651:
2-amino-2-desoxyisochorismic acid (ADIC). Following this, PhzD catalyzes the hydrolytic removal the pyruvate moiety from ADIC to form (5S,6S)-6-amino-5-hydroxy-1,3-cyclohexadieve-1-carboxylic acid (DHHA). In the next step, PhzF catalyzes two steps: the abstraction of a hydrogen from C3 of DHHA, delocalization of the double bond system and reprotonation at C1 as well as enol tautomerization to form the highly unstable 6-amino-5-oxocyclohex-2-ene-1-carboxylic acid (AOCHC). From here two molecules of AOCHC are condensed by PhzB to form the tricyclic compound, hexahydrophenazine-1,6-dicarboxylic acid (HHPDC). The product of this reaction, HHPDC, is unstable and spontaneously undergoes oxidative decarboxylation in an uncatalyzed reaction to form tetrahydrophenazine-1,6-carboxylic acid (THPCA). In the final step of phenazine-1-carboxylic acid synthesis the enzyme PhzG catalyzes the oxidation of THPCA to dihydro-phenazine-1-carboxylic acid. This is the last catalyzed step in the production of PCA, the last step is an uncatalyzed oxidation of DHPCA to PCA. The conversion of PCA to
Pyocyanin is achieved in two enzymatic steps: firstly, PCA is methylated on N5 to 5-methylphenazine-1-carboxylate betaine by the enzyme PhzM using the cofactor S-adenosyl-L-methionine and secondly, PhzS catalyzes the hydroxylative decarboxylation of this substrate to form the final product, Pyocyanin.
24:
323:
196:
33:
477:
472:
643:
744:
producer of ATP but also has numerous other functions such as calcium homeostatic control, the facilitation of receptor-mediated endocytosis and the degradation of proteins. Therefore, the inactivation of vacuolar-ATPase by hydrogen peroxide produced by pyocyanin has huge consequences for the lung. Additional to these effects, another target of pyocyanin is caspase 3-like proteases which can then go on to initiate
569:
801:) are all impaired by pyocyanin, weakening the immune system of the lung. In vivo studies have shown that the growth of fungus is inhibited in the presence of pyocyanin. The fungicidal mechanism is the activation of NAD(P)H to induce a redox-active cascade producing reactive oxygen intermediates. This allows
743:
synthesis and assembly, vesicle transport machinery, and protein sorting machinery all confer an increased sensitivity to pyocyanin which further enhances the effects on cystic fibrosis on the patient. Vacuolar- ATPase in yeast cells is a particularly potent target as it is the main non-mitochondrial
698:
is an important antioxidant modulated by pyocyanin. In particular the pool of the reduced form is depleted while the oxidised form is promoted by hydrogen peroxide which is not dismutated by catalase. In the cystic fibrosis lung, intracellular pyocyanin converts molecular oxygen to the superoxide
629:
at blood pH, it is easily able to cross the cell membrane. There are three different states in which pyocyanin can exist: oxidized (blue), monovalently reduced (colourless) or divalently reduced (red). Mitochondria play an important role in the cycling of pyocyanin between its redox states. Due to
784:
to persist in the cystic fibrosis lung; it is often detected in the sputum of cystic fibrosis patients. Pyocyanin in vitro has the ability to interfere with functions such as ciliary beating and therefore cause epithelial dysfunction as the ciliary are needed to sweep mucus up the throat.
813:
which are already impaired in cystic fibrosis. CFTR channels rely on ATP for two main purposes. Firstly, the binding and hydrolysis of ATP has to occur at two nucleotide binding domains for the channel to move between its open and closed conformation. Secondly, phosphorylation of CFTR by
650:
Pyocyanin biosynthesis begins with the synthesis of the phenazine-1-carboxylic acid (PCA) core. In this reaction the enzyme PhzE catalyzes the loss of the hydroxyl group from C4 of
Chorismic Acid as well as the transfer of an amine group from glutamine to form glutamic acid and
768:, which cause oxidative stress by directly damaging DNA or by targeting other constituents of the cell cycle such as DNA recombination and repair machinery. Pyocyanin contributes to the disproportion of protease and antiprotease activity by disabling α
1241:
Denning G, Iyer S, Reszka K, O'Malley Y, Rasmussen G, Britigan B (2003). "Phenazine-1-carboxylic acid, a secondary metabolite of
Pseudomonas aeruginosa, alters expression of immunomodulatory proteins by human airway epithelial cells".
485:
452:
670:, and more specifically by the Pseudomonas Quinolone Signal (PQS) system involving the transcriptional regulator MvfR (also known as PqsR). Conversion of PCA into pyocyanin is then achieved by the products of
752:. Mitochondrial electron carriers ubiquinone and nicotinic acid are also susceptible to pyocyanin. The cell cycle can be disturbed by the action of pyocyanin, and it can hinder the proliferation of
539:
818:
should occur in order for the channel to be operational. PKA is activated by cAMP which is produced from ATP. Both these processes are impaired when ATP is depleted by pyocyanin.
810:
613:. Pyocyanin is a blue secondary metabolite, turning red below pH 4.9, with the ability to oxidise and reduce other molecules and therefore kill microbes competing against
582:
739:, vesicular transport, and cell growth. An enhanced susceptibility to pyocyanin is seen in cells with certain mutant proteins or complexes. Mutations in genes affecting
1227:
883:
703:
to NADP. This has a doubly negative effect on the lungs. Firstly, the NADPH used by pyocyanin depletes the available substrate for the reaction catalysed by the
372:
1434:
soda and sodB genes encoding manganese- and iron cofactored SOD: demonstration of increased Mn SOD dismutase activity in alginate-producing bacteria"
805:
to have a competitive advantage as it may dominate over other microorganisms in the cystic fibrosis lung. The intracellular concentration of
1500:
1391:
1281:
337:
735:
Pyocyanin is able to target a wide range of cellular components and pathways. Pathways that are affected by pyocyanin include the
993:
secretory product pyocyanin inactivates α1 protease inhibitor: implications for the pathogenesis of cystic fibrosis lung disease"
723:, which usually upregulate NADPH oxidase. When the lung is confronted with pyocyanin, an increased concentration of catalase and
1770:
535:
113:
1244:
1719:
1279:
Muller M (2002). "Pyocyanin inducesoxidative stress in human endothelialcells and modulates the glutathione redox cycle".
531:
1047:"Functional Analysis of Genes for Biosynthesis of Pyocyanin and Phenazine-1-Carboxamide from Pseudomonas aeruginosa PAO1"
1889:
952:
757:
517:
280:
301:
1383:"VMA13 encodes a 54-kDa vacuolar H(+)ATPase subunit required for activity but not assembly of the enzyme complex in
476:
646:
Biosynthesis of pyocyanin from
Pseudomonas aeruginosa. Hydrogens abstracted during next enzymatic step colored red.
589:
1904:
1609:
203:
736:
241:
1438:
1182:
905:
631:
605:
471:
191:
1664:
1554:
997:
853:
827:
806:
609:
153:
1221:
499:
464:
247:
1884:
1869:
1335:
1045:
Mavrodi, D. V.; Bonsall, R. F.; Delaney, S. M.; Soule, M. J.; Phillips, G.; Thomashow, L. S. (2001).
950:
Lau G, Hassett D, Ran H, Kong F (2004). "The role of pyocyanin in
Pseudomonas aeruginosa infection".
724:
543:
45:
1544:
Kanthakumar K, Taylor G, Tsang K, Cundell D, Rutman A, Smith S, Jeffery P, Cole P, Wilson R (1993).
780:
Many studies have concluded that pyocyanin has a derogatory effect in cystic fibrosis which enables
666:, which encode the enzymes required to produce PCA. Transcription of these operons is controlled by
1894:
1766:"Regulation of the cystic fibrosis transmembrane conductance regulator ClK channel by its R domain"
527:
318:
79:
23:
1848:
1636:
1518:
1483:
Sorensen R, Klinger J (1987). "Biological
Effects of Pseudomonas aeruginosa Phenazine Pigments".
1840:
1789:
1746:
1691:
1628:
1581:
1506:
1496:
1465:
1410:
1363:
1298:
1261:
1209:
1154:
1136:
1084:
1066:
1024:
969:
932:
761:
843:
which are effectively able to extrude intracellular pyocyanin in an energy dependent manner.
1830:
1779:
1736:
1728:
1681:
1673:
1618:
1571:
1563:
1488:
1455:
1447:
1400:
1353:
1343:
1290:
1253:
1199:
1191:
1174:"Function analysis of genes for biosynthesis of pyocyanin and phenazine -1-carboxamide from
1144:
1128:
1074:
1058:
1014:
1006:
961:
922:
914:
815:
395:
678:, which are unique genes in the chromosome. Biosynthesis can be impaired by disrupting the
289:
1899:
1530:
864:
832:
622:
173:
1339:
322:
195:
89:
32:
1821:
1741:
1710:
1172:
Mavrodi D, Bonsall, R, Delaney, S, Soule, M, Phillips G & Thomashow, L. S. (2001).
1149:
1116:
716:
667:
642:
560:
133:
1835:
1808:
1686:
1655:
1576:
1545:
1460:
1429:
1405:
1358:
1317:
1294:
1204:
1173:
1079:
1046:
927:
900:
694:
by reducing its gene’s transcription as well as directly targeting the enzyme itself.
1878:
1677:
1451:
1195:
1062:
1019:
988:
887:
794:
790:
712:
704:
184:
1852:
1640:
1567:
1010:
551:
523:
918:
965:
269:
1623:
1600:
753:
695:
1382:
1257:
1660:
pyocyanin increases interleukin-8 expression by human airway epithelial cells"
1132:
859:
786:
765:
626:
426:
164:
1140:
1070:
346:
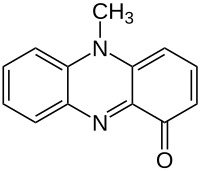
InChI=1S/C13H10N2O/c1-15-10-6-3-2-5-9(10)14-13-11(15)7-4-8-12(13)16/h2-8H,1H3
1381:
Ho M, Hirata R, Umemota N, Ohya Y, Takatsuki A, Stevens T, Anraku Y (1993).
1348:
745:
708:
682:
pathway which is needed for the synthesis of chorismic acid from shikimate.
356:
InChI=1/C13H10N2O/c1-15-10-6-3-2-5-9(10)14-13-11(15)7-4-8-12(13)16/h2-8H,1H3
1793:
1784:
1765:
1750:
1632:
1367:
1302:
1265:
1213:
1158:
1088:
1028:
973:
1844:
1695:
1654:
Denning G, Wollenweber L, Railsback M, Cox C, Stoll L, Britigan B (1998).
1599:
Usher L, Lawson R, Gaery I, Taylor C, Bingle C, Taylor G, Whyte M (2002).
1585:
1510:
1469:
1414:
936:
1732:
749:
740:
720:
691:
509:
256:
204:
1492:
727:
is seen in order to deal with the barrage of radicals being produced.
604:(PCN) is one of the many toxic compounds produced and secreted by the
144:
1764:
Ostedgaard S, Baldursson O, Vermeer D, Welsh M, Robertson A (2001).
1428:
Hassett D, Woodruff W, Wozniak D, Vasil M, Cohen S, Ohman D (1993).
559:
Except where otherwise noted, data are given for materials in their
505:
230:
1605:
exotoxin pyocyanin: a potential mechanism of persistent infection"
700:
641:
124:
112:
102:
1809:"Molecular mechanisms of bacterial virulence elucidated using a
1487:. Antibiotics and Chemotherapy. Vol. 39. pp. 113–124.
1326:
798:
221:
707:
enzyme. Secondly, the superoxide radical generated can inhibit
1709:
Kerr J, Taylor G, Rutman A, Hoiby N, Cole P, Wilson R (1998).
1485:
Basic
Research and Clinical Aspects of Pseudomonas Aeruginosa
547:
306:
809:
is also diminished by pyocyanin causing further damage to
1110:
1108:
1106:
1104:
1102:
1100:
1098:
577:
1715:
pyocyanin and 1-hydroxyphenazine inhbit fungal growth"
1807:
Mahajan-Miklos S, Tan M, Rahme L, Ausubel F (1999).
1040:
1038:
1117:"The structural biology of phenazine biosynthesis"
630:its redox-active properties, pyocyanin generates
268:
901:"Mechanism of the antibiotic action pyocyanine"
88:
617:as well as mammalian cells of the lungs which
1115:Blankenfeldt, Wulf; Parsons, James F (2014).
8:
1226:: CS1 maint: multiple names: authors list (
658:strains carry two nearly identical operons,
1601:"Induction of neutrophil apoptosis by the
321:
194:
172:
15:
1834:
1783:
1740:
1685:
1622:
1575:
1550:pyocyanin on human ciliary beat in vitro"
1459:
1404:
1357:
1347:
1203:
1148:
1078:
1018:
926:
288:
1316:Huimin R, Hassett D & Lau G (2003).
987:Britigin B, Railsback A, Cox D (1999).
876:
789:apoptosis, immunoglobulin release from
377:
342:
317:
246:
1526:
1516:
1219:
185:
1430:"Cloning and characterization of the
1121:Current Opinion in Structural Biology
349:Key: YNCMLFHHXWETLD-UHFFFAOYSA-N
152:
132:
7:
756:. This is done by the generation of
63:Pyocyanin; Pyrocyanine; 5-Methyl-1(5
1392:The Journal of Biological Chemistry
359:Key: YNCMLFHHXWETLD-UHFFFAOYAI
259:
229:
14:
1282:Free Radical Biology and Medicine
1678:10.1128/IAI.66.12.5777-5784.1998
1452:10.1128/jb.175.23.7658-7665.1993
1196:10.1128/JB.183.21.6454-6465.2001
1063:10.1128/JB.183.21.6454-6465.2001
793:, and interleukin release (e.g.
567:
475:
470:
413:
407:
31:
22:
1771:Journal of Biological Chemistry
1568:10.1128/IAI.61.7.2848-2853.1993
1011:10.1128/IAI.67.3.1207-1212.1999
563:(at 25 °C , 100 kPa).
67:)-phenazinone; Sanasin; Sanazin
1245:American Journal of Physiology
899:Hassan H, Fridovich I (1980).
419:
401:
1:
1836:10.1016/S0092-8674(00)80958-7
1720:Journal of Clinical Pathology
1406:10.1016/S0021-9258(17)46842-6
1295:10.1016/S0891-5849(02)01087-0
919:10.1128/JB.141.1.156-163.1980
758:reactive oxygen intermediates
380:CN1C2=CC=CC=C2N=C3C1=CC=CC3=O
966:10.1016/j.molmed.2004.10.002
953:Trends in Molecular Medicine
1624:10.4049/jimmunol.168.4.1861
1921:
1258:10.1152/ajplung.00086.2003
699:free radical by oxidizing
1610:The Journal of Immunology
1133:10.1016/j.sbi.2014.08.013
822:Defence against pyocyanin
557:
451:
446:
388:
368:
333:
72:
60:
44:
39:
30:
21:
1546:"Mechanism of action of
1385:Saccharomyces cerevisiae
737:electron transport chain
654:The chromosomes of most
518:Precautionary statements
1439:Journal of Bacteriology
1349:10.1073/pnas.2332354100
1183:Journal of Bacteriology
1051:Journal of Bacteriology
906:Journal of Bacteriology
831:possesses two specific
632:reactive oxygen species
625:. Since pyocyanin is a
606:Gram negative bacterium
1815:Caenorhabditis elegans
1811:Pseudomonas aeruginosa
1785:10.1074/jbc.R100001200
1713:Pseudomonas aeruginosa
1665:Infection and Immunity
1603:Pseudomonas aeruginosa
1555:Infection and Immunity
1432:Pseudomonas aeruginosa
1320:Pseudomonas aeruginosa
1176:Pseudomonas aeruginosa
998:Infection and Immunity
991:Pseudomonas aeruginosa
854:Pseudomonas aeruginosa
828:Caenorhabditis elegans
772:- protease inhibitor.
690:Pyocyanin inactivates
647:
610:Pseudomonas aeruginosa
1548:Pseudomona aeruginosa
645:
1733:10.1136/jcp.52.5.385
725:superoxide dismutase
621:has infected during
50:5-Methylphenazin-1(5
46:Preferred IUPAC name
1890:Biological pigments
1817:pathogenesis model"
1399:(24): 18286–18292.
1340:2003PNAS..10014315R
1334:(24): 14315–14320.
434: g·mol
18:
1318:"Human targets of
648:
590:Infobox references
16:
1870:Pyocyanin profile
1778:(11): 7689–7692.
1672:(12): 5777–5784.
1502:978-3-8055-4541-9
1493:10.1159/000414339
1289:(11): 1527–1533.
1190:(21): 6454–6465.
1057:(21): 6454–6465.
762:hydrogen peroxide
664:phzA2B2C2D2E2F2G2
660:phzA1B1C1D1E1F1G1
598:Chemical compound
596:
595:
500:Hazard statements
302:CompTox Dashboard
114:Interactive image
1912:
1905:Bacterial toxins
1857:
1856:
1838:
1804:
1798:
1797:
1787:
1761:
1755:
1754:
1744:
1706:
1700:
1699:
1689:
1651:
1645:
1644:
1626:
1617:(4): 1861–1868.
1596:
1590:
1589:
1579:
1562:(7): 2848–2853.
1541:
1535:
1534:
1528:
1524:
1522:
1514:
1480:
1474:
1473:
1463:
1425:
1419:
1418:
1408:
1378:
1372:
1371:
1361:
1351:
1313:
1307:
1306:
1276:
1270:
1269:
1238:
1232:
1231:
1225:
1217:
1207:
1169:
1163:
1162:
1152:
1112:
1093:
1092:
1082:
1042:
1033:
1032:
1022:
1005:(3): 1207–1212.
984:
978:
977:
947:
941:
940:
930:
896:
890:
881:
833:ABC transporters
816:Protein kinase A
580:
574:
571:
570:
553:
549:
545:
541:
537:
533:
529:
525:
511:
507:
479:
474:
433:
421:
415:
409:
403:
396:Chemical formula
326:
325:
310:
308:
292:
272:
261:
250:
233:
206:
198:
187:
176:
156:
136:
116:
92:
35:
26:
19:
1920:
1919:
1915:
1914:
1913:
1911:
1910:
1909:
1875:
1874:
1866:
1861:
1860:
1806:
1805:
1801:
1763:
1762:
1758:
1708:
1707:
1703:
1653:
1652:
1648:
1598:
1597:
1593:
1543:
1542:
1538:
1525:
1515:
1503:
1482:
1481:
1477:
1446:(23): 7658–65.
1427:
1426:
1422:
1380:
1379:
1375:
1315:
1314:
1310:
1278:
1277:
1273:
1252:(3): 584–L592.
1240:
1239:
1235:
1218:
1171:
1170:
1166:
1114:
1113:
1096:
1044:
1043:
1036:
986:
985:
981:
949:
948:
944:
898:
897:
893:
882:
878:
873:
865:Cystic fibrosis
849:
824:
778:
776:Cystic fibrosis
771:
733:
688:
640:
623:cystic fibrosis
599:
592:
587:
586:
585: ?)
576:
572:
568:
564:
520:
502:
488:
467:
431:
418:
412:
406:
398:
384:
381:
376:
375:
364:
361:
360:
357:
351:
350:
347:
341:
340:
329:
311:
304:
295:
275:
262:
236:
216:
179:
159:
139:
119:
106:
95:
82:
68:
56:
55:
12:
11:
5:
1918:
1916:
1908:
1907:
1902:
1897:
1892:
1887:
1877:
1876:
1873:
1872:
1865:
1864:External links
1862:
1859:
1858:
1799:
1756:
1727:(5): 385–387.
1701:
1646:
1591:
1536:
1527:|journal=
1501:
1475:
1420:
1373:
1308:
1271:
1233:
1164:
1094:
1034:
979:
942:
913:(1): 156–163.
891:
875:
874:
872:
869:
868:
867:
862:
857:
848:
845:
823:
820:
785:Additionally,
777:
774:
769:
732:
729:
687:
684:
668:quorum sensing
639:
636:
597:
594:
593:
588:
566:
565:
561:standard state
558:
555:
554:
540:P305+P351+P338
521:
516:
513:
512:
503:
498:
495:
494:
489:
484:
481:
480:
468:
463:
460:
459:
449:
448:
444:
443:
440:
436:
435:
429:
423:
422:
416:
410:
404:
399:
394:
391:
390:
386:
385:
383:
382:
379:
371:
370:
369:
366:
365:
363:
362:
358:
355:
354:
352:
348:
345:
344:
336:
335:
334:
331:
330:
328:
327:
314:
312:
300:
297:
296:
294:
293:
285:
283:
277:
276:
274:
273:
265:
263:
255:
252:
251:
244:
238:
237:
235:
234:
226:
224:
218:
217:
215:
214:
210:
208:
200:
199:
189:
181:
180:
178:
177:
169:
167:
161:
160:
158:
157:
149:
147:
141:
140:
138:
137:
129:
127:
121:
120:
118:
117:
109:
107:
100:
97:
96:
94:
93:
85:
83:
78:
75:
74:
70:
69:
62:
58:
57:
49:
48:
42:
41:
37:
36:
28:
27:
13:
10:
9:
6:
4:
3:
2:
1917:
1906:
1903:
1901:
1898:
1896:
1893:
1891:
1888:
1886:
1883:
1882:
1880:
1871:
1868:
1867:
1863:
1854:
1850:
1846:
1842:
1837:
1832:
1828:
1824:
1823:
1818:
1816:
1812:
1803:
1800:
1795:
1791:
1786:
1781:
1777:
1773:
1772:
1767:
1760:
1757:
1752:
1748:
1743:
1738:
1734:
1730:
1726:
1722:
1721:
1716:
1714:
1705:
1702:
1697:
1693:
1688:
1683:
1679:
1675:
1671:
1667:
1666:
1661:
1659:
1650:
1647:
1642:
1638:
1634:
1630:
1625:
1620:
1616:
1612:
1611:
1606:
1604:
1595:
1592:
1587:
1583:
1578:
1573:
1569:
1565:
1561:
1557:
1556:
1551:
1549:
1540:
1537:
1532:
1520:
1512:
1508:
1504:
1498:
1494:
1490:
1486:
1479:
1476:
1471:
1467:
1462:
1457:
1453:
1449:
1445:
1441:
1440:
1435:
1433:
1424:
1421:
1416:
1412:
1407:
1402:
1398:
1394:
1393:
1388:
1386:
1377:
1374:
1369:
1365:
1360:
1355:
1350:
1345:
1341:
1337:
1333:
1329:
1328:
1323:
1321:
1312:
1309:
1304:
1300:
1296:
1292:
1288:
1284:
1283:
1275:
1272:
1267:
1263:
1259:
1255:
1251:
1247:
1246:
1237:
1234:
1229:
1223:
1215:
1211:
1206:
1201:
1197:
1193:
1189:
1185:
1184:
1179:
1177:
1168:
1165:
1160:
1156:
1151:
1146:
1142:
1138:
1134:
1130:
1126:
1122:
1118:
1111:
1109:
1107:
1105:
1103:
1101:
1099:
1095:
1090:
1086:
1081:
1076:
1072:
1068:
1064:
1060:
1056:
1052:
1048:
1041:
1039:
1035:
1030:
1026:
1021:
1016:
1012:
1008:
1004:
1000:
999:
994:
992:
983:
980:
975:
971:
967:
963:
960:(12): 1–666.
959:
955:
954:
946:
943:
938:
934:
929:
924:
920:
916:
912:
908:
907:
902:
895:
892:
889:
888:Sigma-Aldrich
885:
880:
877:
870:
866:
863:
861:
858:
856:
855:
851:
850:
846:
844:
842:
838:
834:
830:
829:
821:
819:
817:
812:
808:
804:
803:P. aeruginosa
800:
796:
792:
791:B-lymphocytes
788:
783:
782:P. aeruginosa
775:
773:
767:
763:
759:
755:
751:
747:
742:
738:
730:
728:
726:
722:
718:
714:
710:
706:
705:NADPH oxidase
702:
697:
693:
686:Redox warfare
685:
683:
681:
677:
673:
669:
665:
661:
657:
656:P. aeruginosa
652:
644:
637:
635:
633:
628:
624:
620:
619:P. aeruginosa
616:
615:P. aeruginosa
612:
611:
607:
603:
591:
584:
579:
562:
556:
522:
519:
515:
514:
504:
501:
497:
496:
493:
490:
487:
483:
482:
478:
473:
469:
466:
462:
461:
457:
455:
450:
445:
441:
438:
437:
430:
428:
425:
424:
400:
397:
393:
392:
387:
378:
374:
367:
353:
343:
339:
332:
324:
320:
319:DTXSID9041108
316:
315:
313:
303:
299:
298:
291:
287:
286:
284:
282:
279:
278:
271:
267:
266:
264:
258:
254:
253:
249:
245:
243:
240:
239:
232:
228:
227:
225:
223:
220:
219:
212:
211:
209:
207:
202:
201:
197:
193:
190:
188:
186:ECHA InfoCard
183:
182:
175:
171:
170:
168:
166:
163:
162:
155:
154:ChEMBL2289232
151:
150:
148:
146:
143:
142:
135:
131:
130:
128:
126:
123:
122:
115:
111:
110:
108:
104:
99:
98:
91:
87:
86:
84:
81:
77:
76:
71:
66:
59:
53:
47:
43:
38:
34:
29:
25:
20:
1829:(1): 47–56.
1826:
1820:
1814:
1810:
1802:
1775:
1769:
1759:
1724:
1718:
1712:
1704:
1669:
1663:
1657:
1649:
1614:
1608:
1602:
1594:
1559:
1553:
1547:
1539:
1484:
1478:
1443:
1437:
1431:
1423:
1396:
1390:
1384:
1376:
1331:
1325:
1319:
1311:
1286:
1280:
1274:
1249:
1243:
1236:
1222:cite journal
1187:
1181:
1175:
1167:
1124:
1120:
1054:
1050:
1002:
996:
990:
982:
957:
951:
945:
910:
904:
894:
879:
852:
840:
836:
826:
825:
802:
781:
779:
734:
689:
679:
675:
671:
663:
659:
655:
653:
649:
638:Biosynthesis
618:
614:
608:
601:
600:
491:
453:
73:Identifiers
64:
61:Other names
51:
1885:Antibiotics
1658:Pseudomonas
754:lymphocytes
696:Glutathione
486:Signal word
439:Appearance
389:Properties
192:100.213.248
1895:Phenazines
1879:Categories
1322:pyocyanin"
871:References
860:Pyoverdine
787:neutrophil
766:superoxide
760:, such as
711:, such as
627:zwitterion
465:Pictograms
427:Molar mass
290:9OQM399341
165:ChemSpider
134:CHEBI:8653
101:3D model (
80:CAS Number
17:Pyocyanin
1529:ignored (
1519:cite book
1141:0959-440X
1127:: 26–33.
1071:0021-9193
884:Pyocyanin
746:apoptosis
709:cytokines
602:Pyocyanin
536:P301+P312
456:labelling
213:687-347-7
205:EC Number
1853:11207155
1794:11244086
1751:10560362
1641:12207823
1633:11823520
1368:14605211
1303:12446210
1266:12765878
1214:11591691
1159:25215885
1089:11591691
1029:10024562
974:15567330
847:See also
750:necrosis
741:V-ATPase
692:catalase
447:Hazards
1845:9989496
1742:1023078
1696:9826354
1586:8390405
1511:3118778
1470:8244935
1415:8349704
1336:Bibcode
1150:4268259
937:6243619
835:called
731:Targets
583:what is
581: (
432:210.236
257:PubChem
248:D011710
90:85-66-5
1900:Enones
1851:
1843:
1792:
1749:
1739:
1694:
1687:108730
1684:
1639:
1631:
1584:
1577:280930
1574:
1509:
1499:
1468:
1461:206923
1458:
1413:
1366:
1359:283589
1356:
1301:
1264:
1212:
1205:100142
1202:
1157:
1147:
1139:
1087:
1080:100142
1077:
1069:
1027:
1017:
972:
935:
928:293551
925:
578:verify
575:
492:Danger
442:Solid
373:SMILES
231:C01748
145:ChEMBL
40:Names
1849:S2CID
1637:S2CID
1178:PAO1"
1020:96448
989:"The
841:pgp-2
837:pgp-1
721:IFN-γ
717:IL-13
701:NADPH
338:InChI
125:ChEBI
103:JSmol
54:)-one
1841:PMID
1822:Cell
1790:PMID
1747:PMID
1692:PMID
1629:PMID
1582:PMID
1531:help
1507:PMID
1497:ISBN
1466:PMID
1411:PMID
1364:PMID
1327:PNAS
1299:PMID
1262:PMID
1228:link
1210:PMID
1155:PMID
1137:ISSN
1085:PMID
1067:ISSN
1025:PMID
970:PMID
933:PMID
839:and
811:CFTR
799:CCL5
797:and
795:IL-8
764:and
748:and
719:and
713:IL-4
676:phzS
674:and
672:phzS
662:and
552:P501
548:P330
544:P310
532:P280
528:P270
524:P264
510:H318
506:H302
281:UNII
270:6817
242:MeSH
222:KEGG
174:6558
1831:doi
1780:doi
1776:276
1737:PMC
1729:doi
1682:PMC
1674:doi
1619:doi
1615:168
1572:PMC
1564:doi
1489:doi
1456:PMC
1448:doi
1444:175
1401:doi
1397:268
1354:PMC
1344:doi
1332:100
1291:doi
1254:doi
1250:285
1200:PMC
1192:doi
1188:183
1145:PMC
1129:doi
1075:PMC
1059:doi
1055:183
1015:PMC
1007:doi
962:doi
923:PMC
915:doi
911:141
886:at
807:ATP
680:aro
454:GHS
307:EPA
260:CID
1881::
1847:.
1839:.
1827:96
1825:.
1819:.
1788:.
1774:.
1768:.
1745:.
1735:.
1725:52
1723:.
1717:.
1690:.
1680:.
1670:66
1668:.
1662:.
1635:.
1627:.
1613:.
1607:.
1580:.
1570:.
1560:61
1558:.
1552:.
1523::
1521:}}
1517:{{
1505:.
1495:.
1464:.
1454:.
1442:.
1436:.
1409:.
1395:.
1389:.
1362:.
1352:.
1342:.
1330:.
1324:.
1297:.
1287:33
1285:.
1260:.
1248:.
1224:}}
1220:{{
1208:.
1198:.
1186:.
1180:.
1153:.
1143:.
1135:.
1125:29
1123:.
1119:.
1097:^
1083:.
1073:.
1065:.
1053:.
1049:.
1037:^
1023:.
1013:.
1003:67
1001:.
995:.
968:.
958:10
956:.
931:.
921:.
909:.
903:.
715:,
634:.
550:,
546:,
542:,
538:,
534:,
530:,
526:,
508:,
458::
411:10
405:13
1855:.
1833::
1813:-
1796:.
1782::
1753:.
1731::
1711:"
1698:.
1676::
1656:"
1643:.
1621::
1588:.
1566::
1533:)
1513:.
1491::
1472:.
1450::
1417:.
1403::
1387:"
1370:.
1346::
1338::
1305:.
1293::
1268:.
1256::
1230:)
1216:.
1194::
1161:.
1131::
1091:.
1061::
1031:.
1009::
976:.
964::
939:.
917::
770:1
573:Y
420:O
417:2
414:N
408:H
402:C
309:)
305:(
105:)
65:H
52:H
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.