1847:
1601:
2111:
1299:
2940:
2872:
2835:
2783:
2745:
2707:
2669:
2631:
2470:
2379:
1366:
407:
276:
1344:
72:
42:
736:
82:
1505:
52:
1960:
1259:
1436:
732:
1706:
1929:) agents. Halogenation generally provides polyhalogenated pyrroles, but monohalogenation can be performed. As is typical for electrophilic additions to pyrroles, halogenation generally occurs at the 2-position, but can also occur at the 3-position by silation of the nitrogen. This is a useful method for further functionalization of the generally less reactive 3-position.
735:
859:
737:
1768:-serine into ring B (figure 3). Ring A fragment is transferred from the peptidyl carrier protein (PCP) to the Acyl Carrier Protein (ACP) by a KS domain, followed by transfer to malonyl-ACP via decarboxylative Claisen condensation. This fragment is then able to react with the masked carbanion formed from the PLP mediated decarboxylation of
1728:
1156:
1551:, where R is an electron-withdrawing group, and R is an alkane, aryl group, or ester. Examples of disubstituted alkynes have also been seen to form the desired pyrrole in considerable yield. The reaction is proposed to proceed via a silver
2079:
reactions such as -, -, and -cyclizations. Diels-Alder cyclizations can occur with the pyrrole acting as a diene, especially in the presence of an electron-withdrawing group on the nitrogen. Vinylpyrroles can also act as dienes.
2008:-Metalated pyrrole can react with electrophiles at the N or C positions, depending on the coordinating metal. More ionic nitrogen–metal bonds (such as with lithium, sodium, and potassium) and more solvating solvents lead to
753:
1403:
1566:
3323:
Balón, M.; Carmona, M. C.; Muñoz, M. A.; Hidalgo, J. (1989). "The acid-base properties of pyrrole and its benzologs indole and carbazole: a re-examination from the excess acidity method".
1645:
1536:
1759:
1026:
N) is formed by protonation at the 2 position. Substitution of pyrrole with alkyl substituents provides a more basic molecule—for example, tetramethylpyrrole has a conjugate acid p
872:
1781:
1317:
The Knorr pyrrole synthesis involves the reaction of an α-amino ketone or an α-amino-β-ketoester with an activated methylene compound. The method involves the reaction of an α-
1772:-serine, which cyclizes in a dehydration reaction to yield the second pyrrole ring. This intermediate is then modified by methylation (which incorporates a methyl group from
1754:-proline through the nonribosomal peptide synthase (NRPS) pathway (figure 2), wherein the pyrrolidine ring of proline is oxidized twice through FAD to yield pyrrole ring A.
2499:
2248:
2020:
41:
3410:
738:
1210:
include PQQ, makaluvamine M, ryanodine, rhazinilam, lamellarin, prodigiosin, myrmicarin, and sceptrin. The syntheses of pyrrole-containing haemin, synthesized by
2012:-alkylation. Nitrophilic metals, such as MgX, lead to alkylation at C (mainly C2), due to a higher degree of coordination to the nitrogen atom. In the cases of
746:
453:
3621:
1600:
1565:
2084:
2016:-substituted pyrroles, metalation of the carbons is more facile. Alkyl groups can be introduced as electrophiles, or by cross-coupling reactions.
1846:
1402:
1843:
Pyrroles generally react with electrophiles at the α position (C2 or C5), due to the highest degree of stability of the protonated intermediate.
760:
2110:
1644:
71:
1679:
687:
1535:
1003:. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of the heteroatom, with a dipole moment of 1.58
3559:
3363:
3315:
3219:
3122:
Walsh, C. T.; Garneau-Tsodikova, S.; Howard-Jones, A. R. (2006). "Biological formation of pyrroles: Nature's logic and enzymatic machinery".
2422:
2326:
2297:
2272:
2232:
1298:
660:
1776:-methionine onto the alcohol at the 6 position) and oxidation of the primary alcohol to the aldehyde to yield the core A–B ring structures.
3503:
Kaur, Matinder; Choi, Dong Hoon (2015). "Diketopyrrolopyrrole: brilliant red pigment dye-based fluorescent probes and their applications".
81:
2063:
of pyrrole esters and amides produced pyrrolines, with the regioselectivity depending on the position of the electron-withdrawing group.
1397:
cyclization then forms the 5-membered ring, which reacts to eliminate the tosyl group. The last step is tautomerization to the pyrrole.
1365:
925:. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g.,
2516:
1556:
1343:
1362:
In the Paal–Knorr pyrrole synthesis, a 1,4-dicarbonyl compound reacts with ammonia or a primary amine to form a substituted pyrrole.
3394:
3105:
2896:
1735:
The biosynthesis of
Prodigiosin involves the convergent coupling of three pyrrole type rings (labeled A, B, and C in figure 1) from
1687:
1447:
1011:, it has chemical shifts at 6.68 (H2, H5) and 6.22 (H3, H4). Pyrrole is an extremely weak base for an amine, with a conjugate acid p
421:
1758:
1357:
2533:
2131:-methylpyrrolecarboxylic acid, a building-block in pharmaceutical chemistry. Pyrroles are also found in several drugs, including
1462:. The product is a pyrrole with substituents at the 3 and 4 positions. The aldehyde reacts with the diamine to an intermediate di-
1959:
1878:
51:
2540:
2796:
Corwin, Alsoph Henry (1950). "Chapter 6: The
Chemistry of Pyrrole and its Derivatives". In Elderfield, Robert Cooley (ed.).
1504:
1683:
1953:
1831:
reagents that are used in benzene chemistry are not applicable to pyrroles. In contrast, substituted pyrroles (including
1780:
879:
354:
1258:
385:
1949:
2256:
2019:
1973:
1695:
1278:
1143:, "reddish, fiery"), from the reaction used to detect it—the red color that it imparts to wood when moistened with
577:
559:
2564:
Lubell, W.; Saint-Cyr, D.; Dufour-Gallant, J.; Hopewell, R.; Boutard, N.; Kassem, T.; Dörr, A.; Zelli, R. (2013).
1413:
3061:; Howard-Jones, Annaleise R. (2006). "Biological formation of pyrroles: Nature's logic and enzymatic machinery".
2565:
1521:
1467:
1269:
Several syntheses of the pyrrole ring have been described. Three routes dominate, but many other methods exist.
1122:
283:
3058:
1699:
1671:
783:
2645:
3486:"DPP Pigments,Diketopyrrolopyrrole Pigments,DPP Pigments Wholesaler,Diketopyrrolopyrrole Pigments Suppliers"
2351:
1626:
1312:
1164:
607:
2984:
Milgram, Benjamin C.; Eskildsen, Katrine; Richter, Steven M.; Scheidt, W. Robert; Scheidt, Karl A. (2007).
2939:
2871:
2834:
2782:
2744:
2706:
2668:
2630:
2469:
2378:
1948:
can occur with or without a catalyst. 2-Acylpyrroles are also obtained from reaction with nitriles, by the
271:
3038:
2919:[Synthesis of pyrrole derivatives: pyrrole from diethyl succinyl succinate, pyrrole from azines].
1479:
1443:
1382:
984:
3616:
3546:. The Chemistry of Heterocyclic Compounds. Vol. 48. Chichester: John Wiley & Sons. p. 351.
2412:
1724:
Proline can be used as precursor of aromatic pyrroles in secondary natural products, as in prodigiosins.
3589:
Jolicoeur, Benoit; Chapman, Erin E.; Thompson, Alison; Lubell, William D. (2006). "Pyrrole protection".
3305:
2849:
2812:
2607:
233:
2916:
2759:
2438:
3425:
2683:
1113:
are, respectively, 152, 88, 121, and 67 kJ/mol (36, 21, 29, and 16 kcal/mol). The molecule is flat.
2721:
2450:
2359:
2188:
1078:
101:
1889:
1614:
1498:
1378:
402:
140:
1086:
3569:
3404:
2040:
1893:
1675:
1163:
Pyrrole itself is not naturally occurring, but many of its derivatives are found in a variety of
193:
181:
3250:
Bailey, Denis M.; Johnson, Robert E.; Albertson, Noel F. (1971). "Ethyl
Pyrrole-2-Carboxylate".
2953:
Robinson, Gertrude Maud; Robinson, Robert (1918). "LIV. A new synthesis of tetraphenylpyrrole".
174:
3277:
Silverstein, Robert M.; Ryskiewicz, Edward E.; Willard, Constance (1956). "2-Pyrrolealdehyde".
3636:
3555:
3520:
3390:
3359:
3311:
3215:
3188:
3139:
3101:
3078:
3019:
2892:
2512:
2418:
2322:
2293:
2268:
2228:
2151:
1919:
1904:
1471:
1144:
806:
3598:
3547:
3512:
3437:
3332:
3286:
3259:
3178:
3170:
3131:
3070:
3009:
3001:
2963:
2928:
2917:"Synthese von Pyrrolderivaten: Pyrrole aus Succinylobernsteinsäureester, Pyrrole aus Azinen"
2861:
2824:
2771:
2733:
2695:
2657:
2619:
2534:"The Chemical Constituents in Cigarettes and Cigarette Smoke: Priorities for Harm Reduction"
2504:
2458:
2367:
2260:
2104:
1915:
1866:
1832:
1494:
1390:
1168:
1102:
911:
907:
903:
481:
1804:
1529:
948:, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as
363:
3581:
2100:
2096:
2083:
2060:
1630:
1578:
1574:
1559:
1486:
1329:
1203:
945:
796:
302:
253:
3544:
Pyrroles. Part I. The
Synthesis and the Physical and Chemical Aspects of the Pyrrole Ring
3485:
1130:
150:
2852:[Synthesis of furan derivatives from the ester of 2,3-diacetyl-succinic acid],
2454:
2363:
406:
275:
213:
3183:
3158:
3014:
2985:
1987:
1941:
1940:
generally occurs at the 2-position, through the use of various methods. Acylation with
1525:
1333:
850:
598:
549:
3336:
3630:
2993:
2955:
2315:
2076:
1945:
956:
641:
539:
529:
264:
1435:
812:
2912:
2813:"Ueber die Derivate des Acetophenonacetessigesters und des Acetonylacetessigesters"
2193:
2132:
2052:
1991:
1983:
1828:
1682:(which requires NADH or NADPH). This can then either spontaneously cyclize to form
1659:
1622:
1586:
1451:
1217:
Pyrrole is a constituent of tobacco smoke and may contribute to its toxic effects.
1211:
1004:
988:
895:
2288:
Loudon, Marc G. (2002). "Chemistry of
Naphthalene and the Aromatic Heterocycles".
1513:
45:
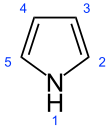
Explicit structural formula of pyrrole, with aromaticity indicated by dashed bonds
3159:"Structure, Chemical Synthesis, and Biosynthesis of Prodiginine Natural Products"
2886:
2340:
2338:
2253:
Nomenclature of
Organic Chemistry: IUPAC Recommendations and Preferred Names 2013
327:
3428:[On the reaction of chloroform with the potassium compound of pyrrole].
3174:
2408:
2198:
2164:
1995:
1859:
1824:
1819:, in that it is easy to alkylate and acylate. Under acidic conditions, pyrroles
1796:
1792:
1638:
1548:
1425:
1416:, an isocyanoacetate reacts with a nitroalkene in a 1,4-addition, followed by 5-
1195:
1172:
972:
899:
772:
1731:
Figure 1: Structure of
Prodigiosin 1 highlighting the A, B, and C pyrrole rings
1512:
Pyrroles bearing multiple substituents have been obtained from the reaction of
3602:
3441:
3380:
2865:
2828:
2623:
2056:
1709:
1582:
1207:
1187:
1183:
506:
244:
3551:
3290:
3263:
2932:
2775:
2737:
2699:
2661:
2508:
2462:
987:
liquid that darkens readily upon exposure to air, and is usually purified by
2986:"Microwave-Assisted Piloty–Robinson Synthesis of 3,4-Disubstituted Pyrroles"
2158:
2140:
2136:
1937:
1874:
1705:
1691:
1667:
1552:
1490:
1459:
1179:
1106:
1094:
1070:
1045:
1000:
960:
829:
625:
374:
3524:
3192:
3143:
3082:
3023:
2103:, a dichlorocyclopropane intermediate is formed, which breaks down to form
1764:
Ring A is then expanded via the polyketide synthase pathway to incorporate
1727:
1442:
The starting materials in the Piloty–Robinson pyrrole synthesis, named for
2264:
991:
immediately before use. Pyrrole has a nutty odor. Pyrrole is a 5-membered
3356:
Industrial
Chemistry of the Aromatics: Raw materials, processes, products
2967:
2762:[On the formation of pyrrole derivatives from isonitrosketones].
1590:
1455:
1429:
1234:
1126:
1074:
1066:
992:
759:
752:
745:
718:
3005:
2035:-substituted 3-bromopyrrole, which can be synthesized by bromination of
1625:. ALA dehydratase catalyzes the condensation of two ALA molecules via a
1266:
Pyrrole can also be formed by catalytic dehydrogenation of pyrrolidine.
17:
3622:
Substitution reaction mechanisms of nitrogen-containing heteroaromatics
3516:
3426:"Ueber die Einwirkung des Chloroforms auf die Kaliumverbindung Pyrrols"
3206:
Wang Jitao ; Zhang
Baoshen ; Wang Yongmei ; Hu Qingmei , eds. (2003).
2292:(4th ed.). New York: Oxford University Press. pp. 1135–1136.
2203:
2176:
2092:
1897:
1885:
1855:
1820:
1816:
1812:
1655:
1618:
1594:
1475:
1245:
1230:
1199:
1090:
968:
841:
837:
519:
314:
284:
2143:. Pyrroles are used as lightfast red, scarlet, and carmine pigments.
3135:
3074:
2724:[Synthesis experiments with the ester of acetoacetic acid].
2371:
2172:
1808:
1544:
1517:
1321:
1238:
833:
224:
1033:
of +3.7. Pyrrole is also weakly acidic at the N–H position, with a p
849:
Except where otherwise noted, data are given for materials in their
2532:
Fowles, Jefferson; Bates, Michael; Noiton, Dominique (March 2000).
2346:
955:
Pyrroles are components of more complex macrocycles, including the
2850:"Synthese von Furfuranderivaten aus dem Diacetbernsteinsäureester"
2347:"The aromatic pathways of porphins, chlorins and bacteriochlorins"
1800:
1726:
1704:
1463:
1386:
1318:
1226:
1171:. Common naturally produced molecules containing pyrroles include
1155:
1154:
1110:
1098:
996:
338:
204:
180:
173:
163:
1089:). In terms of its aromaticity, pyrrole's is modest relative to
3096:
Lehninger, Albert L.; Nelson, David L.; Cox, Michael M. (2000).
2414:
Advanced
Organic Chemistry: Reactions, Mechanisms, and Structure
1634:
1283:
The Hantzsch pyrrole synthesis is the reaction of β-ketoesters (
1191:
1041:
1040:
of 16.5. As a hydrogen bonding Lewis acid it is classified as a
964:
949:
3350:
Franck, Heinz-Gerhard; Stadelhofer, Jürgen Walter (1987).
1982:
of 17.5. Pyrrole can be deprotonated with strong bases such as
1633:(PBG). This later reacts to form, for example, the macrocycles
1532:
process. Similar reactions can be performed using azalactones.
3455:
Corwin, Alsoph Henry (1950). Elderfield, Robert Cooley (ed.).
3214:] (in Chinese) (2nd ed.). Tianjin Nankai University.
1835:
pyrroles) have been used in a broad range of transformations.
1018:
of −3.8. The most thermodynamically stable pyrrolium cation (C
2760:"Ueber die Bildung von Pyrrolderivaten aus Isonitrosoketonen"
2313:
Cox, Michael; Lehninger, Albert L.; Nelson, David R. (2000).
1138:
2109:
2082:
2018:
1994:. Treating this conjugate base with an electrophile such as
1958:
1845:
1643:
1599:
1564:
1534:
1503:
1434:
1401:
1364:
1342:
1297:
1257:
390:
80:
70:
50:
40:
3352:
Industrielle Aromatenchemie: Rohstoffe, Verfahren, Produkte
2474:
See especially pages 67–68, where Runge names the compound
544:
129 to 131 °C (264 to 268 °F; 402 to 404 K)
2417:(6th ed.), New York: Wiley-Interscience, p. 62,
1206:, and porphyrinogens. Other pyrrole-containing secondary
730:
3304:
Bird, C. W.; Cheeseman, G. W. H. (1984).
2227:(97th ed.). Boca Raton: CRC Press. pp. 3–478.
3470:
Mosher, H. S. (1950). Elderfield, Robert Cooley (ed.).
3358:] (in German). Berlin: Springer. pp. 403–404.
2392:
Armarego, Wilfred L. F.; Chai, Christina L. L. (2003).
867:
2610:[New methods of forming pyrrole derivatives].
2031:
Substitution at C3 can be achieved through the use of
1972:
The NH proton in pyrroles is moderately acidic with a
1287:) with ammonia (or primary amines) and α-haloketones (
1406:
Mechanism of the Van Leusen reaction to form pyrroles
2595:(3rd ed.). Liverpool: Longman. p. 194-196.
2648:[Studies in the furan and pyrrole groups].
2439:"Ueber einige Produkte der Steinkohlendestillation"
2161:, a partially saturated analog with one double bond
3617:Synthesis of pyrroles (overview of recent methods)
2314:
2441:[On some products of coal distillation].
2249:International Union of Pure and Applied Chemistry
1539:Synthesis of pyrroles via Diels–Alder cyclization
1225:Pyrrole is prepared industrially by treatment of
3474:. Vol. 1. New York, NY: Wiley. p. 475.
3459:. Vol. 1. New York, NY: Wiley. p. 309.
2800:. Vol. 1. New York, NY: Wiley. p. 287.
1811:reactions. Its reactivity is similar to that of
326:
1569:Synthesis of pyrrole via silver click chemistry
734:
149:
3430:Berichte der Deutschen Chemischen Gesellschaft
3100:(3rd ed.). New York: W. H. Freeman.
2921:Berichte der Deutschen Chemischen Gesellschaft
2854:Berichte der Deutschen Chemischen Gesellschaft
2817:Berichte der Deutschen Chemischen Gesellschaft
2764:Berichte der Deutschen Chemischen Gesellschaft
2722:"Synthetische Versuche mit dem Acetessigester"
2688:Berichte der Deutschen Chemischen Gesellschaft
2650:Berichte der Deutschen Chemischen Gesellschaft
2612:Berichte der Deutschen Chemischen Gesellschaft
2500:Ullmann's Encyclopedia of Industrial Chemistry
1613:The biosynthesis of pyrrole rings begins with
1555:intermediate. This method is analogous to the
777:33.33 °C (91.99 °F; 306.48 K)
8:
3409:: CS1 maint: multiple names: authors list (
2686:[Synthesis of pyrrole derivatives].
1648:Mechanism of biosynthesis of porphobilinogen
1573:One synthetic route to pyrrole involves the
75:Ball-and-stick model of the pyrrole molecule
85:Space-filling model of the pyrrole molecule
3331:(23). Great Britain: Pergamon: 7501–7504.
2979:
2977:
2123:Polypyrrole is of some commercial value.
959:and products derived therefrom, including
405:
274:
252:
29:
3182:
3013:
2646:"Studien in der Furan- und Pyrrol-Gruppe"
2559:
2557:
2492:
2490:
2488:
2107:(the Ciamician–Dennstedt rearrangement).
1604:Synthesis of pyrrole from ammonium mucate
362:
3424:Ciamician, G. L.; Dennstedt, M. (1881).
3389:] (in Ukrainian). pp. 781–782.
3340:suggests that figure's revision to 17.3.
2888:Heterocyclic Chemistry in Drug Discovery
2608:"Neue Bildungsweise von Pyrrolderivaten"
2345:Jusélius, Jonas; Sundholm, Dage (2000).
1712:structure of both proline enantiomers: (
940:
936:
932:
920:
916:
791:550 °C (1,022 °F; 823 K)
2215:
1952:. Pyrrole aldehydes can be formed by a
1747:-methionine, pyruvate, and 2-octenal.
1547:-catalyzed cyclization of alkynes with
1093:but comparable to related heterocycles
458:
426:
401:
3577:
3567:
3402:
2396:(5th ed.). Elsevier. p. 346.
1963:Vilsmeier–Haack formylation of pyrrole
1839:Reaction of pyrrole with electrophiles
1807:reactions, and does not undergo usual
1690:(using NADH or NADPH), or turned into
1680:glutamate-5-semialdehyde dehydrogenase
1466:(R−C=N−N=C−R). In the second step, a -
1332:α to (bonded to the next carbon to) a
265:
2497:Harreus, Albrecht Ludwig. "Pyrrole".
2225:CRC Handbook of Chemistry and Physics
1497:at high temperatures and assisted by
1381:pyrroles are produced by reaction of
534:−23 °C (−9 °F; 250 K)
433:Key: KAESVJOAVNADME-UHFFFAOYSA-N
232:
212:
7:
3307:Comprehensive Heterocyclic Chemistry
2394:Purification of Laboratory Chemicals
2317:Lehninger Principles of Biochemistry
1990:. The resulting alkali pyrrolide is
1585:. The salt is typically heated in a
1129:. In 1857, it was isolated from the
1085: + 2 aromatic system (see
55:Numbered skeletal formula of pyrrole
2167:, the saturated hydrogenated analog
2051:Pyrroles can undergo reductions to
1214:was recognized by the Nobel Prize.
317:
1850:Pyrrole electrophilic substitution
1520:. The reaction mechanism involves
1474:leads to ring closure and loss of
1077:on the nitrogen atom is partially
25:
2127:-Methylpyrrole is a precursor to
2114:Ciamician–Dennstedt rearrangement
1688:pyrroline-5-carboxylate reductase
1686:, which is reduced to proline by
1617:(ALA), which is synthesized from
1543:Pyrroles can also be prepared by
1470:takes place between. Addition of
430:InChI=1S/C4H5N/c1-2-4-5-3-1/h1-5H
2938:
2870:
2833:
2781:
2743:
2705:
2667:
2629:
2468:
2377:
1968:Reaction of deprotonated pyrrole
1779:
1757:
1482:was developed by the Robinsons.
1424:cyclization, elimination of the
1369:The Paal–Knorr pyrrole synthesis
1291:) to give substituted pyrroles (
1133:. Its name comes from the Greek
857:
499:
493:
440:InChI=1/C4H5N/c1-2-4-5-3-1/h1-5H
2171:Derivatives of pyrrole include
1262:Synthesis of pyrrole from furan
853:(at 25 °C , 100 kPa).
2684:"Synthese von Pyrrolderivaten"
2541:New Zealand Ministry of Health
2321:. New York: Worth Publishers.
2257:The Royal Society of Chemistry
1389:in the presence of base, in a
1328:) and a compound containing a
1302:The Hantzsch pyrrole synthesis
1233:in the presence of solid acid
1121:Pyrrole was first detected by
979:Properties, structure, bonding
487:
27:Organic ring compound (C₄H₄NH)
1:
3542:Jones, R. Jones, ed. (1990).
3490:dyes-pigments.standardcon.com
3379:Lyastukhin, Voronov (2006).
3337:10.1016/S0040-4020(01)89212-7
2758:Knorr, L.; Lange, H. (1902).
2591:Gilchrist, Thomas L. (1997).
2443:Annalen der Physik und Chemie
1799:, does not easily react as a
1698:, followed by cyclisation by
1684:1-pyrroline-5-carboxylic acid
1125:in 1834, as a constituent of
1048:lists its acid parameters as
681:108.2 kJ mol (gas)
654:1.903 J K mol
3310:. Pergamon. pp. 39–88.
2175:, a derivative with a fused
2099:, in a -cycloaddition. With
1662:derived from the amino acid
1454:, are two equivalents of an
1358:Paal–Knorr pyrrole synthesis
1352:Paal–Knorr pyrrole synthesis
3175:10.1021/acs.chemrev.6b00024
3040:Practical Organic Chemistry
1854:Pyrroles react easily with
1750:Ring A is synthesized from
1347:The Knorr pyrrole synthesis
1081:into the ring, creating a 4
66:
36:
3653:
3098:Principles of Biochemistry
2223:William M. Haynes (2016).
2075:-substitution can undergo
1795:, pyrrole is difficult to
1696:ornithine aminotransferase
1355:
1310:
1279:Hantzsch pyrrole synthesis
1276:
1273:Hantzsch pyrrole synthesis
1139:
572:17.5 (for the N−H proton)
554:7 mmHg at 23 °C
3603:10.1016/j.tet.2006.08.071
3442:10.1002/cber.188101401240
3059:Garneau-Tsodikova, Sylvie
2866:10.1002/cber.188401702254
2829:10.1002/cber.188401702228
2624:10.1002/cber.189002301243
1522:1,3-dipolar cycloaddition
1478:to form the pyrrole. The
1468:sigmatropic rearrangement
847:
818:
712:
634:
474:
449:
417:
133:
116:
100:
95:
65:
35:
3552:10.1002/recl.19911100712
3505:Chemical Society Reviews
3381:
3291:10.15227/orgsyn.036.0074
3264:10.15227/orgsyn.051.0100
3207:
2933:10.1002/cber.19100430182
2776:10.1002/cber.19020350392
2738:10.1002/jlac.18862360303
2700:10.1002/cber.18840170220
2662:10.1002/cber.19020350263
2570:-Pyrroles (Update 2013)"
2539:. Porirua, New Zealand:
2509:10.1002/14356007.a22_453
2463:10.1002/andp.18341070502
2091:Pyrroles can react with
1954:Vilsmeier–Haack reaction
1787:Reactions and reactivity
1700:ornithine cyclodeaminase
1672:Glutamate-5-semialdehyde
1508:Piloty–Robinson reaction
971:, bacteriochlorins, and
630:0.001225 Pa s
3063:Natural Product Reports
3057:Walsh, Christopher T.;
2503:. Weinheim: Wiley-VCH.
2352:Phys. Chem. Chem. Phys.
2147:Analogs and derivatives
1629:ring synthesis to form
1581:, the ammonium salt of
1313:Knorr pyrrole synthesis
1307:Knorr pyrrole synthesis
983:Pyrrole is a colorless
608:Magnetic susceptibility
3472:Heterocyclic Compounds
3457:Heterocyclic Compounds
3157:Hu, Dennis X. (2016).
2848:Knorr, Ludwig (1884),
2798:Heterocyclic Compounds
2682:Knorr, Ludwig (1884).
2593:Heterocyclic Chemistry
2115:
2088:
2028:
1964:
1950:Houben–Hoesch reaction
1851:
1732:
1721:
1716:)-proline (left) and (
1649:
1605:
1570:
1540:
1509:
1489:is treated first with
1439:
1407:
1383:tosylmethyl isocyanide
1370:
1348:
1303:
1263:
1160:
1069:character because the
741:
708:2242 kJ mol
86:
76:
56:
46:
3321:, although note that
2885:Li, Jie Jack (2013).
2644:Feist, Franz (1902).
2606:Hantzsch, A. (1890).
2437:Runge, F. F. (1834).
2265:10.1039/9781849733069
2189:Simple aromatic rings
2113:
2086:
2067:Cyclization reactions
2022:
1962:
1849:
1730:
1708:
1647:
1603:
1568:
1562:used to form azoles.
1538:
1507:
1499:microwave irradiation
1485:In one modification,
1438:
1414:Barton–Zard synthesis
1405:
1368:
1346:
1301:
1261:
1178:, bile pigments like
1158:
1105:of benzene, pyrrole,
740:
524:0.967 g cm
84:
74:
54:
44:
3239:(in German). Thieme.
3237:Römpp Lexikon Chemie
2968:10.1039/CT9181300639
2574:Science of Synthesis
2543:. pp. 20, 49–65
2154:of pyrrole include:
1678:(ATP-dependent) and
1524:followed by loss of
1151:Occurrence in nature
813:Chemical Safety Data
723:(fire diamond)
102:Preferred IUPAC name
3597:(50): 11531–11563.
2891:. New York: Wiley.
2455:1834AnP...107...65R
2407:Smith, Michael B.;
2364:2000PCCP....2.2145J
2039:-silylpyrrole with
1674:is first formed by
1615:aminolevulinic acid
1379:Van Leusen reaction
1159:Structure of Heme B
619:10 cm mol
514: g·mol
194:Beilstein Reference
32:
3517:10.1039/C4CS00248B
2726:Annalen der Chemie
2720:Knorr, L. (1886).
2152:Structural analogs
2116:
2089:
2029:
1965:
1852:
1793:aromatic character
1733:
1722:
1676:glutamate 5-kinase
1650:
1606:
1571:
1541:
1510:
1440:
1408:
1371:
1349:
1304:
1264:
1161:
1131:pyrolysate of bone
1103:resonance energies
995:heterocycle, like
906:, a five-membered
880:Infobox references
819:Related compounds
742:
87:
77:
57:
47:
30:
3561:978-0-471-62753-1
3387:Organic Chemistry
3365:978-3-662-07876-1
3317:978-0-08-096519-2
3279:Organic Syntheses
3252:Organic Syntheses
3221:978-7-310-00620-5
3212:Organic Chemistry
3169:(14): 7818–7853.
3006:10.1021/jo070389+
3000:(10): 3941–3944.
2811:Paal, C. (1884),
2424:978-0-471-72091-1
2358:(10): 2145–2151.
2328:978-1-57259-153-0
2299:978-0-19-511999-2
2290:Organic Chemistry
2274:978-0-85404-182-4
2234:978-1-4987-5429-3
2002:-methylpyrrole.
1775:
1771:
1767:
1753:
1746:
1742:
1738:
1702:to form proline.
1665:
1472:hydrochloric acid
1385:(TosMIC) with an
1145:hydrochloric acid
888:Chemical compound
886:
885:
825:Related compounds
807:Safety data sheet
386:CompTox Dashboard
182:Interactive image
175:Interactive image
91:
90:
61:
60:
16:(Redirected from
3644:
3606:
3585:
3579:
3575:
3573:
3565:
3529:
3528:
3500:
3494:
3493:
3482:
3476:
3475:
3467:
3461:
3460:
3452:
3446:
3445:
3421:
3415:
3414:
3408:
3400:
3376:
3370:
3369:
3347:
3341:
3339:
3320:
3301:
3295:
3294:
3274:
3268:
3267:
3247:
3241:
3240:
3232:
3226:
3225:
3203:
3197:
3196:
3186:
3163:Chemical Reviews
3154:
3148:
3147:
3136:10.1039/b605245m
3119:
3113:
3111:
3093:
3087:
3086:
3075:10.1039/b605245m
3054:
3048:
3047:
3045:
3034:
3028:
3027:
3017:
2990:
2981:
2972:
2971:
2950:
2944:
2943:
2942:
2936:
2909:
2903:
2902:
2882:
2876:
2875:
2874:
2868:
2860:(2): 2863–2870,
2845:
2839:
2838:
2837:
2831:
2823:(2): 2756–2767,
2808:
2802:
2801:
2793:
2787:
2786:
2785:
2779:
2770:(3): 2998–3008.
2755:
2749:
2748:
2747:
2741:
2717:
2711:
2710:
2709:
2703:
2694:(2): 1635–1642.
2679:
2673:
2672:
2671:
2665:
2656:(2): 1537–1544.
2641:
2635:
2634:
2633:
2627:
2603:
2597:
2596:
2588:
2582:
2581:
2561:
2552:
2551:
2549:
2548:
2538:
2529:
2523:
2522:
2494:
2483:
2473:
2472:
2466:
2434:
2428:
2427:
2404:
2398:
2397:
2389:
2383:
2382:
2381:
2375:
2372:10.1039/b000260g
2342:
2333:
2332:
2320:
2310:
2304:
2303:
2285:
2279:
2278:
2245:
2239:
2238:
2220:
2105:3-chloropyridine
1827:, and thus many
1783:
1773:
1769:
1765:
1761:
1751:
1744:
1740:
1736:
1663:
1660:biosynthetically
1495:benzoyl chloride
1391:Michael addition
1204:bacteriochlorins
1169:natural products
1142:
1141:
1123:F. F. Runge
943:
929:-methylpyrrole,
924:
904:organic compound
870:
864:
861:
860:
797:Explosive limits
762:
755:
748:
733:
704:
677:
650:
635:Thermochemistry
620:
618:
513:
501:
495:
489:
482:Chemical formula
410:
409:
394:
392:
366:
330:
319:
303:Gmelin Reference
286:
278:
267:
256:
236:
216:
184:
177:
153:
67:
37:
33:
21:
3652:
3651:
3647:
3646:
3645:
3643:
3642:
3641:
3627:
3626:
3613:
3588:
3576:
3566:
3562:
3541:
3538:
3536:Further reading
3533:
3532:
3502:
3501:
3497:
3484:
3483:
3479:
3469:
3468:
3464:
3454:
3453:
3449:
3423:
3422:
3418:
3401:
3397:
3383:
3382:Органічна хімія
3378:
3377:
3373:
3366:
3349:
3348:
3344:
3322:
3318:
3303:
3302:
3298:
3276:
3275:
3271:
3249:
3248:
3244:
3234:
3233:
3229:
3222:
3209:
3205:
3204:
3200:
3156:
3155:
3151:
3121:
3120:
3116:
3108:
3095:
3094:
3090:
3056:
3055:
3051:
3043:
3036:
3035:
3031:
2988:
2983:
2982:
2975:
2952:
2951:
2947:
2937:
2911:
2910:
2906:
2899:
2884:
2883:
2879:
2869:
2847:
2846:
2842:
2832:
2810:
2809:
2805:
2795:
2794:
2790:
2780:
2757:
2756:
2752:
2742:
2719:
2718:
2714:
2704:
2681:
2680:
2676:
2666:
2643:
2642:
2638:
2628:
2605:
2604:
2600:
2590:
2589:
2585:
2563:
2562:
2555:
2546:
2544:
2536:
2531:
2530:
2526:
2519:
2496:
2495:
2486:
2467:
2436:
2435:
2431:
2425:
2406:
2405:
2401:
2391:
2390:
2386:
2376:
2344:
2343:
2336:
2329:
2312:
2311:
2307:
2300:
2287:
2286:
2282:
2275:
2259:. p. 141.
2247:
2246:
2242:
2235:
2222:
2221:
2217:
2212:
2185:
2149:
2121:
2119:Commercial uses
2101:dichlorocarbene
2097:dichlorocarbene
2069:
2061:Birch reduction
2059:. For example,
2049:
1980:
1970:
1942:acid anhydrides
1935:
1927:
1923:
1912:
1908:
1901:
1882:
1870:
1863:
1841:
1789:
1631:porphobilinogen
1611:
1579:ammonium mucate
1575:decarboxylation
1560:click chemistry
1487:propionaldehyde
1448:Robert Robinson
1430:tautomerization
1376:
1360:
1354:
1330:methylene group
1315:
1309:
1281:
1275:
1253:
1249:
1242:
1223:
1176:
1153:
1119:
1061:
1054:
1039:
1032:
1025:
1021:
1017:
1010:
981:
946:Porphobilinogen
942:
938:
934:
930:
922:
918:
914:
889:
882:
877:
876:
875: ?)
866:
862:
858:
854:
826:
788:
785:
767:
766:
765:
764:
757:
750:
743:
739:
731:
705:
702:
696:
692:
689:
688:Std enthalpy of
678:
675:
669:
665:
662:
661:Std enthalpy of
651:
644:
616:
614:
611:
596:
586:
568:
511:
498:
492:
484:
470:
467:
462:
457:
456:
445:
442:
441:
435:
434:
431:
425:
424:
413:
395:
388:
369:
349:
333:
320:
305:
296:
259:
239:
219:
196:
187:
167:
156:
143:
129:
128:
112:
111:
28:
23:
22:
15:
12:
11:
5:
3650:
3648:
3640:
3639:
3629:
3628:
3625:
3624:
3619:
3612:
3611:External links
3609:
3608:
3607:
3586:
3578:|journal=
3560:
3537:
3534:
3531:
3530:
3495:
3477:
3462:
3447:
3416:
3395:
3371:
3364:
3342:
3316:
3296:
3269:
3242:
3227:
3220:
3198:
3149:
3130:(4): 517–531.
3124:Nat. Prod. Rep
3114:
3106:
3088:
3069:(4): 517–531.
3049:
3046:. p. 837.
3037:Vogel (1956).
3029:
2973:
2945:
2927:(1): 489–498.
2904:
2897:
2877:
2840:
2803:
2788:
2750:
2732:(3): 290–332.
2712:
2674:
2636:
2598:
2583:
2553:
2524:
2518:978-3527306732
2517:
2484:
2478:(fire oil) or
2429:
2423:
2399:
2384:
2334:
2327:
2305:
2298:
2280:
2273:
2240:
2233:
2214:
2213:
2211:
2208:
2207:
2206:
2201:
2196:
2191:
2184:
2181:
2169:
2168:
2162:
2148:
2145:
2120:
2117:
2071:Pyrroles with
2068:
2065:
2048:
2045:
1988:sodium hydride
1978:
1969:
1966:
1946:acid chlorides
1934:
1931:
1925:
1921:
1910:
1906:
1899:
1880:
1868:
1861:
1840:
1837:
1788:
1785:
1610:
1607:
1526:carbon dioxide
1493:and then with
1410:
1409:
1375:
1372:
1356:Main article:
1353:
1350:
1334:carbonyl group
1311:Main article:
1308:
1305:
1277:Main article:
1274:
1271:
1251:
1247:
1240:
1222:
1219:
1174:
1152:
1149:
1118:
1115:
1059:
1052:
1037:
1030:
1023:
1019:
1015:
1008:
980:
977:
957:porphyrinogens
887:
884:
883:
878:
856:
855:
851:standard state
848:
845:
844:
827:
824:
821:
820:
816:
815:
810:
803:
802:
799:
793:
792:
789:
782:
779:
778:
775:
769:
768:
758:
751:
744:
729:
728:
727:
726:
724:
715:
714:
710:
709:
706:
700:
694:
686:
683:
682:
679:
673:
667:
659:
656:
655:
652:
640:
637:
636:
632:
631:
628:
622:
621:
612:
606:
603:
602:
594:
588:
584:
574:
573:
570:
566:
556:
555:
552:
550:Vapor pressure
546:
545:
542:
536:
535:
532:
526:
525:
522:
516:
515:
509:
503:
502:
496:
490:
485:
480:
477:
476:
472:
471:
469:
468:
465:
463:
460:
452:
451:
450:
447:
446:
444:
443:
439:
438:
436:
432:
429:
428:
420:
419:
418:
415:
414:
412:
411:
398:
396:
384:
381:
380:
377:
371:
370:
368:
367:
359:
357:
351:
350:
348:
347:
343:
341:
335:
334:
332:
331:
323:
321:
313:
310:
309:
306:
301:
298:
297:
295:
294:
290:
288:
280:
279:
269:
261:
260:
258:
257:
249:
247:
241:
240:
238:
237:
229:
227:
221:
220:
218:
217:
209:
207:
201:
200:
197:
192:
189:
188:
186:
185:
178:
170:
168:
161:
158:
157:
155:
154:
146:
144:
139:
136:
135:
131:
130:
127:
126:
123:
119:
118:
114:
113:
105:
104:
98:
97:
93:
92:
89:
88:
78:
63:
62:
59:
58:
48:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
3649:
3638:
3635:
3634:
3632:
3623:
3620:
3618:
3615:
3614:
3610:
3604:
3600:
3596:
3592:
3587:
3583:
3571:
3563:
3557:
3553:
3549:
3545:
3540:
3539:
3535:
3526:
3522:
3518:
3514:
3510:
3506:
3499:
3496:
3491:
3487:
3481:
3478:
3473:
3466:
3463:
3458:
3451:
3448:
3443:
3439:
3436:: 1153–1162.
3435:
3431:
3427:
3420:
3417:
3412:
3406:
3398:
3396:966-7022-19-6
3392:
3388:
3384:
3375:
3372:
3367:
3361:
3357:
3353:
3346:
3343:
3338:
3334:
3330:
3326:
3319:
3313:
3309:
3308:
3300:
3297:
3292:
3288:
3284:
3280:
3273:
3270:
3265:
3261:
3257:
3253:
3246:
3243:
3238:
3231:
3228:
3223:
3217:
3213:
3202:
3199:
3194:
3190:
3185:
3180:
3176:
3172:
3168:
3164:
3160:
3153:
3150:
3145:
3141:
3137:
3133:
3129:
3125:
3118:
3115:
3109:
3107:1-57259-153-6
3103:
3099:
3092:
3089:
3084:
3080:
3076:
3072:
3068:
3064:
3060:
3053:
3050:
3042:
3041:
3033:
3030:
3025:
3021:
3016:
3011:
3007:
3003:
2999:
2996:
2995:
2994:J. Org. Chem.
2987:
2980:
2978:
2974:
2969:
2965:
2961:
2958:
2957:
2956:J. Chem. Soc.
2949:
2946:
2941:
2934:
2930:
2926:
2922:
2918:
2914:
2913:Piloty, Oskar
2908:
2905:
2900:
2898:9781118354421
2894:
2890:
2889:
2881:
2878:
2873:
2867:
2863:
2859:
2855:
2851:
2844:
2841:
2836:
2830:
2826:
2822:
2818:
2814:
2807:
2804:
2799:
2792:
2789:
2784:
2777:
2773:
2769:
2765:
2761:
2754:
2751:
2746:
2739:
2735:
2731:
2727:
2723:
2716:
2713:
2708:
2701:
2697:
2693:
2689:
2685:
2678:
2675:
2670:
2663:
2659:
2655:
2651:
2647:
2640:
2637:
2632:
2625:
2621:
2618:: 1474–1476.
2617:
2613:
2609:
2602:
2599:
2594:
2587:
2584:
2580:(1): 157–388.
2579:
2575:
2571:
2569:
2560:
2558:
2554:
2542:
2535:
2528:
2525:
2520:
2514:
2510:
2506:
2502:
2501:
2493:
2491:
2489:
2485:
2481:
2477:
2471:
2464:
2460:
2456:
2452:
2448:
2444:
2440:
2433:
2430:
2426:
2420:
2416:
2415:
2410:
2403:
2400:
2395:
2388:
2385:
2380:
2373:
2369:
2365:
2361:
2357:
2354:
2353:
2348:
2341:
2339:
2335:
2330:
2324:
2319:
2318:
2309:
2306:
2301:
2295:
2291:
2284:
2281:
2276:
2270:
2266:
2262:
2258:
2254:
2250:
2244:
2241:
2236:
2230:
2226:
2219:
2216:
2209:
2205:
2202:
2200:
2197:
2195:
2192:
2190:
2187:
2186:
2182:
2180:
2178:
2174:
2166:
2163:
2160:
2157:
2156:
2155:
2153:
2146:
2144:
2142:
2138:
2134:
2130:
2126:
2118:
2112:
2108:
2106:
2102:
2098:
2094:
2085:
2081:
2078:
2077:cycloaddition
2074:
2066:
2064:
2062:
2058:
2054:
2046:
2044:
2042:
2038:
2034:
2026:
2021:
2017:
2015:
2011:
2007:
2003:
2001:
1997:
1993:
1989:
1985:
1981:
1977:
1967:
1961:
1957:
1955:
1951:
1947:
1943:
1939:
1932:
1930:
1928:
1917:
1913:
1902:
1895:
1891:
1887:
1883:
1876:
1872:
1864:
1857:
1848:
1844:
1838:
1836:
1834:
1830:
1829:electrophilic
1826:
1822:
1818:
1814:
1810:
1806:
1802:
1798:
1794:
1786:
1784:
1782:
1777:
1762:
1760:
1755:
1748:
1729:
1725:
1719:
1715:
1711:
1707:
1703:
1701:
1697:
1693:
1689:
1685:
1681:
1677:
1673:
1669:
1661:
1657:
1653:
1646:
1642:
1640:
1636:
1632:
1628:
1624:
1620:
1616:
1608:
1602:
1598:
1596:
1592:
1588:
1584:
1580:
1576:
1567:
1563:
1561:
1558:
1554:
1550:
1546:
1537:
1533:
1531:
1527:
1523:
1519:
1515:
1506:
1502:
1500:
1496:
1492:
1488:
1483:
1481:
1477:
1473:
1469:
1465:
1461:
1457:
1453:
1449:
1445:
1437:
1433:
1431:
1427:
1423:
1419:
1415:
1404:
1400:
1399:
1398:
1396:
1392:
1388:
1384:
1380:
1374:Other methods
1373:
1367:
1363:
1359:
1351:
1345:
1341:
1339:
1335:
1331:
1327:
1323:
1320:
1314:
1306:
1300:
1296:
1294:
1290:
1286:
1280:
1272:
1270:
1267:
1260:
1256:
1254:
1243:
1236:
1232:
1228:
1220:
1218:
1215:
1213:
1209:
1205:
1201:
1197:
1193:
1189:
1185:
1181:
1177:
1170:
1166:
1157:
1150:
1148:
1146:
1136:
1132:
1128:
1124:
1116:
1114:
1112:
1108:
1104:
1100:
1096:
1092:
1088:
1087:Hückel's rule
1084:
1080:
1076:
1072:
1068:
1063:
1058:
1051:
1047:
1043:
1036:
1029:
1014:
1006:
1002:
998:
994:
990:
986:
978:
976:
974:
970:
966:
962:
958:
953:
951:
947:
928:
913:
909:
905:
901:
897:
893:
881:
874:
869:
852:
846:
843:
839:
835:
831:
828:
823:
822:
817:
814:
811:
808:
805:
804:
800:
798:
795:
794:
790:
787:
781:
780:
776:
774:
771:
770:
763:
756:
749:
725:
722:
721:
717:
716:
711:
707:
699:
691:
685:
684:
680:
672:
664:
658:
657:
653:
648:
643:
642:Heat capacity
639:
638:
633:
629:
627:
624:
623:
613:
609:
605:
604:
600:
593:
589:
583:
579:
576:
575:
571:
565:
561:
558:
557:
553:
551:
548:
547:
543:
541:
540:Boiling point
538:
537:
533:
531:
530:Melting point
528:
527:
523:
521:
518:
517:
510:
508:
505:
504:
486:
483:
479:
478:
473:
464:
459:
455:
448:
437:
427:
423:
416:
408:
404:
403:DTXSID5021910
400:
399:
397:
387:
383:
382:
378:
376:
373:
372:
365:
361:
360:
358:
356:
353:
352:
345:
344:
342:
340:
337:
336:
329:
325:
324:
322:
316:
312:
311:
307:
304:
300:
299:
292:
291:
289:
287:
282:
281:
277:
273:
270:
268:
266:ECHA InfoCard
263:
262:
255:
251:
250:
248:
246:
243:
242:
235:
231:
230:
228:
226:
223:
222:
215:
211:
210:
208:
206:
203:
202:
198:
195:
191:
190:
183:
179:
176:
172:
171:
169:
165:
160:
159:
152:
148:
147:
145:
142:
138:
137:
132:
124:
121:
120:
115:
109:
103:
99:
94:
83:
79:
73:
69:
68:
64:
53:
49:
43:
39:
38:
34:
19:
3594:
3590:
3543:
3511:(1): 58–77.
3508:
3504:
3498:
3489:
3480:
3471:
3465:
3456:
3450:
3433:
3429:
3419:
3386:
3374:
3355:
3351:
3345:
3328:
3324:
3306:
3299:
3282:
3278:
3272:
3255:
3251:
3245:
3236:
3230:
3211:
3201:
3166:
3162:
3152:
3127:
3123:
3117:
3097:
3091:
3066:
3062:
3052:
3039:
3032:
2997:
2992:
2959:
2954:
2948:
2924:
2920:
2907:
2887:
2880:
2857:
2853:
2843:
2820:
2816:
2806:
2797:
2791:
2767:
2763:
2753:
2729:
2725:
2715:
2691:
2687:
2677:
2653:
2649:
2639:
2615:
2611:
2601:
2592:
2586:
2577:
2573:
2567:
2545:. Retrieved
2527:
2498:
2479:
2475:
2449:(5): 65–78.
2446:
2442:
2432:
2413:
2409:March, Jerry
2402:
2393:
2387:
2355:
2350:
2316:
2308:
2289:
2283:
2252:
2243:
2224:
2218:
2194:Tetrapyrrole
2170:
2150:
2133:atorvastatin
2128:
2124:
2122:
2090:
2072:
2070:
2053:pyrrolidines
2050:
2036:
2032:
2030:
2024:
2013:
2009:
2005:
2004:
1999:
1992:nucleophilic
1984:butyllithium
1975:
1971:
1936:
1886:halogenating
1853:
1842:
1790:
1778:
1763:
1756:
1749:
1734:
1723:
1717:
1713:
1710:Zwitterionic
1654:
1651:
1623:succinyl-CoA
1612:
1609:Biosynthesis
1587:distillation
1572:
1557:azide–alkyne
1542:
1511:
1484:
1452:Oskar Piloty
1441:
1421:
1417:
1411:
1394:
1377:
1361:
1337:
1325:
1316:
1292:
1288:
1284:
1282:
1268:
1265:
1224:
1216:
1212:Hans Fischer
1162:
1134:
1120:
1082:
1065:Pyrrole has
1064:
1056:
1049:
1034:
1027:
1012:
989:distillation
982:
973:chlorophylls
954:
926:
896:heterocyclic
891:
890:
784:Autoignition
719:
697:
670:
646:
591:
581:
563:
339:RTECS number
134:Identifiers
117:Other names
107:
3591:Tetrahedron
3325:Tetrahedron
2962:: 639–645.
2199:Polypyrrole
2165:Pyrrolidine
2027:-metalation
1996:iodomethane
1875:sulfonating
1825:polypyrrole
1805:Diels–Alder
1797:hydrogenate
1791:Due to its
1639:chlorophyll
1589:setup with
1549:isonitriles
1530:Diels–Alder
1528:by a retro-
1426:nitro group
1208:metabolites
1196:chlorophyll
1079:delocalized
1055:= 1.38 and
786:temperature
773:Flash point
475:Properties
379:1992, 1993
272:100.003.387
234:ChEMBL16225
214:CHEBI:19203
3235:"Pyrrol".
2547:2012-09-23
2482:(red oil).
2210:References
2095:, such as
2087:Pyrrole DA
2057:pyrrolines
2047:Reductions
1823:easily to
1739:-proline,
1627:Knorr-type
1583:mucic acid
1514:münchnones
1188:porphyrins
1186:, and the
1184:biliverdin
1071:lone pairs
961:porphyrins
801:3.1–14.8%
690:combustion
507:Molar mass
364:86S1ZD6L2C
245:ChemSpider
162:3D model (
141:CAS Number
3580:ignored (
3570:cite book
3405:cite book
2159:Pyrroline
2141:sunitinib
2137:ketorolac
1938:Acylation
1933:Acylation
1856:nitrating
1833:protected
1743:-serine,
1720:)-proline
1692:ornithine
1668:glutamate
1553:acetylide
1491:hydrazine
1480:mechanism
1460:hydrazine
1235:catalysts
1221:Synthesis
1180:bilirubin
1173:vitamin B
1165:cofactors
1107:thiophene
1095:thiophene
1075:electrons
1046:ECW model
1042:hard acid
1007:. In CDCl
1001:thiophene
910:with the
830:Phosphole
663:formation
626:Viscosity
461:N1C=CC=C1
375:UN number
346:UX9275000
293:203-724-7
285:EC Number
3637:Pyrroles
3631:Category
3525:25186723
3193:27314508
3144:16874387
3083:16874387
3024:17432915
2915:(1910).
2411:(2007),
2251:(2014).
2183:See also
2093:carbenes
2023:Pyrrole
1591:glycerol
1456:aldehyde
1444:Gertrude
1200:chlorins
1127:coal tar
1067:aromatic
1062:= 0.68.
1044:and the
993:aromatic
985:volatile
969:chlorins
900:aromatic
720:NFPA 704
713:Hazards
610:(χ)
597:0.4 for
578:Basicity
151:109-97-7
110:-Pyrrole
31:Pyrrole
18:Pyrroles
3258:: 100.
3184:5555159
3015:1939979
2451:Bibcode
2360:Bibcode
2204:Azonine
2177:benzene
2055:and to
1884:), and
1821:oxidize
1817:aniline
1813:benzene
1656:Proline
1619:glycine
1595:solvent
1518:alkynes
1476:ammonia
1412:By the
1237:, like
1231:ammonia
1135:pyrrhos
1117:History
1091:benzene
912:formula
892:Pyrrole
873:what is
871: (
842:stibole
838:bismole
590:13.6 (p
560:Acidity
520:Density
315:PubChem
125:Imidole
3558:
3523:
3393:
3362:
3314:
3285:: 74.
3218:
3191:
3181:
3142:
3104:
3081:
3022:
3012:
2989:(Note)
2895:
2515:
2480:Rothöl
2476:Pyrrol
2421:
2325:
2296:
2271:
2231:
2179:ring.
2173:indole
2139:, and
1998:gives
1914:, and
1888:(e.g.
1858:(e.g.
1809:olefin
1545:silver
1428:, and
1393:. A 5-
1322:ketone
1140:πυρρός
1109:, and
1101:. The
967:, the
868:verify
865:
834:arsole
809:(SDS)
512:67.091
466:1cccc1
454:SMILES
225:ChEMBL
96:Names
3385:[
3354:[
3210:[
3044:(PDF)
2537:(PDF)
1879:Py·SO
1801:diene
1593:as a
1464:imine
1387:enone
1319:amino
1229:with
1227:furan
1111:furan
1099:furan
997:furan
894:is a
615:−47.6
422:InChI
308:1705
205:ChEBI
199:1159
164:JSmol
122:Azole
3582:help
3556:ISBN
3521:PMID
3411:link
3391:ISBN
3360:ISBN
3312:ISBN
3216:ISBN
3208:有机化学
3189:PMID
3140:PMID
3102:ISBN
3079:PMID
3020:PMID
2893:ISBN
2578:2013
2513:ISBN
2419:ISBN
2323:ISBN
2294:ISBN
2269:ISBN
2229:ISBN
1986:and
1944:and
1815:and
1637:and
1635:heme
1621:and
1516:and
1458:and
1450:and
1446:and
1418:endo
1395:endo
1244:and
1192:heme
1182:and
1167:and
1097:and
999:and
965:heme
950:heme
908:ring
599:C.A.
355:UNII
328:8027
254:7736
3599:doi
3548:doi
3513:doi
3438:doi
3333:doi
3287:doi
3260:doi
3179:PMC
3171:doi
3167:116
3132:doi
3071:doi
3010:PMC
3002:doi
2964:doi
2960:113
2929:doi
2862:doi
2825:doi
2772:doi
2734:doi
2730:236
2696:doi
2658:doi
2620:doi
2505:doi
2459:doi
2368:doi
2261:doi
2041:NBS
1956:.
1894:NBS
1890:NCS
1873:),
1860:HNO
1803:in
1694:by
1670:.
1658:is
1641:.
1577:of
1422:dig
1340:).
1295:).
1239:SiO
1190:of
1073:of
963:of
939:NCH
701:298
674:298
391:EPA
318:CID
3633::
3595:62
3593:.
3574::
3572:}}
3568:{{
3554:.
3519:.
3509:44
3507:.
3488:.
3434:14
3432:.
3407:}}
3403:{{
3329:45
3327:.
3283:36
3281:.
3256:51
3254:.
3187:.
3177:.
3165:.
3161:.
3138:.
3128:23
3126:.
3077:.
3067:23
3065:.
3018:.
3008:.
2998:72
2991:.
2976:^
2925:43
2923:.
2858:17
2856:,
2821:17
2819:,
2815:,
2768:35
2766:.
2728:.
2692:17
2690:.
2654:35
2652:.
2616:23
2614:.
2576:.
2572:.
2566:"1
2556:^
2511:.
2487:^
2457:.
2447:31
2445:.
2366:.
2349:.
2337:^
2267:.
2255:.
2135:,
2043:.
1916:KI
1909:Cl
1905:SO
1903:,
1898:Br
1896:,
1892:,
1867:Ac
1652:.
1597:.
1501::
1432:.
1255:.
1246:Al
1202:,
1198:,
1194:,
1175:12
1147:.
975:.
952:.
944:.
923:NH
902:,
898:,
840:,
836:,
832:,
693:(Δ
666:(Δ
601:)
587:)
580:(p
569:)
562:(p
3605:.
3601::
3584:)
3564:.
3550::
3527:.
3515::
3492:.
3444:.
3440::
3413:)
3399:.
3368:.
3335::
3293:.
3289::
3266:.
3262::
3224:.
3195:.
3173::
3146:.
3134::
3112:.
3110:.
3085:.
3073::
3026:.
3004::
2970:.
2966::
2935:.
2931::
2901:.
2864::
2827::
2778:.
2774::
2740:.
2736::
2702:.
2698::
2664:.
2660::
2626:.
2622::
2568:H
2550:.
2521:.
2507::
2465:.
2461::
2453::
2374:.
2370::
2362::
2356:2
2331:.
2302:.
2277:.
2263::
2237:.
2129:N
2125:N
2073:N
2037:N
2033:N
2025:C
2014:N
2010:N
2006:N
2000:N
1979:a
1976:K
1974:p
1926:2
1924:O
1922:2
1920:H
1918:/
1911:2
1907:2
1900:2
1881:3
1877:(
1871:O
1869:2
1865:/
1862:3
1774:L
1770:L
1766:L
1752:L
1745:L
1741:L
1737:L
1718:R
1714:S
1666:-
1664:L
1420:-
1338:2
1336:(
1326:1
1324:(
1293:3
1289:2
1285:1
1252:3
1250:O
1248:2
1241:2
1137:(
1083:n
1060:A
1057:C
1053:A
1050:E
1038:a
1035:K
1031:a
1028:K
1024:6
1022:H
1020:4
1016:a
1013:K
1009:3
1005:D
941:3
937:4
935:H
933:4
931:C
927:N
921:4
919:H
917:4
915:C
863:Y
761:0
754:2
747:2
703:)
698:H
695:c
676:)
671:H
668:f
649:)
647:C
645:(
617:×
595:a
592:K
585:b
582:K
567:a
564:K
500:N
497:5
494:H
491:4
488:C
393:)
389:(
166:)
108:H
106:1
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.