1014:
cellular proteins. Lomustin also affects a variety of cellular events including ribosomal and nucleoplastic messenger RNA processing, DNA base structure, and DNA polymerase activity. However, the clinical application of lomustine is restricted due to dose-related toxicities such as hematologic and pulmonary toxicity. Lomustine is also a lipid soluble, which allows it to permeate the blood brain barrier well. This quality made it a reasonable candidate for the chemotherapy of intrinsic brain tumors. Lomustine is administered orally in six-to-eight-week intervals and with nadirs at five weeks after administration due to its delayed myelosuppressive properties.
1087:
are in stage two, and four are in stage one. The conditions that the active trials are researching include
Anaplastic Astrocytoma, Untreated Childhood Medulloblastoma, High Grade Glioma: Glioblastoma, Brain Neoplasm, Central Nervous System Neoplasm, High Grade Glioma: Gliosarcoma, Medulloblastomas, Oligodendroglioma, Acute T Cell Leukemia Lymphoma, Bone Cancer, Breast Neoplasms, Colorectal Neoplasms, Lung Neoplasms, Malignancies Multiple, Metastatic Cancer, Pancreatic Cancer, Refractory Cancer, Renal Cancer, Resistant Cancer, Diffuse Intrinsic Pontine Gliomas, Ependymoma, Recurrent Brain Tumors, and MGMT Methylated Glioblastoma.
1010:
intermediates in vivo. These products are electrophilic, and they attack nucleophilic sites on DNA and RNA to form alkylated products. Unlike other anticancer agents like mitomycin C, streptonigrin, bleomycin, and the anthracyclines, lomustine does not require bioactivation to react with cellular targets. Lomustine is a chloroethlyating compound and it causes alkylation and cross-linking of RNA and DNA at the O6 position of guanine-containing bases. If the DNA and RNA is not repaired, this cross-linking causes breakage during replication and eventually causes cell death via apoptosis.
536:
513:
38:
1057:
absorb the price increases. The average age of people diagnosed with glioblastoma is 65, which included many
Medicare patients who have Part D coverage. Under Part D coverage, patients are responsible for up to 25% of the price of a brand-name drug. Because lomustine is used as a control arm for many clinical trials, the denied insurance coverage and skyrocketing cost caused some trials to face difficulty enrolling and maintaining patients.
992:
29:
1028:
recurrent high-grade gliomas. It is also approved for treatment of
Hodgkin’s lymphoma in combination with other chemotherapies, following disease progression with initial chemotherapy. It is the standard of care for recurrent glioblastoma multiforme in Europe, and it is frequently used as a control arm in recurrent glioblastoma multiforme trials.
1042:
Biotechnology took over as the sole distributor of lomustine in the United States. NextSource recognized the medical importance of lomustine, so they acquired the manufacturing rights from
Bristol-Myers-Squibb in partnership with Corden Pharma. They relaunched the product under the name of Gleostine the next year.
1056:
benefit made the decision to leave the program. In July 2021, the list price for the highest dose of lomustine was 1900% higher than it was in 2013, when it was being manufactured and sold by
Bristol-Myers Squibb. Since there were no approved generic alternatives to lomustine, patients were forced to
906:
Fatal toxicity may occur with overdosage of
Lomustine. Overdoses may lead to heightened myelosupression, abdominal pain, diarrhea, vomiting, anorexia, extreme fatigue, dizziness, liver failure, and shortness of breath. No antidotes for this drug currently exist. Non-fatal overdose management includes
1086:
Lomustine has been part of eighty-eight clinical trials. Twenty five trials are currently active, thirty eight have been completed, eleven have been terminated, four have been withdrawn, and the status of ten trials is unknown. Of the currently active trials, nine of them are in stage three, twelve
1027:
Lomustine was approved by the FDA to treat high-grade gliomas in 1976. Lomustine, alone or in combination with other chemotherapeutic drugs, was the standard of care following surgery and/or radiation up until the early twenty-first century. In the United States, lomustine is currently approved for
852:
Certain side effects from lumostine require immediate medical attention. These commonly include, but are not limited to, bleeding gums, chest pain, shortness of breath, sores or white spots in the mouth, fever, edema of the legs, abnormal bleeding or bruising, tar-like stools, blood in the urine or
1064:
to manufacture a generic form of lomustine. The agreement allowed the drug to be manufactured in the United States, which made it more accessible to patients and lowered the cost of brain cancer treatment by approximately 90%. The manufacturing agreement also resulted in lomustine once again being
1013:
Lomustine also has other biologic effects including inhibition of DNA synthesis and some cell phase specificity. In general, nitrosureas lack cross-resistance with other alkylating agents. Since lomustine is a nitrosurea, it inhibits several processes such as carbomylation and the modification of
931:
Lomustine is manufactured by using continuous flow manufacturing. It requires two flow reactors, with an intermediate purification to change reaction solvents. The first step in the synthesis of
Lomustine is a fast reaction at room temperature. The reaction is the carbamylation of cyclohexylamine
897:
Lomustine causes myelosupression in a delayed, dose-dependent, and cumulative fashion. This delayed decrease in bone marrow activity generally occurs in the 4-6 week window after the drug's administration. Patients are expected to show thrombocytopenia and leukopenia during this period. Patients'
823:
Lomustine is used as an "off-label" veterinary treatment for cancers in cats and dogs. Clinical trials have demonstrated the drug's success in treating progressive lymphomas, mast cell tumors, and brain cancers. The chemotherapy has also been used to treat sarcomas and spinal cord tumors in these
775:
Lomustine is an alkylating chemotherapy drug that is indicated by the FDA for the treatment of patients with brain tumors (primary and metastatic), following any necessary surgery and radiation, as well as for treatment of progressive
Hodgkin’s lymphoma. Lomustine is approved for the treatment of
880:
Lomustine can cause or worsen pulmonary infiltrates and fibrosis in patients. Pulmonary toxicity generally occurs after at least 6 months of treatment with lumostine. Lung function should be monitored via Forced Vital
Capacity (FVC) or Carbon Monoxide Diffusing Capacity (DLCO) tests to determine
836:
Lomustine is available in 5 mg (yellow capsule), 10 mg (white capsule), 40 mg (white & green capsule), and 100 mg (green capsule) gel capsules, referred to as
Gleostine. Lomustine dosing is calculated based on body surface area. One dose of the drug is administered orally
814:
While there are no clinical studies on lomustine use in the 65+ age group, clinicians are recommended to exercise caution in prescribing this drug to geriatric patients. Lomustine causes high levels of organ toxicity, which must be taken into account when determining dosing for elderly patients.
1041:
Lomustine was manufactured in limited supply by Bristol-Myers Squibb prior to 2013. This resulted in a short supply of a key chemotherapeutic drug used in the treatment of brain cancer and Hodgkin’s lymphoma. In 2013, Bristol-Myers Squibb discontinued production of lomustine. NextSource
1009:
Cell-cycle specific chemotherapy drugs only affect cells when they are dividing, whereas cell-cycle non-specific drugs affect cells when they are at rest. Lomustine is a cell cycle non-specific, highly lipophilic alkylating agent which produces chloroethyl carbenium ions and carbamylating
827:
Lomustine may be administered orally or by injection in cats and dogs. This chemotherapy has been observed to have a variety of side effects in animals, paralleling those in humans, including but not limited to bone marrow immunosuppression, gastrointestinal issues, and hepatotoxicity.
840:
Lomustine is highly toxic; as such, only one dose is dispensed at a time in order to lower overdose risk. Due to the cytotoxic nature of lomustine, the drug must be dosed, administered, and disposed of with special precautions including wearing gloves to prevent dermal exposure.
837:
every 6 weeks, generally at a dosage of 130 mg/m for all patients. The dose may be lowered based on the patients blood counts and immune strength, but is still administered every 6 weeks. Lomustine must be taken on an empty stomach of at least two hours.
797:
No research currently exists on the effects of lomustine and its metabolites on breastfed infants. However, patients on Lomustine are advised not to breastfeed during the course of treatment due to the potential for serious adverse reactions to the drug.
784:
While current data is only based on animal studies, there is reason to believe that lomustine use during pregnancy can cause harm to a fetus, potentially leading to miscarriages or birth defects. Patients are advised not to take lomustine while pregnant.
922:
Lomustine is contraindicated in the administration of most live vaccines during treatment, due to infection risk. Receiving these vaccines during the course of lomustine is highly discouraged due to the immunosuppression caused by this chemotherapy.
873:
Hepatotoxicity occurs due to increased levels of liver transaminases, alkaline phosphatase, and bilirubin with lumostine use. Liver enzymes and function should be monitored during use and dose should be adjusted based on toxicity levels.
856:
Other side effects that may occur as the body adjusts to this drug do not require medical attention. These include, but are not limited to, hair loss, inflammation of the mouth, trouble with speaking, blurred vision, and shakiness.
1762:
Temerk Y, Ibrahim M, Ibrahim H, Kotb M (2016-05-15). "Interactions of an anticancer drug lomustine with single and double stranded DNA at physiological conditions analyzed by electrochemical and spectroscopic methods".
788:
Patients hoping to conceive should be aware that lumostine may have the capacity to reduce fertility. Patients are advised to use birth control with partners while taking lomustine due to the potential for fetal harm.
758:, has the ability to created interstrand cross-links (ICLs) in DNA. As with other nitrosoureas, it may also inhibit several key enzymatic processes by carbamoylation of amino acids in proteins. Lomustine is
898:
complete blood counts should be consistently monitored during treatment in order to mitigate the myelosupressive risks of the drug and prevent fatal complications such as infections and internal bleeding.
918:
There are 407 FDA-approved drugs which may interact with lomustine. Many of these interactions are due to severe side-effects of this chemotherapy, which are incompatible other drugs' known side effects.
776:
brain tumors, breast cancer, lung cancer, Hodgkin’s lymphoma, and melanoma by Health Canada. Lomustine is also used as an anti-cancer drug in several European countries, including the United Kingdom.
806:
There is insufficient clinical data surrounding the use of lomustine in pediatric populations. Current protocols use the same dosing and treatment protocols for pediatric patents as adult patients.
877:
Lomustine causes progressive kidney shrinkage and failure with long-term use. Renal enzymes and function should be monitored during use and dose should be adjusted based on toxicity levels.
118:
988:-butanol and a 1-(2-chloroethyl)-3-cyclohexylurea radical, which reacts with the nitrosyl in a termination step to form lomustine (1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea).
676:
2051:
70:
2751:
1200:
759:
632:
889:
Long term use of lomustine is linked to secondary malignancies including acute leukemia and myelodysplasia, due to the drug's DNA alkylating properties.
3756:
1065:
included in the Medicare federal drug discount program. Continuity Pharma was able to develop the generic form of lomustine due to the technology of
980:-butoxide radical then reacts with the nitrogen-hydrogen bond on the chlorine side of the carbonyl group of 1-(2-chloroethyl)-3-cyclohexylurea
3712:
2044:
1073:. Pending FDA approval, the foundation will oversee the manufacturing and distribution of the drug to patients across the United States.
3334:
3761:
2486:
2442:
2320:
2245:
1267:
881:
patients risk of developing pulmonary toxicities. Patients who develop pulmonary fibrosis should discontinue treatment immediately.
697:
652:
1558:
2037:
2967:
2761:
713:
279:
148:
3791:
3380:
2382:
2272:
1505:
944:
intermediate (Diab et al.). This reaction involves the interaction between the lone pair on the nitrogen of cyclohexylamine
1176:
3786:
3302:
3285:
3160:
2924:
2302:
1982:
3034:
2630:
1052:
at the beginning of 2021. This was the first time in history that a company with a drug eligible for coverage under the
412:
608:
3729:
3236:
2029:
492:
1367:
3781:
2416:
1066:
743:
as a second-line option. It has also been used in veterinary practice as a treatment for cancers in cats and dogs.
3592:
3482:
3472:
870:
Lomustine use is linked to a variety of organ toxicities hepatotoxicity, nephrotoxicity, and pulmonary toxicity.
1045:
849:
Lumostine causes a variety of side effects including gastrointestinal, ocular, neurologic, and other disorders.
3776:
3639:
3175:
2425:
2014:
1384:
1049:
1346:
732:
361:
1695:
972:
in aqueous solution. The reaction involves the radical homolytic bond breaking of tert-butyl nitrates into a
3717:
3624:
3457:
3172:
2658:
2506:
2433:
1070:
102:
531:
3537:
3527:
3260:
3102:
218:
1932:
1798:
Weller M, Le Rhun E (July 2020). "How did lomustine become standard of care in recurrent glioblastoma?".
1105:"FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)"
3771:
3766:
2809:
2626:
2596:
2456:
1410:"Lomustine (CCNU) and prednisone chemotherapy for high-grade completely excised canine mast cell tumors"
1133:
Lee FY, Workman P, Roberts JT, Bleehen NM (1985). "Clinical pharmacokinetics of oral CCNU (lomustine)".
716:
352:
1061:
1529:
481:
3207:
3084:
2605:
2521:
1104:
258:
3492:
3392:
3371:
3092:
2914:
2746:
2477:
2373:
952:
This results in a tetrahedral intermediate, in which a proton from the nitrogen of cyclohexylamine
508:
307:
1283:
37:
2550:
1833:
1708:
1158:
110:
3684:
2580:
2560:
2545:
1957:
3659:
3248:
2082:
2020:
1887:
1825:
1780:
1700:
1613:
1481:
1431:
1263:
1150:
1069:. Continuity Pharma provides the active pharmaceutical ingredients (API) for lomustine to the
174:
162:
50:
1732:
461:
3664:
3436:
3151:
2785:
2770:
2491:
1877:
1867:
1815:
1807:
1772:
1744:
1690:
1680:
1603:
1595:
1421:
1142:
960:. The second step in the synthesis is the nitrosation of 1-(2-chloroethyl)-3-cyclohexylurea
740:
548:
200:
1958:"Rogue drug maker first inflates the price of lomustine, then says No to Medicare coverage"
1452:
907:
hospitalization and antibiotic treatment to address myelosuppressive effects of lomustine.
421:
316:
3118:
3080:
2871:
2293:
2208:
1530:"Gleostine, CCNU (lomustine) dosing, indications, interactions, adverse effects, and more"
1053:
401:
228:
208:
1669:"Flow synthesis kinetics for lomustine, an anti-cancer active pharmaceutical ingredient"
535:
512:
3723:
3272:
2863:
2357:
2221:
2108:
2078:
2065:
1882:
1855:
1608:
1583:
1426:
1409:
940:
in the presence of triethylamine (TEA) to form the 1-(2- chloroethyl)-3-cyclohexylurea
728:
1582:
Wirsching HG, Tritschler I, Palla A, Renner C, Weller M, Tabatabai G (December 2014).
3750:
3567:
2942:
2815:
2696:
2639:
2397:
2346:
2010:
1837:
1712:
619:
524:
187:
94:
1162:
3644:
3608:
3507:
3497:
3451:
3447:
3409:
3317:
3107:
3046:
3042:
3023:
3018:
2997:
2986:
2800:
2794:
2780:
2775:
2706:
2701:
2654:
2540:
2407:
2387:
2316:
2265:
2255:
2069:
2061:
1776:
1731:
Jaman Z, Sobreira TJ, Mufti A, Ferreira CR, Cooks RG, Thompson DH (February 2019).
1234:
991:
720:
131:
126:
1748:
341:
80:
3689:
3669:
3654:
3649:
3634:
3597:
3572:
3517:
3487:
3431:
3404:
3277:
3218:
3190:
3180:
3133:
3097:
3013:
2975:
2957:
2952:
2932:
2909:
2880:
2858:
2838:
2828:
2679:
2664:
2644:
2530:
2461:
2447:
2402:
2335:
2330:
2307:
2282:
2250:
2193:
2156:
2133:
2118:
2113:
2098:
1872:
736:
88:
2024:
1811:
660:
InChI=1S/C9H16ClN3O2/c10-6-7-13(12-15)9(14)11-8-4-2-1-3-5-8/h8H,1-7H2,(H,11,14)
3679:
3587:
3582:
3562:
3557:
3552:
3547:
3542:
3512:
3502:
3467:
3462:
3442:
3397:
3385:
3360:
3355:
3345:
3327:
3322:
3312:
3307:
3265:
3253:
3241:
3213:
3200:
3195:
3165:
3128:
3123:
3061:
3056:
3002:
2947:
2899:
2885:
2833:
2820:
2789:
2722:
2711:
2684:
2674:
2669:
2615:
2570:
2565:
2466:
2430:
2362:
2352:
2341:
2325:
2277:
2260:
2236:
2188:
2176:
2128:
1908:
1820:
1262:(5th ed.). Philadelphia: Lippincott Williams & Wilkins. p. 300.
1060:
In December 2021, the Glioblastom Foundation announced a new partnership with
747:
584:
392:
240:
1784:
1704:
3694:
3674:
3629:
3619:
3613:
3532:
3477:
3426:
3350:
3340:
3295:
3282:
3223:
3185:
3113:
3051:
2992:
2981:
2937:
2894:
2853:
2847:
2805:
2732:
2727:
2717:
2689:
2649:
2610:
2585:
2575:
2535:
2181:
2171:
2166:
2161:
2123:
1284:"Gleostine (lomustine) Capsules, for Oral Use. Full Prescribing Information"
724:
74:
1891:
1829:
1617:
1435:
1154:
20:
3603:
3577:
3522:
3290:
3228:
3008:
2904:
2876:
2555:
2392:
1733:"Rapid on-demand synthesis of lomustine under continuous flow conditions"
1599:
910:
Only one dose of the drug is dispensed at a time to lower overdose risk.
372:
1638:
381:
2511:
2412:
2226:
2087:
1685:
1668:
1146:
327:
28:
2151:
1909:"The Gleostine® (lomustine) brand has replaced the BMS CeeNU product"
472:
1584:"The management of lomustine overdose in malignant glioma patients"
441:
990:
607:
598:
452:
233:
1856:"Current FDA-Approved Therapies for High-Grade Malignant Gliomas"
432:
168:
2033:
181:
1453:"Lomustine for dogs and cats: Uses, Dosage & Side Effects"
1177:"BC Cancer Agency Cancer Drug Manual. Lomustine (CCNU; CeeNU)"
1112:
956:
is transferred to the nitrogen of 1-chloro-2-isocyanatoethane
948:
and the nitrosyl carbon center on 1-chloro-2-isocyanatoethane
755:
751:
739:, which is its primary use, although it is also used to treat
1447:
1445:
497:
1210:. Bristol-Myers Squibb Australia Pty Ltd. 30 September 2015
731:. It is a highly lipid-soluble drug, thus it crosses the
157:
1983:"New Deal Halts Price Gouging of Brain Cancer Patients"
683:
1553:
1551:
1549:
3419:
3370:
3150:
3143:
3079:
3033:
2966:
2923:
2760:
2745:
2625:
2595:
2520:
2505:
2476:
2372:
2292:
2235:
2218:
2207:
2143:
2096:
2077:
1559:"Lomustine (Oral Route) Side Effects - Mayo Clinic"
618:
596:
583:
547:
542:
523:
491:
471:
451:
431:
411:
391:
371:
360:
351:
326:
306:
270:
257:
239:
227:
217:
207:
199:
147:
142:
117:
101:
87:
69:
59:
49:
44:
1633:
1631:
1629:
1627:
1696:20.500.11820/7466f1c6-1f4d-402c-a53e-d4d40f73f3ff
1506:"Lomustine (Oral Route) Proper Use - Mayo Clinic"
1476:
1474:
1472:
340:
315:
1849:
1847:
2045:
1726:
1724:
1722:
1260:Principles and Practice of Pediatric Oncology
976:butoxide radical and a nitrosyl radical. The
8:
1903:
1901:
1662:
1660:
1658:
1482:"Lomustine - Chemotherapy Drugs - Chemocare"
735:. This property makes it ideal for treating
64:1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea
19:
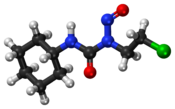
1340:
1338:
1336:
1334:
1332:
1330:
1328:
1326:
1324:
3147:
2757:
2517:
2232:
2215:
2093:
2052:
2038:
2030:
1737:Organic Process Research & Development
1322:
1320:
1318:
1316:
1314:
1312:
1310:
1308:
1306:
1304:
534:
511:
400:
2013:at the U.S. National Library of Medicine
1881:
1871:
1819:
1694:
1684:
1667:Diab S, Raiyat M, Gerogiorgis DI (2021).
1607:
1425:
420:
1933:"FDA Approves Name Change for Lomustine"
995:Reaction scheme for lomustine synthesis.
1347:"Gleostine (Lomustine) FDA Access Data"
1096:
657:
637:
507:
380:
284:
93:
1765:Journal of Electroanalytical Chemistry
1201:"PRODUCT INFORMATION CeeNU(lomustine)"
525:
18:
1368:"BC Cancer Agency Cancer Drug Manual"
1229:
1227:
1225:
480:
460:
79:
7:
1854:Fisher JP, Adamson DC (March 2021).
1673:Reaction Chemistry & Engineering
1135:Cancer Chemotherapy and Pharmacology
130:
2321:ribonucleotide reductase inhibitors
1408:Hay JK, Larson VS (December 2019).
1258:Pizzo PA, Poplack DG, eds. (2006).
1048:decided to withdraw lomustine from
819:Chemotherapy in veterinary medicine
440:
331:
2487:Ribonucleotide reductase inhibitor
2443:Ribonucleotide reductase inhibitor
1235:"Lomustine | VCA Animal Hospitals"
14:
2246:Dihydrofolate reductase inhibitor
3757:Alkylating antineoplastic agents
568:
565:
559:
36:
27:
1414:The Canadian Veterinary Journal
1289:. NextSource Biotechnology, LLC
936:by 1-chloro-2-isocyanatoethane
665:Key:GQYIWUVLTXOXAJ-UHFFFAOYSA-N
2383:Thymidylate synthase inhibitor
2273:Thymidylate synthase inhibitor
1777:10.1016/j.jelechem.2016.03.020
771:Chemotherapy in human medicine
574:
553:
245:Monoxydroxylated metabolites,
1:
2303:Adenosine deaminase inhibitor
2144:Block microtubule disassembly
1939:. Haymarket Media. 2014-08-08
1749:10.1021/acs.oprd.8b00387.s001
1067:continuous flow manufacturing
727:and is in the same family as
55:Gleostine, CCNU, CeeNu, CuuNu
853:stool, and extreme fatigue.
746:Lomustine is a bifunctional
1873:10.3390/biomedicines9030324
723:. It is closely related to
3808:
1812:10.1016/j.ctrv.2020.102029
1046:NextSource Pharmaceuticals
543:Chemical and physical data
3762:IARC Group 2A carcinogens
3707:
3593:Omacetaxine mepesuccinate
3483:Ciltacabtagene autoleucel
3473:Brexucabtagene autoleucel
1457:www.wedgewoodpharmacy.com
673:
648:
628:
275:
266:16–48 hours (metabolites)
35:
26:
3640:Talimogene laherparepvec
2507:Topoisomerase inhibitors
2426:DNA polymerase inhibitor
2015:Medical Subject Headings
1800:Cancer Treatment Reviews
1389:www.cancerresearchuk.org
640:O=C(NC1CCCCC1)N(N=O)CCCl
624:90 °C (194 °F)
3458:Axicabtagene ciloleucel
2062:chemotherapeutic agents
1588:Neuro-Oncology Practice
1071:Glioblastoma Foundation
893:Delayed myelosupression
3625:Sitimagene ceradenovec
3538:Idecabtagene vicleucel
3103:Methyl aminolevulinate
1534:reference.medscape.com
1208:TGA eBusiness Services
996:
885:Secondary malignancies
793:Breastfeeding patients
760:cell-cycle nonspecific
704:; original brand name
3792:Chloroethyl compounds
3114:Porphyrin derivatives
2810:Melphalan flufenamide
2457:Hypomethylating agent
2066:antineoplastic agents
1037:Pricing controversies
994:
968:-butyl nitrite (TBN)
3787:Cyclohexyl compounds
3443:Asparagine depleters
3372:Receptor antagonists
3286:+abiraterone acetate
1345:FDA (January 2016).
717:nitrosourea compound
3493:Denileukin diftitox
3393:Retinoid X receptor
3093:Aminolevulinic acid
2915:Triethylenemelamine
2747:Crosslinking of DNA
2478:Deoxyribonucleotide
2417:+gimeracil/oteracil
1032:Society and culture
1005:Mechanism of action
733:blood–brain barrier
177:(Prescription only)
23:
3734:Never to phase III
2551:Etirinotecan pegol
1821:20.500.12210/75164
1686:10.1039/D1RE00184A
1600:10.1093/nop/npu023
1563:www.mayoclinic.org
1510:www.mayoclinic.org
1147:10.1007/bf00434350
997:
810:Geriatric patients
802:Pediatric patients
708:, now marketed as
3782:Cancer treatments
3744:
3743:
3703:
3702:
3660:Tigilanol tiglate
3152:Enzyme inhibitors
3075:
3074:
3071:
3070:
2771:Nitrogen mustards
2741:
2740:
2501:
2500:
2203:
2202:
2021:Diseases Database
1962:The Cancer Letter
1679:(10): 1819–1828.
1420:(12): 1326–1330.
1062:Continuity Pharma
914:Drug interactions
780:Pregnant patients
750:, alkylates both
700:; abbreviated as
691:
690:
609:Interactive image
493:CompTox Dashboard
290:-(2-Chloroethyl)-
249:-4-hydroxy-CCNU,
185:
172:
160:
65:
16:Chemical compound
3799:
3665:Tisagenlecleucel
3437:Arsenic trioxide
3148:
3081:Photosensitizers
2872:Alkyl sulfonates
2786:Cyclophosphamide
2758:
2697:Anthracenediones
2518:
2492:Hydroxycarbamide
2233:
2216:
2094:
2054:
2047:
2040:
2031:
1998:
1997:
1995:
1994:
1979:
1973:
1972:
1970:
1969:
1954:
1948:
1947:
1945:
1944:
1929:
1923:
1922:
1920:
1919:
1905:
1896:
1895:
1885:
1875:
1851:
1842:
1841:
1823:
1795:
1789:
1788:
1759:
1753:
1752:
1728:
1717:
1716:
1698:
1688:
1664:
1653:
1652:
1650:
1649:
1635:
1622:
1621:
1611:
1579:
1573:
1572:
1570:
1569:
1555:
1544:
1543:
1541:
1540:
1526:
1520:
1519:
1517:
1516:
1502:
1496:
1495:
1493:
1492:
1478:
1467:
1466:
1464:
1463:
1449:
1440:
1439:
1429:
1405:
1399:
1398:
1396:
1395:
1381:
1375:
1374:
1372:
1364:
1358:
1357:
1351:
1342:
1299:
1298:
1296:
1294:
1288:
1280:
1274:
1273:
1255:
1249:
1248:
1246:
1245:
1231:
1220:
1219:
1217:
1215:
1205:
1197:
1191:
1190:
1188:
1186:
1181:
1173:
1167:
1166:
1130:
1124:
1123:
1121:
1119:
1109:nctr-crs.fda.gov
1101:
748:alkylating agent
741:Hodgkin lymphoma
687:
686:
679:
611:
591:
576:
570:
567:
561:
555:
538:
527:
516:
515:
501:
499:
484:
464:
444:
424:
404:
384:
364:
344:
334:
333:
319:
262:
190:
183:
180:
170:
167:
159:
156:
134:
97:
83:
63:
40:
31:
24:
22:
3807:
3806:
3802:
3801:
3800:
3798:
3797:
3796:
3777:Organochlorides
3747:
3746:
3745:
3740:
3739:
3724:Clinical trials
3699:
3420:Other/ungrouped
3415:
3366:
3139:
3119:Porfimer sodium
3067:
3029:
2962:
2919:
2749:
2737:
2621:
2591:
2509:
2497:
2472:
2368:
2288:
2224:
2222:antimetabolites
2220:
2219:DNA precursors/
2211:
2209:DNA replication
2199:
2139:
2109:Vinca alkaloids
2085:
2073:
2058:
2007:
2002:
2001:
1992:
1990:
1981:
1980:
1976:
1967:
1965:
1956:
1955:
1951:
1942:
1940:
1931:
1930:
1926:
1917:
1915:
1907:
1906:
1899:
1853:
1852:
1845:
1797:
1796:
1792:
1761:
1760:
1756:
1730:
1729:
1720:
1666:
1665:
1656:
1647:
1645:
1643:go.drugbank.com
1637:
1636:
1625:
1581:
1580:
1576:
1567:
1565:
1557:
1556:
1547:
1538:
1536:
1528:
1527:
1523:
1514:
1512:
1504:
1503:
1499:
1490:
1488:
1480:
1479:
1470:
1461:
1459:
1451:
1450:
1443:
1407:
1406:
1402:
1393:
1391:
1383:
1382:
1378:
1373:. June 5, 2018.
1370:
1366:
1365:
1361:
1349:
1344:
1343:
1302:
1292:
1290:
1286:
1282:
1281:
1277:
1270:
1257:
1256:
1252:
1243:
1241:
1233:
1232:
1223:
1213:
1211:
1203:
1199:
1198:
1194:
1184:
1182:
1179:
1175:
1174:
1170:
1132:
1131:
1127:
1117:
1115:
1103:
1102:
1098:
1093:
1084:
1082:Clinical trials
1079:
1054:Medicare Part D
1039:
1034:
1025:
1020:
1007:
1002:
929:
916:
904:
895:
887:
868:
863:
847:
834:
821:
812:
804:
795:
782:
773:
768:
682:
680:
677:(what is this?)
674:
669:
666:
661:
656:
655:
644:
641:
636:
635:
614:
589:
579:
573:
564:
558:
519:
495:
487:
467:
447:
427:
407:
387:
367:
347:
330:
322:
302:
299:
283:
282:
260:
253:-4-hydroxy-CCNU
219:Protein binding
209:Bioavailability
201:Pharmacokinetic
195:
188:
138:
104:
17:
12:
11:
5:
3805:
3803:
3795:
3794:
3789:
3784:
3779:
3774:
3769:
3764:
3759:
3749:
3748:
3742:
3741:
3738:
3737:
3736:
3735:
3732:
3721:
3715:
3709:
3708:
3705:
3704:
3701:
3700:
3698:
3697:
3692:
3687:
3682:
3677:
3672:
3667:
3662:
3657:
3652:
3647:
3642:
3637:
3632:
3627:
3622:
3617:
3611:
3600:
3595:
3590:
3585:
3580:
3575:
3570:
3565:
3560:
3555:
3550:
3545:
3540:
3535:
3530:
3525:
3520:
3515:
3510:
3505:
3500:
3495:
3490:
3485:
3480:
3475:
3470:
3465:
3460:
3455:
3439:
3434:
3429:
3423:
3421:
3417:
3416:
3414:
3413:
3401:
3389:
3376:
3374:
3368:
3367:
3365:
3364:
3358:
3353:
3348:
3343:
3331:
3325:
3320:
3315:
3310:
3299:
3293:
3288:
3280:
3273:PARP inhibitor
3269:
3257:
3245:
3233:
3232:
3231:
3226:
3221:
3216:
3204:
3198:
3193:
3188:
3183:
3169:
3156:
3154:
3145:
3141:
3140:
3138:
3137:
3131:
3126:
3121:
3110:
3105:
3100:
3095:
3089:
3087:
3077:
3076:
3073:
3072:
3069:
3068:
3066:
3065:
3059:
3054:
3049:
3039:
3037:
3031:
3030:
3028:
3027:
3021:
3016:
3005:
3000:
2995:
2990:
2978:
2972:
2970:
2964:
2963:
2961:
2960:
2955:
2950:
2945:
2940:
2935:
2929:
2927:
2925:Platinum-based
2921:
2920:
2918:
2917:
2912:
2907:
2902:
2890:
2889:
2883:
2867:
2866:
2861:
2856:
2851:
2841:
2836:
2824:
2823:
2818:
2813:
2803:
2798:
2792:
2783:
2778:
2766:
2764:
2755:
2743:
2742:
2739:
2738:
2736:
2735:
2730:
2725:
2720:
2715:
2709:
2704:
2693:
2687:
2682:
2677:
2672:
2667:
2662:
2652:
2647:
2640:Anthracyclines
2635:
2633:
2623:
2622:
2620:
2619:
2613:
2601:
2599:
2593:
2592:
2590:
2589:
2583:
2578:
2573:
2568:
2563:
2558:
2553:
2548:
2543:
2538:
2526:
2524:
2515:
2503:
2502:
2499:
2498:
2496:
2495:
2482:
2480:
2474:
2473:
2471:
2470:
2464:
2452:
2451:
2438:
2437:
2421:
2420:
2410:
2405:
2400:
2395:
2390:
2378:
2376:
2370:
2369:
2367:
2366:
2360:
2358:Mercaptopurine
2349:
2344:
2339:
2333:
2328:
2312:
2311:
2298:
2296:
2290:
2289:
2287:
2286:
2280:
2269:
2263:
2258:
2253:
2241:
2239:
2230:
2213:
2205:
2204:
2201:
2200:
2198:
2197:
2185:
2179:
2174:
2169:
2164:
2159:
2147:
2145:
2141:
2140:
2138:
2137:
2131:
2126:
2121:
2116:
2104:
2102:
2091:
2075:
2074:
2060:Intracellular
2059:
2057:
2056:
2049:
2042:
2034:
2028:
2027:
2018:
2006:
2005:External links
2003:
2000:
1999:
1974:
1949:
1924:
1897:
1843:
1790:
1754:
1743:(3): 334–341.
1718:
1654:
1623:
1594:(4): 178–183.
1574:
1545:
1521:
1497:
1468:
1441:
1400:
1376:
1359:
1300:
1275:
1268:
1250:
1221:
1192:
1168:
1141:(2): 125–131.
1125:
1095:
1094:
1092:
1089:
1083:
1080:
1078:
1075:
1038:
1035:
1033:
1030:
1024:
1021:
1019:
1016:
1006:
1003:
1001:
998:
928:
925:
915:
912:
903:
900:
894:
891:
886:
883:
867:
864:
862:
859:
846:
843:
833:
832:Administration
830:
820:
817:
811:
808:
803:
800:
794:
791:
781:
778:
772:
769:
767:
764:
729:streptozotocin
689:
688:
671:
670:
668:
667:
664:
662:
659:
651:
650:
649:
646:
645:
643:
642:
639:
631:
630:
629:
626:
625:
622:
616:
615:
613:
612:
604:
602:
594:
593:
587:
581:
580:
577:
571:
562:
556:
551:
545:
544:
540:
539:
529:
521:
520:
518:
517:
504:
502:
489:
488:
486:
485:
477:
475:
469:
468:
466:
465:
457:
455:
449:
448:
446:
445:
437:
435:
429:
428:
426:
425:
417:
415:
409:
408:
406:
405:
397:
395:
389:
388:
386:
385:
377:
375:
369:
368:
366:
365:
357:
355:
349:
348:
346:
345:
337:
335:
324:
323:
321:
320:
312:
310:
304:
303:
301:
300:
286:
278:
277:
276:
273:
272:
268:
267:
264:
255:
254:
243:
237:
236:
231:
225:
224:
221:
215:
214:
211:
205:
204:
197:
196:
194:
193:
178:
165:
153:
151:
145:
144:
140:
139:
137:
136:
123:
121:
115:
114:
107:
105:administration
99:
98:
91:
85:
84:
77:
67:
66:
61:
57:
56:
53:
47:
46:
42:
41:
33:
32:
15:
13:
10:
9:
6:
4:
3:
2:
3804:
3793:
3790:
3788:
3785:
3783:
3780:
3778:
3775:
3773:
3770:
3768:
3765:
3763:
3760:
3758:
3755:
3754:
3752:
3733:
3731:
3728:
3727:
3725:
3722:
3719:
3716:
3714:
3711:
3710:
3706:
3696:
3693:
3691:
3688:
3686:
3683:
3681:
3678:
3676:
3673:
3671:
3668:
3666:
3663:
3661:
3658:
3656:
3653:
3651:
3648:
3646:
3643:
3641:
3638:
3636:
3633:
3631:
3628:
3626:
3623:
3621:
3618:
3615:
3612:
3610:
3606:
3605:
3601:
3599:
3596:
3594:
3591:
3589:
3586:
3584:
3581:
3579:
3576:
3574:
3571:
3569:
3568:Lurbinectedin
3566:
3564:
3561:
3559:
3556:
3554:
3551:
3549:
3546:
3544:
3541:
3539:
3536:
3534:
3531:
3529:
3526:
3524:
3521:
3519:
3516:
3514:
3511:
3509:
3506:
3504:
3501:
3499:
3496:
3494:
3491:
3489:
3486:
3484:
3481:
3479:
3476:
3474:
3471:
3469:
3466:
3464:
3461:
3459:
3456:
3453:
3449:
3445:
3444:
3440:
3438:
3435:
3433:
3430:
3428:
3425:
3424:
3422:
3418:
3411:
3407:
3406:
3402:
3399:
3395:
3394:
3390:
3387:
3383:
3382:
3378:
3377:
3375:
3373:
3369:
3362:
3359:
3357:
3354:
3352:
3349:
3347:
3344:
3342:
3338:
3336:
3332:
3329:
3326:
3324:
3321:
3319:
3316:
3314:
3311:
3309:
3305:
3304:
3300:
3297:
3294:
3292:
3289:
3287:
3284:
3281:
3279:
3275:
3274:
3270:
3267:
3263:
3262:
3258:
3255:
3251:
3250:
3246:
3243:
3239:
3238:
3234:
3230:
3227:
3225:
3222:
3220:
3217:
3215:
3212:
3211:
3210:
3209:
3205:
3202:
3199:
3197:
3194:
3192:
3189:
3187:
3184:
3182:
3178:
3177:
3174:
3170:
3167:
3163:
3162:
3158:
3157:
3155:
3153:
3149:
3146:
3142:
3135:
3132:
3130:
3127:
3125:
3122:
3120:
3116:
3115:
3111:
3109:
3106:
3104:
3101:
3099:
3096:
3094:
3091:
3090:
3088:
3086:
3082:
3078:
3063:
3060:
3058:
3055:
3053:
3050:
3048:
3044:
3041:
3040:
3038:
3036:
3035:Intercalation
3032:
3025:
3022:
3020:
3017:
3015:
3011:
3010:
3006:
3004:
3001:
2999:
2996:
2994:
2991:
2988:
2984:
2983:
2979:
2977:
2974:
2973:
2971:
2969:
2965:
2959:
2956:
2954:
2951:
2949:
2946:
2944:
2943:Dicycloplatin
2941:
2939:
2936:
2934:
2931:
2930:
2928:
2926:
2922:
2916:
2913:
2911:
2908:
2906:
2903:
2901:
2898:
2896:
2892:
2891:
2887:
2884:
2882:
2878:
2875:
2873:
2869:
2868:
2865:
2862:
2860:
2857:
2855:
2852:
2849:
2845:
2842:
2840:
2837:
2835:
2832:
2830:
2826:
2825:
2822:
2819:
2817:
2816:Prednimustine
2814:
2811:
2807:
2804:
2802:
2799:
2796:
2793:
2791:
2787:
2784:
2782:
2779:
2777:
2774:
2772:
2768:
2767:
2765:
2763:
2759:
2756:
2753:
2748:
2744:
2734:
2731:
2729:
2726:
2724:
2721:
2719:
2716:
2713:
2710:
2708:
2705:
2703:
2699:
2698:
2694:
2691:
2688:
2686:
2683:
2681:
2678:
2676:
2673:
2671:
2668:
2666:
2663:
2660:
2656:
2653:
2651:
2648:
2646:
2642:
2641:
2637:
2636:
2634:
2632:
2631:Intercalation
2628:
2624:
2617:
2614:
2612:
2608:
2607:
2603:
2602:
2600:
2598:
2594:
2587:
2584:
2582:
2579:
2577:
2574:
2572:
2569:
2567:
2564:
2562:
2559:
2557:
2554:
2552:
2549:
2547:
2544:
2542:
2539:
2537:
2533:
2532:
2528:
2527:
2525:
2523:
2519:
2516:
2513:
2508:
2504:
2493:
2489:
2488:
2484:
2483:
2481:
2479:
2475:
2468:
2465:
2463:
2459:
2458:
2454:
2453:
2449:
2445:
2444:
2440:
2439:
2435:
2434:+daunorubicin
2432:
2428:
2427:
2423:
2422:
2418:
2414:
2411:
2409:
2406:
2404:
2401:
2399:
2398:Doxifluridine
2396:
2394:
2391:
2389:
2385:
2384:
2380:
2379:
2377:
2375:
2371:
2364:
2361:
2359:
2355:
2354:
2350:
2348:
2347:Rabacfosadine
2345:
2343:
2340:
2337:
2334:
2332:
2329:
2327:
2323:
2322:
2318:
2314:
2313:
2309:
2305:
2304:
2300:
2299:
2297:
2295:
2291:
2284:
2281:
2279:
2275:
2274:
2270:
2267:
2264:
2262:
2259:
2257:
2254:
2252:
2248:
2247:
2243:
2242:
2240:
2238:
2234:
2231:
2228:
2223:
2217:
2214:
2210:
2206:
2195:
2191:
2190:
2186:
2183:
2180:
2178:
2175:
2173:
2170:
2168:
2165:
2163:
2160:
2158:
2154:
2153:
2149:
2148:
2146:
2142:
2135:
2132:
2130:
2127:
2125:
2122:
2120:
2117:
2115:
2111:
2110:
2106:
2105:
2103:
2100:
2095:
2092:
2089:
2084:
2080:
2076:
2071:
2067:
2063:
2055:
2050:
2048:
2043:
2041:
2036:
2035:
2032:
2026:
2022:
2019:
2016:
2012:
2009:
2008:
2004:
1988:
1984:
1978:
1975:
1963:
1959:
1953:
1950:
1938:
1934:
1928:
1925:
1914:
1910:
1904:
1902:
1898:
1893:
1889:
1884:
1879:
1874:
1869:
1865:
1861:
1857:
1850:
1848:
1844:
1839:
1835:
1831:
1827:
1822:
1817:
1813:
1809:
1805:
1801:
1794:
1791:
1786:
1782:
1778:
1774:
1770:
1766:
1758:
1755:
1750:
1746:
1742:
1738:
1734:
1727:
1725:
1723:
1719:
1714:
1710:
1706:
1702:
1697:
1692:
1687:
1682:
1678:
1674:
1670:
1663:
1661:
1659:
1655:
1644:
1640:
1634:
1632:
1630:
1628:
1624:
1619:
1615:
1610:
1605:
1601:
1597:
1593:
1589:
1585:
1578:
1575:
1564:
1560:
1554:
1552:
1550:
1546:
1535:
1531:
1525:
1522:
1511:
1507:
1501:
1498:
1487:
1486:chemocare.com
1483:
1477:
1475:
1473:
1469:
1458:
1454:
1448:
1446:
1442:
1437:
1433:
1428:
1423:
1419:
1415:
1411:
1404:
1401:
1390:
1386:
1380:
1377:
1369:
1363:
1360:
1355:
1348:
1341:
1339:
1337:
1335:
1333:
1331:
1329:
1327:
1325:
1323:
1321:
1319:
1317:
1315:
1313:
1311:
1309:
1307:
1305:
1301:
1285:
1279:
1276:
1271:
1269:9780781754927
1265:
1261:
1254:
1251:
1240:
1236:
1230:
1228:
1226:
1222:
1209:
1202:
1196:
1193:
1178:
1172:
1169:
1164:
1160:
1156:
1152:
1148:
1144:
1140:
1136:
1129:
1126:
1114:
1110:
1106:
1100:
1097:
1090:
1088:
1081:
1076:
1074:
1072:
1068:
1063:
1058:
1055:
1051:
1047:
1043:
1036:
1031:
1029:
1022:
1017:
1015:
1011:
1004:
999:
993:
989:
987:
983:
979:
975:
971:
967:
963:
959:
955:
951:
947:
943:
939:
935:
927:Manufacturing
926:
924:
920:
913:
911:
908:
902:Overdose risk
901:
899:
892:
890:
884:
882:
878:
875:
871:
865:
860:
858:
854:
850:
844:
842:
838:
831:
829:
825:
818:
816:
809:
807:
801:
799:
792:
790:
786:
779:
777:
770:
765:
763:
761:
757:
753:
749:
744:
742:
738:
734:
730:
726:
722:
718:
715:
711:
707:
703:
699:
695:
685:
678:
672:
663:
658:
654:
647:
638:
634:
627:
623:
621:
620:Melting point
617:
610:
606:
605:
603:
600:
595:
588:
586:
582:
552:
550:
546:
541:
537:
533:
530:
528:
526:ECHA InfoCard
522:
514:
510:
509:DTXSID2023222
506:
505:
503:
494:
490:
483:
479:
478:
476:
474:
470:
463:
459:
458:
456:
454:
450:
443:
439:
438:
436:
434:
430:
423:
419:
418:
416:
414:
410:
403:
399:
398:
396:
394:
390:
383:
379:
378:
376:
374:
370:
363:
359:
358:
356:
354:
350:
343:
339:
338:
336:
329:
325:
318:
314:
313:
311:
309:
305:
297:
293:
289:
285:
281:
274:
269:
265:
263:
256:
252:
248:
244:
242:
238:
235:
232:
230:
226:
222:
220:
216:
212:
210:
206:
202:
198:
191:
179:
176:
166:
164:
155:
154:
152:
150:
146:
141:
133:
128:
125:
124:
122:
120:
116:
112:
108:
106:
100:
96:
92:
90:
86:
82:
78:
76:
72:
68:
62:
58:
54:
52:
48:
45:Clinical data
43:
39:
34:
30:
25:
3772:Nitrosoureas
3767:Nitrosamines
3645:Tazemetostat
3609:Alitretinoin
3602:
3528:Estramustine
3508:Elsamitrucin
3498:Eflornithine
3452:Pegaspargase
3448:Asparaginase
3441:
3410:Testolactone
3403:
3391:
3379:
3333:
3318:Panobinostat
3301:
3271:
3259:
3247:
3235:
3206:
3171:
3159:
3112:
3108:Padeliporfin
3047:Dactinomycin
3043:Streptomyces
3024:Temozolomide
3019:Mitozolomide
3007:
2998:Mitobronitol
2987:Procarbazine
2980:
2968:Nonclassical
2893:
2870:
2864:Streptozocin
2843:
2829:Nitrosoureas
2827:
2801:Chlorambucil
2795:Trofosfamide
2781:Chlormethine
2776:Bendamustine
2769:
2707:Mitoxantrone
2702:Losoxantrone
2695:
2655:Daunorubicin
2638:
2604:
2541:Camptothecin
2529:
2485:
2455:
2441:
2424:
2408:Fluorouracil
2388:Capecitabine
2381:
2351:
2315:
2301:
2271:
2266:Pralatrexate
2256:Methotrexate
2244:
2187:
2150:
2107:
1991:. Retrieved
1989:. 2021-12-22
1986:
1977:
1966:. Retrieved
1964:. 2021-07-16
1961:
1952:
1941:. Retrieved
1936:
1927:
1916:. Retrieved
1912:
1863:
1860:Biomedicines
1859:
1803:
1799:
1793:
1768:
1764:
1757:
1740:
1736:
1676:
1672:
1646:. Retrieved
1642:
1591:
1587:
1577:
1566:. Retrieved
1562:
1537:. Retrieved
1533:
1524:
1513:. Retrieved
1509:
1500:
1489:. Retrieved
1485:
1460:. Retrieved
1456:
1417:
1413:
1403:
1392:. Retrieved
1388:
1379:
1362:
1353:
1291:. Retrieved
1278:
1259:
1253:
1242:. Retrieved
1238:
1212:. Retrieved
1207:
1195:
1183:. Retrieved
1171:
1138:
1134:
1128:
1116:. Retrieved
1108:
1099:
1085:
1059:
1044:
1040:
1026:
1023:FDA approval
1012:
1008:
1000:Pharmacology
985:
981:
977:
973:
969:
965:
961:
957:
953:
949:
945:
941:
937:
933:
930:
921:
917:
909:
905:
896:
888:
879:
876:
872:
869:
855:
851:
848:
845:Side Effects
839:
835:
826:
822:
813:
805:
796:
787:
783:
774:
766:Medical uses
745:
737:brain tumors
721:chemotherapy
709:
705:
701:
693:
692:
681:
675:
298:-nitrosourea
295:
294:-cyclohexyl-
291:
287:
259:Elimination
250:
246:
149:Legal status
143:Legal status
3720:from market
3690:Vorasidenib
3670:Trabectedin
3655:Tiazofurine
3650:Tebentafusp
3635:Tagraxofusp
3598:Plitidepsin
3573:Mitoguazone
3518:Epacadostat
3488:Demecolcine
3432:Aflibercept
3405:Sex steroid
3278:Fuzuloparib
3219:Carfilzomib
3191:Palbociclib
3181:Abemaciclib
3134:Verteporfin
3098:Efaproxiral
3014:Dacarbazine
2976:Altretamine
2958:Satraplatin
2953:Oxaliplatin
2933:Carboplatin
2910:Triaziquone
2881:Mannosulfan
2859:Ranimustine
2839:Fotemustine
2680:Pirarubicin
2665:Doxorubicin
2659:+cytarabine
2645:Aclarubicin
2606:Podophyllum
2531:Camptotheca
2462:Azacitidine
2448:Gemcitabine
2403:Floxuridine
2336:Fludarabine
2331:Clofarabine
2317:Halogenated
2308:Pentostatin
2283:Raltitrexed
2251:Aminopterin
2194:Ixabepilone
2189:Epothilones
2157:Cabazitaxel
2134:Vinorelbine
2119:Vincristine
2114:Vinblastine
2099:microtubule
1639:"Lomustine"
1385:"Lomustine"
592: g·mol
532:100.032.585
271:Identifiers
241:Metabolites
89:MedlinePlus
60:Other names
51:Trade names
3751:Categories
3685:Verdinexor
3680:Venetoclax
3588:Oblimersen
3583:Navitoclax
3563:Lucanthone
3558:Lonidamine
3553:Lifileucel
3548:Ivosidenib
3543:Imetelstat
3513:Enasidenib
3503:Elesclomol
3468:Bexarotene
3463:Belzutifan
3398:Bexarotene
3386:Atrasentan
3361:Umbralisib
3356:Idelalisib
3346:Copanlisib
3328:Vorinostat
3323:Romidepsin
3313:Entinostat
3308:Belinostat
3266:Masoprocol
3254:Tiazofurin
3242:Anagrelide
3214:Bortezomib
3201:Seliciclib
3196:Ribociclib
3176:inhibitors
3166:Tipifarnib
3129:Temoporfin
3124:Talaporfin
3062:Plicamycin
3057:Mitomycins
3003:Pipobroman
2982:Hydrazines
2948:Nedaplatin
2900:Carboquone
2895:Aziridines
2886:Treosulfan
2834:Carmustine
2821:Uramustine
2790:Ifosfamide
2762:Alkylating
2723:Bisantrene
2712:Pixantrone
2685:Valrubicin
2675:Idarubicin
2670:Epirubicin
2616:Teniposide
2571:Lurtotecan
2566:Irinotecan
2467:Decitabine
2431:Cytarabine
2374:Pyrimidine
2363:Tioguanine
2353:Thiopurine
2342:Nelarabine
2326:Cladribine
2278:Pemetrexed
2261:Pemetrexed
2237:Folic acid
2177:Paclitaxel
2129:Vinflunine
1993:2023-05-22
1987:Accesswire
1968:2023-05-22
1943:2023-05-22
1918:2023-05-22
1866:(3): 324.
1806:: 102029.
1648:2023-05-22
1568:2023-05-22
1539:2023-05-22
1515:2023-05-22
1491:2023-05-22
1462:2023-05-22
1394:2023-05-22
1244:2023-05-22
1091:References
866:Toxicities
714:alkylating
597:3D model (
585:Molar mass
462:CHEBI:6520
422:7BRF0Z81KG
393:ChemSpider
353:IUPHAR/BPS
317:13010-47-4
308:CAS Number
280:IUPAC name
229:Metabolism
3730:Phase III
3718:Withdrawn
3695:Vosaroxin
3675:Veliparib
3630:Sotorasib
3620:Selinexor
3614:Tretinoin
3604:Retinoids
3533:Glasdegib
3478:Celecoxib
3427:Adagrasib
3351:Duvelisib
3341:Alpelisib
3296:Rucaparib
3283:Niraparib
3224:Oprozomib
3186:Alvocidib
3052:Bleomycin
3009:Triazenes
2993:Etoglucid
2938:Cisplatin
2854:Nimustine
2848:Semustine
2844:Lomustine
2806:Melphalan
2733:Menogaril
2728:Crisnatol
2718:Amsacrine
2690:Zorubicin
2650:Amrubicin
2611:Etoposide
2586:Topotecan
2581:Silatecan
2576:Rubitecan
2561:Gimatecan
2546:Cositecan
2536:Belotecan
2212:inhibitor
2182:Tesetaxel
2172:Ortataxel
2167:Larotaxel
2162:Docetaxel
2124:Vindesine
2011:Lomustine
1913:gleostine
1838:218648915
1785:1572-6657
1771:: 62–71.
1713:237643008
1705:2058-9883
824:animals.
725:semustine
710:Gleostine
694:Lomustine
482:ChEMBL514
261:half-life
103:Routes of
81:Monograph
75:Drugs.com
21:Lomustine
3578:Mitotane
3523:Eribulin
3291:Olaparib
3229:Ixazomib
2905:Thiotepa
2877:Busulfan
2556:Exatecan
2393:Carmofur
2101:assembly
2064: /
1892:33810154
1830:32408220
1618:26034630
1436:31814640
1214:23 April
1163:29619378
1077:Research
1050:Medicare
984:to form
719:used in
712:) is an
684:(verify)
373:DrugBank
119:ATC code
111:capsules
2512:S phase
2413:Tegafur
2227:S phase
2152:Taxanes
2088:M phase
2023:(DDB):
1883:8004675
1609:4369719
1427:6855235
1354:fda.gov
1293:15 July
1185:15 July
1155:3971475
1018:History
549:Formula
382:DB01206
328:PubChem
192:Rx-only
189:WARNING
161::
135:)
129: (
127:L01AD02
95:a682207
3713:WHO-EM
3337:(Pi3K)
2294:Purine
2097:Block
2017:(MeSH)
1890:
1880:
1836:
1828:
1783:
1711:
1703:
1616:
1606:
1434:
1424:
1266:
1161:
1153:
1118:22 Oct
861:Safety
633:SMILES
590:233.70
473:ChEMBL
442:D00363
186:
173:
163:℞-only
109:Oral (
3249:IMPDI
3144:Other
2025:29525
1834:S2CID
1709:S2CID
1371:(PDF)
1350:(PDF)
1287:(PDF)
1204:(PDF)
1180:(PDF)
1159:S2CID
974:tert-
706:CeeNU
653:InChI
599:JSmol
453:ChEBI
247:trans
234:Liver
213:~100%
3335:PIKI
3303:HDAC
2752:CCNS
1888:PMID
1826:PMID
1781:ISSN
1701:ISSN
1614:PMID
1432:PMID
1295:2016
1264:ISBN
1216:2018
1187:2016
1151:PMID
1120:2023
986:tert
978:tert
966:tert
950:(2).
754:and
702:CCNU
433:KEGG
413:UNII
402:3813
362:7214
342:3950
203:data
71:AHFS
3381:ERA
3237:PhI
3208:PrI
3173:CDK
3085:PDT
2083:MIs
2079:SPs
2070:L01
1937:MPR
1878:PMC
1868:doi
1816:hdl
1808:doi
1773:doi
1769:769
1745:doi
1691:hdl
1681:doi
1604:PMC
1596:doi
1422:PMC
1239:Vca
1143:doi
1113:FDA
982:(3)
970:(4)
964:by
962:(3)
958:(2)
954:(1)
946:(1)
942:(3)
938:(2)
934:(1)
756:RNA
752:DNA
698:INN
498:EPA
332:CID
251:cis
223:50%
175:POM
132:WHO
3753::
3726::
3261:LI
3161:FI
2627:II
2597:II
2419:))
1985:.
1960:.
1935:.
1911:.
1900:^
1886:.
1876:.
1862:.
1858:.
1846:^
1832:.
1824:.
1814:.
1804:87
1802:.
1779:.
1767:.
1741:23
1739:.
1735:.
1721:^
1707:.
1699:.
1689:.
1675:.
1671:.
1657:^
1641:.
1626:^
1612:.
1602:.
1590:.
1586:.
1561:.
1548:^
1532:.
1508:.
1484:.
1471:^
1455:.
1444:^
1430:.
1418:60
1416:.
1412:.
1387:.
1352:.
1303:^
1237:.
1224:^
1206:.
1157:.
1149:.
1139:14
1137:.
1111:.
1107:.
762:.
566:Cl
563:16
292:N'
182:US
169:UK
158:CA
3616:)
3607:(
3454:)
3450:/
3446:(
3412:)
3408:(
3400:)
3396:(
3388:)
3384:(
3363:)
3339:(
3330:)
3306:(
3298:)
3276:(
3268:)
3264:(
3256:)
3252:(
3244:)
3240:(
3203:)
3179:(
3168:)
3164:(
3136:)
3117:(
3083:/
3064:)
3045:(
3026:)
3012:(
2989:)
2985:(
2897::
2888:)
2879:(
2874::
2850:)
2846:(
2831::
2812:)
2808:(
2797:)
2788:(
2773::
2754:)
2750:(
2714:)
2700:(
2692:)
2661:)
2657:(
2643:(
2629:+
2618:)
2609:(
2588:)
2534:(
2522:I
2514:)
2510:(
2494:)
2490:(
2469:)
2460:(
2450:)
2446:(
2436:)
2429:(
2415:(
2386:(
2365:)
2356:(
2338:)
2324:(
2319:/
2310:)
2306:(
2285:)
2276:(
2268:)
2249:(
2229:)
2225:(
2196:)
2192:(
2184:)
2155:(
2136:)
2112:(
2090:)
2086:(
2081:/
2072:)
2068:(
2053:e
2046:t
2039:v
1996:.
1971:.
1946:.
1921:.
1894:.
1870::
1864:9
1840:.
1818::
1810::
1787:.
1775::
1751:.
1747::
1715:.
1693::
1683::
1677:6
1651:.
1620:.
1598::
1592:1
1571:.
1542:.
1518:.
1494:.
1465:.
1438:.
1397:.
1356:.
1297:.
1272:.
1247:.
1218:.
1189:.
1165:.
1145::
1122:.
696:(
601:)
578:2
575:O
572:3
569:N
560:H
557:9
554:C
500:)
496:(
296:N
288:N
184::
171::
113:)
73:/
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.