709:
to a maximum daily dose of 850 mg. Recent research has suggested on the basis of recent efficacy and toxicity data that this 850-mg restriction should be removed. The evidence to date, which is in their research, suggests that a regimen of 20 mg/kg/day of pentavalent antimony, without an upper limit on the daily dose, is more efficacious and is not substantially more toxic than regimens with lower daily doses. It is recommend treating all forms of leishmaniasis with a full 20 mg/kg/day of pentavalent antimony. Treatment of cutaneous leishmaniasis usually lasts for 20 days and visceral and mucosal leishmaniasis for 28 days.
402:
379:
40:
1889:
477:
708:
As dosage regimens for treating leishmaniasis have evolved, the daily dose of antimony and the duration of therapy have been progressively increased to combat unresponsiveness to therapy. In the 1980s, the use of 20 mg/kg/day (instead of 10 mg/kg/day) of antimony was recommended, but only
749:
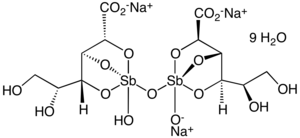
The chemical structure of sodium stibogluconate is somewhat ambiguous, and the structure shown above is idealized. Its solutions may contain multiple antimony compounds, although this heterogeneity may be unimportant. It has been speculated that the active species contains only a single antimony
650:
should be monitored twice weekly; there is no need to stop treatment if the amylase remains less than four times the upper limit of normal; if the amylase rises above the cut-off, then treatment should be interrupted until the amylase falls to less than twice the upper limit of normal, whereupon
1929:
92:
535:
519:
InChI=1S/2C6H10O7.3Na.3O.2Sb/c2*7-1-2(8)3(9)4(10)5(11)6(12)13;;;;;;;;/h2*2-5,7-8,10H,1H2,(H,12,13);;;;;;;;/q2*-2;3*+1;;;;2*+2/p-2/t2-,3?,4+,5-;2-,3-,4+,5-;;;;;;;;/m11......../s1
1411:
1186:
Herwaldt BL, Berman JD (March 1992). "Recommendations for treating leishmaniasis with sodium stibogluconate (Pentostam) and review of pertinent clinical studies".
952:
Herwaldt BL, Berman JD (March 1992). "Recommendations for treating leishmaniasis with sodium stibogluconate (Pentostam) and review of pertinent clinical studies".
662:(i.e., injected directly into the area of infected skin) and again, this is exceedingly painful and does not give results superior to intravenous administration.
766:
The mechanism of sodium stibogluconate is poorly understood, but is thought to stem from the inhibition of macromolecular synthesis via a reduction in available
62:
625:. One of the practical problems is that after a few doses it can become exceedingly difficult to find a vein in which to inject the drug. The insertion of a
491:
712:
The dose of sodium stibogluconate is by slow intravenous infusion (at least five minutes with cardiac monitoring). The injection are stopped if there is
702:
902:
655:(ECG) monitoring while the medicine is injected is advisable and changes quickly reverse after the drug is stopped or the infusion rate is decreased.
1229:
930:
626:
658:
The drug can be given intramuscularly but is exceedingly painful when given by this route. It can also be given intralesionally when treating
1843:
1404:
629:(PICC) does not prevent the problem and can instead exacerbate it: the entire vein along the course of the PICC line can become inflamed and
606:
1070:
845:
582:
Side effects are common and include loss of appetite, nausea, muscle pains, headache, and feeling tired. Serious side effect may include an
1919:
1636:
1144:
1111:
1138:
1105:
896:
511:
1397:
156:
132:
1301:
Rees PH, Keating MI, Kager PA, Hockmeyer WT (August 1980). "Renal clearance of pentavalent antimony (sodium stibogluconate)".
1909:
1914:
1879:
270:
1860:
610:
358:
605:
Sodium stibogluconate has been studied as early as 1937 and has been in medical use since the 1940s. It is on the
1158:
721:
572:
877:
Control of the leishmaniasis: report of a meeting of the WHO Expert
Committee on the Control of Leishmaniases
1848:
1458:
875:
809:
805:
734:
659:
80:
1221:
397:
1804:
1586:
771:
767:
738:
599:
922:
327:
1924:
1743:
1590:
817:
813:
1389:
1504:
374:
1644:
1625:
1575:
784:
652:
177:
1488:
347:
1449:
1326:
1062:
837:
563:. This includes leishmaniasis of the cutaneous, visceral, and mucosal types. Some combination of
72:
1706:
720:
during the Second World War when a treatment was urgently required for Allied troops during the
758:
Pentavalent antimony does not appear to accumulate in the body and is excreted by the kidneys.
1611:
1375:
1318:
1283:
1203:
1134:
1101:
1017:
969:
892:
775:
259:
52:
1126:
1093:
1365:
1357:
1310:
1273:
1263:
1195:
1166:
1007:
961:
884:
583:
414:
186:
287:
279:
194:
1893:
1774:
1728:
1036:
717:
694:
576:
115:
401:
378:
1854:
1782:
1751:
1680:
1648:
1579:
1538:
1530:
1515:
1480:
1278:
1251:
690:
39:
1370:
1345:
1314:
796:(ATP and GTP) as well as between a 34–60% increase of label incorporation into purine
31:
1903:
1786:
1759:
1694:
1684:
1567:
1440:
1420:
698:
595:
560:
390:
219:
1330:
633:. Large doses of sodium stibogluconate are often administered as dilute solutions.
1810:
1800:
1720:
1710:
1542:
1519:
1470:
1431:
793:
640:
636:
587:
338:
110:
105:
100:
665:
Sodium stibogluconate can also cause a reduced appetite, metallic taste in mouth,
1346:"Biochemical mechanisms of the antileishmanial activity of sodium stibogluconate"
727:
The duration of treatment is usually 10 to 21 days and depends on the species of
1824:
1814:
1791:
1755:
1698:
1615:
1605:
1601:
1498:
1494:
1484:
1012:
995:
801:
678:
568:
564:
651:
treatment can be resumed. Cardiac conduction disturbances are less common, but
1763:
1732:
1716:
1688:
1672:
1552:
1548:
1268:
1199:
965:
797:
779:
729:
630:
453:
250:
1663:
1476:
1466:
674:
591:
66:
1287:
1163:
World Health
Organization model list of essential medicines: 21st list 2019
1021:
1379:
1322:
1207:
973:
1820:
1621:
1427:
1424:
1361:
670:
230:
17:
1171:
239:
1508:
888:
643:
205:
789:
788:
and demonstrated a 56–65% reduction in incorporation of a label into
716:
or central chest pain. The chemotherapeutic index was established by
666:
647:
318:
590:. Sodium stibogluconate is less safe than some other options during
307:
713:
622:
476:
467:
677:, headache, tiredness, joint pains, muscle aches, dizziness, and
298:
1393:
594:. It is not believed to result in any problems if used during
363:
1131:
Trypanosomatid
Diseases: Molecular Routes to Drug Discovery
782:. Bermann et al. studied the effects of stibogluconate on
1252:"Pentavalent antimonials: new perspectives for old drugs"
1175:. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
607:
542:
499:...O=2(O1(=O)OC((O)CO)(O)(O1)C()=O)O((O)(O2)C()=O)(O)CO
1877:
1188:
The
American Journal of Tropical Medicine and Hygiene
954:
The
American Journal of Tropical Medicine and Hygiene
923:"Our Formulary Infectious Diseases Laboratories CDC"
639:
is a common deleterious effect of the drug, and the
1773:
1742:
1671:
1662:
1635:
1566:
1529:
1457:
1448:
1439:
465:
452:
413:
408:
389:
357:
337:
317:
297:
269:
249:
229:
204:
176:
147:
131:
126:
91:
79:
61:
51:
46:
609:. In the United States, it is available from the
575:, however, may be recommended due to issues with
1250:Frézard F, Demicheli C, Ribeiro RR (June 2009).
218:
1063:"Sodium Stibogluconate use while Breastfeeding"
820:) following 4 hour exposure to stibogluconate.
193:
185:
621:Sodium stibogluconate is exceedingly toxic to
1930:World Health Organization essential medicines
1405:
1344:Berman JD, Waddell D, Hanson BD (June 1985).
883:. World Health Organization. p. 55,186.
8:
559:among others, is a medication used to treat
346:
30:
140:In general: ℞ (Prescription only)
114:
1668:
1454:
1445:
1412:
1398:
1390:
1094:"Legacies of the Past: Chemical Medicines"
1000:Asian Pacific Journal of Tropical Medicine
703:Centers for Disease Control and Prevention
693:as Pentostam, where it is manufactured by
689:Sodium stibogluconate is available in the
400:
377:
258:
1369:
1277:
1267:
1170:
1011:
996:"Worldwide risk factors in leishmaniasis"
917:
915:
874:Organization, World Health (March 2010).
869:
867:
865:
863:
774:, likely secondary to inhibition of the
286:
278:
989:
987:
985:
983:
1884:
829:
516:
496:
373:
238:
161:
627:peripherally inserted central catheter
391:
29:
1350:Antimicrobial Agents and Chemotherapy
1165:. Geneva: World Health Organization.
1133:. John Wiley & Sons. p. 17.
1100:. John Wiley & Sons. p. 58.
1073:from the original on 20 December 2016
933:from the original on 16 December 2016
848:from the original on 20 December 2016
326:
71:
7:
1043:. BMJ Group and Pharmaceutical Press
104:
306:
209:
1232:from the original on 20 April 2009
994:Oryan A, Akbari M (October 2016).
701:on a named-patient basis from the
598:. Sodium stibogluconate is in the
25:
1125:Jäger T, Koch O, Flohé L (2013).
1887:
1147:from the original on 2016-12-20.
1114:from the original on 2016-12-20.
908:from the original on 2016-06-08.
443:
425:
38:
524:Key:RTLKTTNTVTVWPV-UQCYVGCHSA-L
437:
431:
419:
1:
1315:10.1016/s0140-6736(80)90120-8
579:. It is given by injection.
555:, sold under the brand name
1035:Joint Formulary Committee.
1013:10.1016/j.apjtm.2016.06.021
733:and the type of infection (
611:Centers for Disease Control
57:Pentostam, Stiboson, others
1946:
1920:Drugs developed by GSK plc
1041:British National Formulary
697:. It is available in the
409:Chemical and physical data
1838:
1269:10.3390/molecules14072317
1200:10.4269/ajtmh.1992.46.296
1159:World Health Organization
1098:Drug Discovery: A History
966:10.4269/ajtmh.1992.46.296
532:
507:
487:
152:
87:intravenous, intramusclar
37:
573:liposomal amphotericin B
73:International Drug Names
1587:Pentavalent antimonials
1459:African trypanosomiasis
1037:"Sodium Stibogluconate"
838:"Sodium Stibogluconate"
660:cutaneous leishmaniasis
600:pentavalent antimonials
168:-(oxydistibylidyne)bis
1910:Antimony(V) compounds
1595:Sodium stibogluconate
1591:Meglumine antimoniate
929:. 22 September 2016.
602:class of medication.
553:Sodium stibogluconate
32:Sodium stibogluconate
1915:Antiprotozoal agents
1505:naphthalenesulfonate
1362:10.1128/aac.27.6.916
1228:. 14 January 2009.
1226:The Daily Telegraph
785:Leishmania mexicana
762:Mechanism of action
584:irregular heartbeat
34:
27:Pharmaceutical drug
1865:Never to phase III
1092:Sneader W (2005).
745:Chemical structure
722:invasion of Sicily
653:electrocardiograph
1875:
1874:
1834:
1833:
1658:
1657:
1612:phosphorylcholine
1562:
1561:
1309:(8188): 226–229.
1222:"Leonard Goodwin"
776:citric acid cycle
550:
549:
478:Interactive image
359:CompTox Dashboard
285:nonhydrate:
192:nonhydrate:
16:(Redirected from
1937:
1892:
1891:
1890:
1883:
1669:
1455:
1446:
1414:
1407:
1400:
1391:
1384:
1383:
1373:
1341:
1335:
1334:
1298:
1292:
1291:
1281:
1271:
1262:(7): 2317–2336.
1247:
1241:
1240:
1238:
1237:
1218:
1212:
1211:
1183:
1177:
1176:
1174:
1155:
1149:
1148:
1122:
1116:
1115:
1089:
1083:
1082:
1080:
1078:
1059:
1053:
1052:
1050:
1048:
1032:
1026:
1025:
1015:
991:
978:
977:
949:
943:
942:
940:
938:
919:
910:
909:
907:
882:
871:
858:
857:
855:
853:
834:
754:Pharmacokinetics
546:
545:
538:
480:
460:
445:
439:
433:
427:
421:
404:
393:
382:
381:
367:
365:
350:
330:
310:
290:
282:
262:
242:
222:
212:
211:
197:
189:
118:
108:
75:
42:
35:
33:
21:
1945:
1944:
1940:
1939:
1938:
1936:
1935:
1934:
1900:
1899:
1898:
1888:
1886:
1878:
1876:
1871:
1870:
1855:Clinical trials
1830:
1795:
1775:Dientamoebiasis
1769:
1738:
1654:
1631:
1558:
1525:
1512:
1450:Trypanosomiasis
1435:
1418:
1388:
1387:
1343:
1342:
1338:
1300:
1299:
1295:
1249:
1248:
1244:
1235:
1233:
1220:
1219:
1215:
1185:
1184:
1180:
1157:
1156:
1152:
1141:
1124:
1123:
1119:
1108:
1091:
1090:
1086:
1076:
1074:
1061:
1060:
1056:
1046:
1044:
1034:
1033:
1029:
1006:(10): 925–932.
993:
992:
981:
951:
950:
946:
936:
934:
921:
920:
913:
905:
899:
880:
873:
872:
861:
851:
849:
836:
835:
831:
826:
764:
756:
747:
718:Leonard Goodwin
695:GlaxoSmithKline
687:
619:
541:
539:
536:(what is this?)
533:
528:
525:
520:
515:
514:
503:
500:
495:
494:
483:
458:
448:
442:
436:
430:
424:
385:
361:
353:
333:
313:
293:
265:
245:
225:
208:
200:
172:
169:
160:
159:
143:
122:
82:
28:
23:
22:
15:
12:
11:
5:
1943:
1941:
1933:
1932:
1927:
1922:
1917:
1912:
1902:
1901:
1897:
1896:
1873:
1872:
1869:
1868:
1867:
1866:
1863:
1852:
1846:
1840:
1839:
1836:
1835:
1832:
1831:
1829:
1828:
1818:
1808:
1797:
1796:
1789:
1783:nitroimidazole
1779:
1777:
1771:
1770:
1768:
1767:
1752:nitroimidazole
1748:
1746:
1744:Trichomoniasis
1740:
1739:
1737:
1736:
1725:
1724:
1714:
1703:
1702:
1692:
1681:nitroimidazole
1677:
1675:
1666:
1660:
1659:
1656:
1655:
1653:
1652:
1649:Amphotericin B
1641:
1639:
1633:
1632:
1630:
1629:
1619:
1609:
1584:
1583:
1580:Amphotericin B
1572:
1570:
1564:
1563:
1560:
1559:
1557:
1556:
1546:
1539:nitroimidazole
1535:
1533:
1531:Chagas disease
1527:
1526:
1524:
1523:
1516:nitroimidazole
1513:
1502:
1492:
1474:
1463:
1461:
1452:
1443:
1437:
1436:
1421:Antiparasitics
1419:
1417:
1416:
1409:
1402:
1394:
1386:
1385:
1356:(6): 916–920.
1336:
1293:
1242:
1213:
1194:(3): 296–306.
1178:
1150:
1139:
1117:
1106:
1084:
1054:
1027:
979:
960:(3): 296–306.
944:
911:
897:
859:
828:
827:
825:
822:
763:
760:
755:
752:
746:
743:
691:United Kingdom
686:
683:
618:
615:
548:
547:
530:
529:
527:
526:
523:
521:
518:
510:
509:
508:
505:
504:
502:
501:
498:
490:
489:
488:
485:
484:
482:
481:
473:
471:
463:
462:
456:
450:
449:
446:
440:
434:
428:
422:
417:
411:
410:
406:
405:
395:
387:
386:
384:
383:
375:DTXSID50894911
370:
368:
355:
354:
352:
351:
343:
341:
335:
334:
332:
331:
323:
321:
315:
314:
312:
311:
303:
301:
295:
294:
292:
291:
283:
275:
273:
267:
266:
264:
263:
255:
253:
247:
246:
244:
243:
235:
233:
227:
226:
224:
223:
215:
213:
202:
201:
199:
198:
190:
182:
180:
174:
173:
171:
170:
163:
155:
154:
153:
150:
149:
145:
144:
142:
141:
137:
135:
129:
128:
124:
123:
121:
120:
97:
95:
89:
88:
85:
83:administration
77:
76:
69:
59:
58:
55:
49:
48:
44:
43:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1942:
1931:
1928:
1926:
1923:
1921:
1918:
1916:
1913:
1911:
1908:
1907:
1905:
1895:
1885:
1881:
1864:
1862:
1859:
1858:
1856:
1853:
1850:
1847:
1845:
1842:
1841:
1837:
1826:
1822:
1819:
1816:
1812:
1809:
1806:
1802:
1799:
1798:
1793:
1790:
1788:
1787:Metronidazole
1784:
1781:
1780:
1778:
1776:
1772:
1765:
1761:
1760:Metronidazole
1757:
1753:
1750:
1749:
1747:
1745:
1741:
1734:
1730:
1729:aminoacridine
1727:
1726:
1722:
1718:
1715:
1712:
1708:
1705:
1704:
1700:
1696:
1695:benzimidazole
1693:
1690:
1686:
1685:Metronidazole
1682:
1679:
1678:
1676:
1674:
1670:
1667:
1665:
1661:
1650:
1646:
1643:
1642:
1640:
1638:
1634:
1627:
1623:
1620:
1617:
1613:
1610:
1607:
1603:
1600:
1599:
1598:
1596:
1592:
1588:
1581:
1577:
1574:
1573:
1571:
1569:
1568:Leishmaniasis
1565:
1554:
1550:
1547:
1544:
1540:
1537:
1536:
1534:
1532:
1528:
1521:
1517:
1514:
1510:
1506:
1503:
1500:
1496:
1493:
1490:
1486:
1482:
1478:
1475:
1472:
1468:
1465:
1464:
1462:
1460:
1456:
1453:
1451:
1447:
1444:
1442:
1441:Discicristata
1438:
1433:
1429:
1426:
1422:
1415:
1410:
1408:
1403:
1401:
1396:
1395:
1392:
1381:
1377:
1372:
1367:
1363:
1359:
1355:
1351:
1347:
1340:
1337:
1332:
1328:
1324:
1320:
1316:
1312:
1308:
1304:
1297:
1294:
1289:
1285:
1280:
1275:
1270:
1265:
1261:
1257:
1253:
1246:
1243:
1231:
1227:
1223:
1217:
1214:
1209:
1205:
1201:
1197:
1193:
1189:
1182:
1179:
1173:
1168:
1164:
1160:
1154:
1151:
1146:
1142:
1140:9783527670406
1136:
1132:
1128:
1121:
1118:
1113:
1109:
1107:9780470015520
1103:
1099:
1095:
1088:
1085:
1072:
1068:
1064:
1058:
1055:
1042:
1038:
1031:
1028:
1023:
1019:
1014:
1009:
1005:
1001:
997:
990:
988:
986:
984:
980:
975:
971:
967:
963:
959:
955:
948:
945:
932:
928:
924:
918:
916:
912:
904:
900:
898:9789241209496
894:
890:
886:
879:
878:
870:
868:
866:
864:
860:
847:
843:
839:
833:
830:
823:
821:
819:
815:
811:
807:
803:
799:
795:
794:triphosphates
791:
787:
786:
781:
777:
773:
769:
761:
759:
753:
751:
744:
742:
740:
736:
732:
731:
725:
723:
719:
715:
710:
706:
704:
700:
699:United States
696:
692:
684:
682:
680:
676:
672:
668:
663:
661:
656:
654:
649:
645:
642:
638:
634:
632:
628:
624:
616:
614:
612:
608:
603:
601:
597:
596:breastfeeding
593:
589:
585:
580:
578:
574:
570:
566:
562:
561:leishmaniasis
558:
554:
544:
537:
531:
522:
517:
513:
506:
497:
493:
486:
479:
475:
474:
472:
469:
464:
457:
455:
451:
418:
416:
412:
407:
403:
399:
396:
394:
392:ECHA InfoCard
388:
380:
376:
372:
371:
369:
360:
356:
349:
345:
344:
342:
340:
336:
329:
325:
324:
322:
320:
316:
309:
305:
304:
302:
300:
296:
289:
284:
281:
277:
276:
274:
272:
268:
261:
257:
256:
254:
252:
248:
241:
237:
236:
234:
232:
228:
221:
217:
216:
214:
207:
203:
196:
191:
188:
184:
183:
181:
179:
175:
167:
162:
158:
151:
146:
139:
138:
136:
134:
130:
125:
117:
112:
107:
102:
99:
98:
96:
94:
90:
86:
84:
78:
74:
70:
68:
64:
60:
56:
54:
50:
47:Clinical data
45:
41:
36:
19:
1925:Orphan drugs
1811:tetracycline
1801:oxyquinoline
1721:Furazolidone
1711:Nitazoxanide
1594:
1585:
1543:Benznidazole
1520:Fexinidazole
1489:Tryparsamide
1471:Eflornithine
1423:directed at
1353:
1349:
1339:
1306:
1302:
1296:
1259:
1255:
1245:
1234:. Retrieved
1225:
1216:
1191:
1187:
1181:
1172:10665/325771
1162:
1153:
1130:
1120:
1097:
1087:
1075:. Retrieved
1066:
1057:
1045:. Retrieved
1040:
1030:
1003:
999:
957:
953:
947:
935:. Retrieved
926:
876:
850:. Retrieved
841:
832:
802:diphosphates
783:
765:
757:
748:
728:
726:
711:
707:
688:
664:
657:
637:Pancreatitis
635:
620:
617:Side effects
604:
588:pancreatitis
581:
556:
552:
551:
540:
534:
339:NIAID ChemDB
328:ChEMBL367144
165:
133:Legal status
127:Legal status
1851:from market
1825:Paromomycin
1815:Doxycycline
1792:Secnidazole
1756:Carnidazole
1699:Albendazole
1626:Paromomycin
1616:Miltefosine
1606:Pentamidine
1602:benzamidine
1499:Pentamidine
1495:benzamidine
1485:Melarsoprol
1047:29 December
927:www.cdc.gov
889:10665/44412
792:nucleoside
679:anaphylaxis
569:paromomycin
565:miltefosine
461: g·mol
398:100.170.909
187:219717-42-7
148:Identifiers
53:Trade names
1904:Categories
1805:Iodoquinol
1764:Tinidazole
1733:Quinacrine
1717:nitrofuran
1707:thiazolide
1689:Tinidazole
1673:Giardiasis
1553:Nifurtimox
1549:nitrofuran
1236:2009-01-18
1127:"Foreward"
1077:7 December
937:7 December
852:7 December
824:References
800:mono- and
798:nucleoside
780:glycolysis
730:Leishmania
577:resistance
466:3D model (
454:Molar mass
288:APJ6285Y89
280:V083S0159D
251:ChemSpider
195:16037-91-5
178:CAS Number
164:2,4:2',4'-
157:IUPAC name
1861:Phase III
1849:Withdrawn
1821:neomycin
1664:Trichozoa
1477:arsenical
1467:ornithine
1428:parasites
1256:Molecules
1067:Drugs.com
842:Drugs.com
735:cutaneous
675:diarrhoea
631:thrombose
592:pregnancy
557:Pentostam
81:Routes of
67:Drugs.com
18:Pentostam
1894:Medicine
1622:neomycin
1425:excavata
1331:23764245
1288:19633606
1230:Archived
1161:(2019).
1145:Archived
1112:Archived
1071:Archived
1022:27794384
931:Archived
903:Archived
846:Archived
750:centre.
739:visceral
714:coughing
671:vomiting
543:(verify)
260:21106382
231:DrugBank
220:16685683
111:QP51AB02
93:ATC code
1645:polyene
1576:polyene
1509:Suramin
1380:2411217
1323:6105394
1279:6254722
1208:1313656
974:1313656
705:(CDC).
644:amylase
459:910.899
415:Formula
240:DB05630
206:PubChem
113: (
109:)
103: (
101:P01CB02
1880:Portal
1844:WHO-EM
1481:Atoxyl
1378:
1371:180186
1368:
1329:
1321:
1303:Lancet
1286:
1276:
1206:
1137:
1104:
1020:
972:
895:
816:, and
790:purine
685:Dosing
667:nausea
648:lipase
492:SMILES
348:007935
319:ChEMBL
308:D00582
1327:S2CID
906:(PDF)
881:(PDF)
641:serum
623:veins
512:InChI
468:JSmol
1376:PMID
1319:PMID
1284:PMID
1204:PMID
1135:ISBN
1102:ISBN
1079:2016
1049:2020
1018:PMID
970:PMID
939:2016
893:ISBN
854:2016
778:and
770:and
571:and
299:KEGG
271:UNII
63:AHFS
1637:PAM
1432:P01
1366:PMC
1358:doi
1311:doi
1274:PMC
1264:doi
1196:doi
1167:hdl
1008:doi
962:doi
885:hdl
818:GDP
814:ADP
810:GMP
806:AMP
772:GTP
768:ATP
741:).
737:or
646:or
586:or
364:EPA
210:CID
116:WHO
106:WHO
1906::
1857::
1762:,
1758:,
1687:,
1597:)
1593:,
1487:,
1483:,
1374:.
1364:.
1354:27
1352:.
1348:.
1325:.
1317:.
1305:.
1282:.
1272:.
1260:14
1258:.
1254:.
1224:.
1202:.
1192:46
1190:.
1143:.
1129:.
1110:.
1096:.
1069:.
1065:.
1039:.
1016:.
1002:.
998:.
982:^
968:.
958:46
956:.
925:.
914:^
901:.
891:.
862:^
844:.
840:.
812:,
808:,
724:.
681:.
673:,
669:,
613:.
567:,
444:Sb
441:26
432:Na
429:38
423:12
1882::
1827:)
1823:(
1817:)
1813:(
1807:)
1803:(
1794:)
1785:(
1766:)
1754:(
1735:)
1731:(
1723:)
1719:(
1713:)
1709:(
1701:)
1697:(
1691:)
1683:(
1651:)
1647:(
1628:)
1624:(
1618:)
1614:(
1608:)
1604:(
1589:(
1582:)
1578:(
1555:)
1551:(
1545:)
1541:(
1522:)
1518:(
1511:)
1507:(
1501:)
1497:(
1491:)
1479:(
1473:)
1469:(
1434:)
1430:(
1413:e
1406:t
1399:v
1382:.
1360::
1333:.
1313::
1307:2
1290:.
1266::
1239:.
1210:.
1198::
1169::
1081:.
1051:.
1024:.
1010::
1004:9
976:.
964::
941:.
887::
856:.
804:(
470:)
447:2
438:O
435:3
426:H
420:C
366:)
362:(
166:O
119:)
65:/
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.