarium-153
1743:lutetium-177
1713:actinium-225
1682:
1477:Therapeutic
1433:. Retrieved
1424:
1415:
1393:. Retrieved
1384:
1336:. Retrieved
1323:
1283:. Retrieved
1279:the original
1274:
1265:
1253:. Retrieved
1233:
1226:
1214:. Retrieved
1202:
1193:
1181:. Retrieved
1172:
1162:
1152:16 September
1150:. Retrieved
1137:
1125:
1090:
1086:
1038:
1034:
1024:
1002:. Retrieved
989:
953:. Retrieved
940:
877:
873:
863:
851:. Retrieved
839:
813:. Retrieved
804:
776:. Retrieved
767:
758:
746:. Retrieved
735:
726:
712:
708:
704:
689:
681:
677:
670:
659:
651:
623:
614:
607:
604:Medical uses
591:
581:dotatate or
576:
549:radioisotope
540:
536:
528:
527:
177:Legal status
171:Legal status
139:somatostatin
89:License data
29:
1833:Macrocycles
1758:rhenium-186
1718:bismuth-213
1582:Tositumomab
1338:11 December
1275:ecancer.org
1041:(1): 6–32.
1004:11 December
955:11 December
853:11 December
696:orphan drug
537:Lu dotatate
455: g·mol
269:437608-50-9
234:Identifiers
126:Intravenous
61:Trade names
1817:Categories
1779:See also:
1773:yttrium-90
1753:radium-223
1738:iodine-131
1728:erbium-169
1557:antibodies
1542:Iobenguane
1528:Adrenergic
1506:Lexidronam
1493:palliation
1255:7 November
1183:2 November
880:: 102819.
815:8 November
718:References
685:Erasmus MC
636:solution (
634:amino acid
579:yttrium-90
562:, used in
460:3D model (
444:Molar mass
334:AE221IM3BB
260:CAS Number
243:IUPAC name
133:Drug class
610:indicated
541:Lutathera
119:Routes of
108:Lutathera
81:Monograph
75:Drugs.com
65:Lutathera
18:Lutathera
1807:Medicine
1733:gold-198
1435:23 April
1429:Archived
1395:25 April
1389:Archived
1332:Archived
1249:Archived
1210:Archived
1177:Archived
1146:Archived
1117:23389427
1065:22052063
998:Archived
949:Archived
906:24106690
847:Archived
809:Archived
805:DailyMed
772:Archived
742:Archived
642:infusion
560:dotatate
556:lutetium
305:DrugBank
294:71587735
147:ATC code
103:DailyMed
1285:2 April
1216:22 July
1108:3622744
1056:3280861
897:3784148
671:The US
648:History
630:kidneys
592:The US
583:DOTATOC
568:cancers
553:element
551:of the
543:, is a
402:Formula
314:DB13985
280:PubChem
163:)
157: (
155:V10XX04
105::
1793:Portal
1530:tumors
1241:
1115:
1105:
1063:
1053:
904:
894:
778:9 July
748:29 May
486:SMILES
354:D11033
213:℞-only
211:
198:
141:analog
101:
1491:Pain
1328:(FDA)
1324:U.S.
1206:(EMA)
1142:(FDA)
1138:U.S.
1134:(PDF)
994:(FDA)
990:U.S.
945:(FDA)
941:U.S.
843:(EMA)
768:(emc)
558:with
547:of a
535:) or
506:InChI
462:JSmol
1554:CD20
1437:2024
1397:2024
1340:2019
1287:2018
1257:2020
1239:ISBN
1218:2020
1185:2020
1154:2020
1113:PMID
1061:PMID
1006:2019
957:2019
902:PMID
878:2013
855:2019
817:2020
780:2021
750:2022
694:and
652:The
345:KEGG
325:UNII
71:AHFS
1483:V10
1103:PMC
1095:doi
1051:PMC
1043:doi
1035:Gut
892:PMC
882:doi
533:INN
453:.58
451:607
370:EPA
284:CID
200:POM
160:WHO
37:INN
1819::
1694:Au
1689:Re
1679:Lu
1674:Er
1669:Dy
1664:Sm
1649:Sr
1626:Bi
1621:Ac
1611:Ra
1513:Sr
1501:Sm
1423:.
1383:.
1358:^
1322:.
1295:^
1273:.
1247:.
1201:.
1175:.
1171:.
1136:.
1111:.
1101:.
1091:40
1089:.
1085:.
1073:^
1059:.
1049:.
1039:61
1037:.
1033:.
988:.
975:^
939:.
914:^
900:.
890:.
876:.
872:.
838:.
825:^
803:.
788:^
770:.
766:.
734:.
702:.
600:.
431:19
425:14
419:Lu
416:87
410:65
219:EU
207:US
194:UK
186:CA
98:US
1795::
1685:)
1681:(
1659:I
1654:Y
1644:P
1617:)
1613:(
1577:I
1565:Y
1537:I
1519:)
1515:(
1485:)
1481:(
1470:e
1463:t
1456:v
1439:.
1410:.
1399:.
1353:.
1342:.
1289:.
1259:.
1220:.
1187:.
1156:.
1119:.
1097::
1067:.
1045::
1019:.
1008:.
970:.
959:.
908:.
884::
857:.
819:.
782:.
752:.
531:(
464:)
449:1
437:2
434:S
428:O
422:N
413:H
407:C
372:)
368:(
221::
209::
196::
73:/
39::
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.