418:
179:
119:
411:
397:
404:
335:(Hybrid NRP-PK and PK-NRP). Since nonribosomal peptide assembly lines use carrier proteins similar to those use in polyketide synthases, convergence of the two systems evolved to form hybrids, resulting in polypeptides with nitrogen in the skeletal structure and complex function groups similar to those found in amino acids.
94:. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.
207:
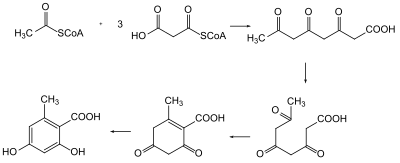
or methylmalonyl-CoA). The condensation reaction is accompanied by the decarboxylation of the extender unit, yielding a beta-keto functional group and releasing a carbon dioxide. The first condensation yields an acetoacetyl group, a diketide. Subsequent condensations yield triketides, tetraketide,
174:
Polyketides are synthesized by multienzyme polypeptides that resemble eukaryotic fatty acid synthase but are often much larger. They include acyl-carrier domains plus an assortment of enzymatic units that can function in an iterative fashion, repeating the same elongation/modification steps (as in
263:
that releases the polyketide via hydrating the thioester linkage (as in fatty acid synthesis) creating a linear polyketide scaffold. However, if water is not able to reach the active site, the hydrating reaction will not occur and an intramolecular reaction is more probable creating a macrocyclic
806:
has opened avenues for creating polyketides not found in nature. For example, the modular nature of PKSs allows for domains to be replaced, added or deleted. Introducing diversity in assembly lines enables the discovery of new polyketides with increased bioactivity or new bioactivity.
149:
Polyketides can be produced in bacteria, fungi, plants, and certain marine organisms. Earlier discovery of naturally occurring polyketides involved the isolation of the compounds being produced by the specific organism using organic chemistry purification methods based on
243:
for the stepwise condensation of the starter unit and extender units) are almost invariably modified. Each polyketide synthases is unique to each polyketide chain because they contain different combinations of domains that reduce the carbonyl group to a hydroxyl (via a
114:
with barium hydroxide causing the pyrone ring to open into a triketide. Further studies in 1903 by Collie on the triketone polyketide intermediate noted the condensation occurring amongst compounds with multiple keten groups coining the term polyketides.
383:
There are more than 10,000 known polyketides, 1% of which are known to have potential for drug activity. Polyketides comprise 20% of the top-selling pharmaceuticals with combined worldwide revenues of over USD 18 billion per year.
140:
and demonstrate the head-to-tail linkage of acetic acids to form the polyketide. In the 1980s and 1990s, advancements in genetics allowed for isolation of the genes associated to polyketides to understand the biosynthesis.
319:
Polyketide synthases are also broadly divided into three classes: Type I PKSs (multimodular megasynthases that are non-iterative, often producing macrolides, polyethers, and polyenes), Type II PKSs (dissociated
1779:
1255:
2089:
Brockmann H, Henkel W (1951). "Pikromycin, ein bitter schmeckendes
Antibioticum aus Actinomyceten" [Pikromycin, a bitter tasting antibiotic from an actinomycete].
853:
130:
It wasn't until 1955 that the biosynthesis of polyketides were understood. Arthur Birch used radioisotope labeling of carbon in acetate to trace the biosynthesis of
102:
Naturally produced polyketides by various plants and organisms have been used by humans since before studies on them began in the 19th and 20th century. In 1893,
284:, sometimes proceeded by the enol tautomers of the polyketide. These enzymes are not part of the domains of the polyketide synthase. Instead, they are found in
2009:
Baerson SR, Rimando AM (2007-01-11). "A Plethora of
Polyketides: Structures, Biological Activities, and Enzymes". In Rimando AM, Baerson SR (eds.).
86:. First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of
2315:
2237:
2028:
1476:
2220:
Caro Y, Venkatachalam M, Lebeau J, et al. (2016). "Pigments and
Colorants from Filamentous Fungi". In Merillon JM, Ramawat KG (eds.).
2320:
1268:
1103:
Johnston C, Ibrahim A, Magarvey N (2012-08-01). "Informatic strategies for the discovery of polyketides and nonribosomal peptides".
158:
of the genes to understand the biosynthesis. In addition, further advancements in biotechnology have allowed for the use of
1192:"Biotechnology of polyketides: new breath of life for the novel antibiotic genetic pathways discovery through metagenomics"
231:
PKSs are multi-domain enzymes or enzyme complex consisting of various domains. The polyketide chains produced by a minimal
1809:"Biosynthesis of antifungal and antibacterial polyketides by Burkholderia gladioli in coculture with Rhizopus microsporus"
1319:
Moore BS, Hertweck C (February 2002). "Biosynthesis and attachment of novel bacterial polyketide synthase starter units".
417:
2120:"Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides"
2046:
2255:"Flavonoids and Related Members of the Aromatic Polyketide Group in Human Health and Disease: Do They Really Work?"
1555:
Robinson JA (May 1991). "Polyketide synthase complexes: their structure and function in antibiotic biosynthesis".
1017:"Studies in relation to biosynthesis. VII. 2-Hydroxy-6-methylbenzoic acid in Penicillium griseofulvum Dierckx"
272:
Further possible modifications to the polyketide scaffolds can be made. This can include glycosylation via a
175:
fatty acid synthesis), or in a sequential fashion so as to generate more heterogeneous types of polyketides.
1692:
Shen B (April 2003). "Polyketide biosynthesis beyond the type I, II and III polyketide synthase paradigms".
155:
1407:"Modular type I polyketide synthase acyl carrier protein domains share a common N-terminally extended fold"
217:
131:
1850:"Huanglongmycin A-C, Cytotoxic Polyketides Biosynthesized by a Putative Type II Polyketide Synthase From
538:
348:
87:
43:
296:
Polyketides are a structurally diverse family. There are various subclasses of polyketides including:
2310:
2131:
1865:
1611:
1564:
1418:
1260:
828:
765:
332:
91:
259:
Termination of the polyketide scaffold biosynthesis can also vary. It is sometimes accompanied by a
253:
803:
273:
245:
232:
192:
151:
136:
2202:
2071:
2015:. ACS Symposium Series. Vol. 955. Washington, DC: American Chemical Society. pp. 2–14.
1250:
111:
59:
178:
595:
2286:
2233:
2224:. Reference Series in Phytochemistry. Cham: Springer International Publishing. pp. 1–70.
2194:
2159:
2063:
2024:
1991:
1942:
1893:
1830:
1761:
1709:
1674:
1639:
1600:"Bioinformatics Prediction of Polyketide Synthase Gene Clusters from Mycosphaerella fijiensis"
1580:
1537:
1482:
1472:
1444:
1387:
1336:
1301:
1264:
1221:
1169:
1120:
1085:
1036:
997:
943:
904:
750:
Polyketides can be used for industrial purposes, such as pigmentation and dietary flavonoids.
621:
356:
325:
297:
31:
264:
polyketide. Another possibility is spontaneous hydrolysis without the aid of a thioesterase.
2276:
2266:
2225:
2186:
2149:
2139:
2100:
2055:
2016:
1981:
1973:
1932:
1924:
1883:
1873:
1820:
1751:
1743:
1701:
1666:
1629:
1619:
1572:
1527:
1517:
1434:
1426:
1377:
1367:
1328:
1293:
1211:
1203:
1159:
1151:
1112:
1075:
1067:
1028:
987:
979:
935:
896:
867:
833:
103:
47:
2177:
Li S, Yang B, Tan GY, et al. (June 2021). "Polyketide pesticides from actinomycetes".
1356:"Biosynthesis of aromatic polyketides in microorganisms using type II polyketide synthases"
687:
236:
195:(PKSs). The core biosynthesis involves stepwise condensation of a starter unit (typically
123:
63:
39:
2135:
1869:
1615:
1568:
1557:
Philosophical
Transactions of the Royal Society of London. Series B, Biological Sciences
1422:
2281:
2254:
2154:
2119:
1986:
1961:
1937:
1912:
1888:
1849:
1756:
1731:
1634:
1599:
1532:
1501:
1439:
1406:
1382:
1355:
1284:
Staunton J, Weissman KJ (August 2001). "Polyketide biosynthesis: a millennium review".
1246:
1216:
1191:
1080:
1055:
992:
967:
770:
662:
491:
481:
360:
331:
In addition to these subclasses, there also exist polyketides that are hybridized with
1705:
885:"VII.—The formation of orcinol and other condensation products from dehydracetic acid"
118:
2304:
2206:
2075:
2010:
1164:
1139:
811:
677:
368:
277:
200:
163:
1207:
863:
2229:
2044:
Weissman K, Leadlay B (2005). "Combinatorial biosynthesis of reduced polyketides".
1155:
572:
541:
511:
485:
477:
443:
437:
425:
364:
285:
260:
240:
159:
1522:
2190:
1624:
1747:
1242:
858:
725:
691:
682:
653:
628:
578:
431:
372:
249:
213:
204:
2124:
Proceedings of the
National Academy of Sciences of the United States of America
2020:
1430:
1372:
2271:
2091:
731:
672:
657:
624:
600:
590:
585:
562:
522:
494:
474:
467:
447:
352:
344:
209:
196:
2104:
1878:
1486:
1124:
1040:
968:"The type I fatty acid and polyketide synthases: a tale of two megasynthases"
947:
908:
862:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
2144:
1016:
871:
785:
706:
667:
642:
632:
610:
556:
549:
525:
507:
501:
462:
410:
309:
301:
2290:
2198:
2163:
2067:
1995:
1977:
1946:
1897:
1834:
1765:
1713:
1678:
1643:
1576:
1541:
1448:
1391:
1340:
1305:
1225:
1173:
1089:
1001:
17:
1584:
1466:
814:
allows for discovery of new natural polyketides and their assembly lines.
396:
939:
900:
823:
790:
780:
721:
605:
565:
545:
515:
225:
221:
2059:
1670:
1657:
Katz L (November 1997). "Manipulation of
Modular Polyketide Synthases".
403:
2012:
Polyketides: Biosynthesis, Biological
Activity, and Genetic Engineering
1116:
1071:
1056:"A sea of biosynthesis: marine natural products meet the molecular age"
532:
321:
313:
305:
281:
107:
1825:
1808:
1725:
1723:
1032:
324:
with iterative action, often producing aromatics), and Type III PKSs (
1928:
1332:
1297:
983:
51:
923:
884:
166:
to find new polyketides using similar enzymes to known polyketides.
280:. Similarly, cyclization and aromatization can be introduced via a
646:
636:
1732:"Evolution and Diversity of Assembly-Line Polyketide Synthases"
154:. Later technology allowed for the isolation of the genes and
1405:
Moretto L, Heylen R, Holroyd N, et al. (February 2019).
1911:
Chan YA, Podevels AM, Kevany BM, Thomas MG (January 2009).
90:
caused by its complex biosynthesis which resembles that of
2253:
Tauchen J, Huml L, Rimpelova S, Jurášek M (August 2020).
2181:. Chemical Biotechnology ● Pharmaceutical Biotechnology.
1807:
Ross C, Opel V, Scherlach K, Hertweck C (December 2014).
1256:
1190:
Gomes ES, Schuch V, de Macedo Lemos EG (December 2013).
1960:
Kim HJ, Choi SH, Jeon BS, et al. (December 2014).
288:
in the genome close to the polyketide synthase genes.
2118:
Gagne SJ, Stout JM, Liu E, et al. (July 2012).
1913:"Biosynthesis of polyketide synthase extender units"
1730:
Nivina A, Yuet KP, Hsu J, Khosla C (December 2019).
1140:"Biosynthesis of polyketides in heterologous hosts"
1354:Wang J, Zhang R, Chen X, et al. (May 2020).
924:"CLXXI.—Derivatives of the multiple keten group"
705:Polyketides can be used for crop protection as
27:Natural organic compounds derived from a chain
1015:Birch AJ, Massy-Westropp RA, Moye CJ (1955).
928:Journal of the Chemical Society, Transactions
889:Journal of the Chemical Society, Transactions
8:
1848:Jiang L, Pu H, Xiang J, et al. (2018).
1802:
1800:
961:
959:
957:
1144:Microbiology and Molecular Biology Reviews
2280:
2270:
2153:
2143:
1985:
1936:
1887:
1877:
1824:
1755:
1633:
1623:
1531:
1521:
1438:
1381:
1371:
1215:
1163:
1079:
991:
1962:"Chemoenzymatic synthesis of spinosyn A"
177:
117:
80:
76:
69:
1500:Risdian C, Mozef T, Wink J (May 2019).
1237:
1235:
846:
328:, producing small aromatic molecules).
208:etc. Other starter units attached to a
1460:
1458:
470:, the first isolated macrolide (1951)
7:
1185:
1183:
1694:Current Opinion in Chemical Biology
1138:Pfeifer BA, Khosla C (March 2001).
1054:Lane AL, Moore BS (February 2011).
859:Compendium of Chemical Terminology
106:synthesized detectable amounts of
25:
1196:Brazilian Journal of Microbiology
966:Smith S, Tsai SC (October 2007).
2179:Current Opinion in Biotechnology
1502:"Biosynthesis of Polyketides in
416:
409:
402:
395:
203:) with an extender unit (either
1780:"5.13E: Polyketide Antibiotics"
1598:Noar RD, Daub ME (2016-07-07).
1208:10.1590/s1517-83822013000400002
1021:Australian Journal of Chemistry
2230:10.1007/978-3-319-19456-1_26-1
1471:. Royal Society of Chemistry.
1156:10.1128/MMBR.65.1.106-118.2001
132:2-hydroxy-6-methylbenzoic acid
1:
2316:NADH dehydrogenase inhibitors
1706:10.1016/S1367-5931(03)00020-6
1523:10.3390/microorganisms7050124
182:Biosynthesis of carminic acid
126:from polyketide intermediate.
2191:10.1016/j.copbio.2021.05.006
1625:10.1371/journal.pone.0158471
1468:Natural product biosynthesis
191:Polyketides are produced by
2047:Nature Reviews Microbiology
1748:10.1021/acs.chemrev.9b00525
883:Collie N, Myers WS (1893).
442:
436:
430:
424:
2337:
2021:10.1021/bk-2007-0955.ch001
1431:10.1038/s41598-019-38747-9
1373:10.1186/s12934-020-01367-4
252:), or a methylene (via an
2272:10.3390/molecules25173846
645:and the pochonin family (
2321:Plant toxin insecticides
2105:10.1002/cber.19510840306
1879:10.3389/fchem.2018.00254
1465:Walsh C, Tang Y (2017).
1360:Microbial Cell Factories
810:Furthermore, the use of
2145:10.1073/pnas.1200330109
1917:Natural Product Reports
1321:Natural Product Reports
1286:Natural Product Reports
1060:Natural Product Reports
972:Natural Product Reports
872:10.1351/goldbook.P04734
375:are in commercial use.
369:animal growth promoters
156:heterologous expression
1978:10.1002/anie.201407806
1858:Frontiers in Chemistry
1577:10.1098/rstb.1991.0038
326:chalcone synthase-like
268:Post-tailoring enzymes
218:cyclohexanecarboxylate
183:
127:
1261:John Wiley & Sons
766:hydroxyanthraquinones
577:The antibiotic agent
333:nonribosomal peptides
181:
121:
88:secondary metabolites
940:10.1039/CT9079101806
901:10.1039/CT8936300122
829:Nonribosomal peptide
248:), an olefin (via a
193:polyketide synthases
92:fatty acid synthesis
2136:2012PNAS..10912811G
2130:(31): 12811–12816.
2060:10.1038/nrmicro1287
1972:(49): 13553–13557.
1870:2018FrCh....6..254J
1742:(24): 12524–12547.
1616:2016PLoSO..1158471N
1569:1991RSPTB.332..107R
1423:2019NatSR...9.2325M
804:Protein engineering
391:
276:or oxidation via a
274:glucosyltransferase
233:polyketide synthase
187:Polyketide synthase
152:bioactivity screens
137:Penicillium patulum
2222:Fungal Metabolites
1819:(Suppl 3): 48–55.
1784:Biology LibreTexts
1411:Scientific Reports
1117:10.1039/C2MD20120H
1072:10.1039/C0NP90032J
922:Collie JN (1907).
625:tacrolimus (FK506)
622:immunosuppressants
387:
184:
128:
44:precursor molecule
2239:978-3-319-19456-1
2030:978-0-8412-3978-4
1966:Angewandte Chemie
1826:10.1111/myc.12246
1671:10.1021/cr960025+
1563:(1263): 107–114.
1478:978-1-78801-131-0
1033:10.1071/ch9550539
690:(intermediate in
454:
453:
440:, an antibiotic.
434:, an antibiotic.
428:, an antibiotic.
357:anticholesteremic
308:ring containing,
235:(consisting of a
112:dehydracetic acid
60:its reduced forms
32:organic chemistry
16:(Redirected from
2328:
2295:
2294:
2284:
2274:
2250:
2244:
2243:
2217:
2211:
2210:
2174:
2168:
2167:
2157:
2147:
2115:
2109:
2108:
2086:
2080:
2079:
2041:
2035:
2034:
2006:
2000:
1999:
1989:
1957:
1951:
1950:
1940:
1929:10.1039/b801658p
1908:
1902:
1901:
1891:
1881:
1845:
1839:
1838:
1828:
1804:
1795:
1794:
1792:
1791:
1776:
1770:
1769:
1759:
1736:Chemical Reviews
1727:
1718:
1717:
1689:
1683:
1682:
1665:(7): 2557–2576.
1659:Chemical Reviews
1654:
1648:
1647:
1637:
1627:
1595:
1589:
1588:
1552:
1546:
1545:
1535:
1525:
1497:
1491:
1490:
1462:
1453:
1452:
1442:
1402:
1396:
1395:
1385:
1375:
1351:
1345:
1344:
1333:10.1039/B003939J
1316:
1310:
1309:
1298:10.1039/a909079g
1281:
1275:
1274:
1259:(4th ed.).
1239:
1230:
1229:
1219:
1202:(4): 1007–1034.
1187:
1178:
1177:
1167:
1135:
1129:
1128:
1100:
1094:
1093:
1083:
1051:
1045:
1044:
1012:
1006:
1005:
995:
984:10.1039/B603600G
978:(5): 1041–1072.
963:
952:
951:
919:
913:
912:
880:
874:
851:
834:ThYme (database)
724:or spinosyn (an
420:
413:
406:
399:
392:
386:
300:, macrolactones/
122:Biosynthesis of
104:J. Norman Collie
85:
72:
57:
46:consisting of a
40:natural products
21:
2336:
2335:
2331:
2330:
2329:
2327:
2326:
2325:
2301:
2300:
2299:
2298:
2252:
2251:
2247:
2240:
2219:
2218:
2214:
2176:
2175:
2171:
2117:
2116:
2112:
2088:
2087:
2083:
2054:(12): 925–936.
2043:
2042:
2038:
2031:
2008:
2007:
2003:
1959:
1958:
1954:
1910:
1909:
1905:
1847:
1846:
1842:
1806:
1805:
1798:
1789:
1787:
1778:
1777:
1773:
1729:
1728:
1721:
1691:
1690:
1686:
1656:
1655:
1651:
1610:(7): e0158471.
1597:
1596:
1592:
1554:
1553:
1549:
1499:
1498:
1494:
1479:
1464:
1463:
1456:
1404:
1403:
1399:
1353:
1352:
1348:
1318:
1317:
1313:
1283:
1282:
1278:
1271:
1263:. p. 688.
1241:
1240:
1233:
1189:
1188:
1181:
1137:
1136:
1132:
1102:
1101:
1097:
1053:
1052:
1048:
1014:
1013:
1009:
965:
964:
955:
921:
920:
916:
882:
881:
877:
852:
848:
843:
820:
801:
771:naphthoquinones
756:
748:
715:
703:
688:Olivetolic acid
656:lowering agent
635:(rapamycin) (a
631:inhibitor) and
492:antihelminthics
459:
381:
341:
294:
270:
237:acyltransferase
189:
172:
147:
124:orsellinic acid
100:
84:
78:
74:
71:
67:
55:
50:of alternating
42:derived from a
38:are a class of
28:
23:
22:
15:
12:
11:
5:
2334:
2332:
2324:
2323:
2318:
2313:
2303:
2302:
2297:
2296:
2245:
2238:
2212:
2169:
2110:
2099:(3): 284–288.
2081:
2036:
2029:
2001:
1952:
1903:
1840:
1796:
1771:
1719:
1700:(2): 285–295.
1684:
1649:
1590:
1547:
1510:Microorganisms
1492:
1477:
1454:
1397:
1346:
1311:
1292:(4): 380–416.
1276:
1269:
1231:
1179:
1150:(1): 106–118.
1130:
1111:(8): 932–937.
1095:
1066:(2): 411–428.
1046:
1027:(4): 539–544.
1007:
953:
914:
875:
845:
844:
842:
839:
838:
837:
831:
826:
819:
816:
800:
797:
796:
795:
794:
793:
788:
783:
775:
774:
773:
768:
763:
755:
752:
747:
744:
743:
742:
741:
740:
737:
734:
729:
714:
711:
702:
699:
698:
697:
696:
695:
685:
680:
675:
670:
665:
663:Discodermolide
660:
650:
640:
615:
614:
613:
608:
603:
598:
593:
583:
582:
581:
570:
569:
568:
554:
553:
552:
530:
529:
528:
519:
499:
498:
497:
488:
482:clarithromycin
478:erythromycin A
471:
458:
455:
452:
451:
441:
435:
429:
422:
421:
414:
407:
400:
380:
377:
361:antiparasitics
340:
337:
293:
292:Classification
290:
269:
266:
254:enoylreductase
188:
185:
171:
168:
146:
143:
99:
96:
75:[−C(=O)−CH
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2333:
2322:
2319:
2317:
2314:
2312:
2309:
2308:
2306:
2292:
2288:
2283:
2278:
2273:
2268:
2264:
2260:
2256:
2249:
2246:
2241:
2235:
2231:
2227:
2223:
2216:
2213:
2208:
2204:
2200:
2196:
2192:
2188:
2184:
2180:
2173:
2170:
2165:
2161:
2156:
2151:
2146:
2141:
2137:
2133:
2129:
2125:
2121:
2114:
2111:
2106:
2102:
2098:
2095:(in German).
2094:
2093:
2085:
2082:
2077:
2073:
2069:
2065:
2061:
2057:
2053:
2049:
2048:
2040:
2037:
2032:
2026:
2022:
2018:
2014:
2013:
2005:
2002:
1997:
1993:
1988:
1983:
1979:
1975:
1971:
1967:
1963:
1956:
1953:
1948:
1944:
1939:
1934:
1930:
1926:
1923:(1): 90–114.
1922:
1918:
1914:
1907:
1904:
1899:
1895:
1890:
1885:
1880:
1875:
1871:
1867:
1863:
1859:
1855:
1853:
1844:
1841:
1836:
1832:
1827:
1822:
1818:
1814:
1810:
1803:
1801:
1797:
1785:
1781:
1775:
1772:
1767:
1763:
1758:
1753:
1749:
1745:
1741:
1737:
1733:
1726:
1724:
1720:
1715:
1711:
1707:
1703:
1699:
1695:
1688:
1685:
1680:
1676:
1672:
1668:
1664:
1660:
1653:
1650:
1645:
1641:
1636:
1631:
1626:
1621:
1617:
1613:
1609:
1605:
1601:
1594:
1591:
1586:
1582:
1578:
1574:
1570:
1566:
1562:
1558:
1551:
1548:
1543:
1539:
1534:
1529:
1524:
1519:
1515:
1511:
1507:
1505:
1496:
1493:
1488:
1484:
1480:
1474:
1470:
1469:
1461:
1459:
1455:
1450:
1446:
1441:
1436:
1432:
1428:
1424:
1420:
1416:
1412:
1408:
1401:
1398:
1393:
1389:
1384:
1379:
1374:
1369:
1365:
1361:
1357:
1350:
1347:
1342:
1338:
1334:
1330:
1326:
1322:
1315:
1312:
1307:
1303:
1299:
1295:
1291:
1287:
1280:
1277:
1272:
1270:9780470547847
1266:
1262:
1258:
1257:
1252:
1248:
1244:
1238:
1236:
1232:
1227:
1223:
1218:
1213:
1209:
1205:
1201:
1197:
1193:
1186:
1184:
1180:
1175:
1171:
1166:
1161:
1157:
1153:
1149:
1145:
1141:
1134:
1131:
1126:
1122:
1118:
1114:
1110:
1106:
1099:
1096:
1091:
1087:
1082:
1077:
1073:
1069:
1065:
1061:
1057:
1050:
1047:
1042:
1038:
1034:
1030:
1026:
1022:
1018:
1011:
1008:
1003:
999:
994:
989:
985:
981:
977:
973:
969:
962:
960:
958:
954:
949:
945:
941:
937:
934:: 1806–1813.
933:
929:
925:
918:
915:
910:
906:
902:
898:
894:
890:
886:
879:
876:
873:
869:
865:
861:
860:
855:
850:
847:
840:
835:
832:
830:
827:
825:
822:
821:
817:
815:
813:
812:genome mining
808:
805:
799:Biotechnology
798:
792:
789:
787:
784:
782:
779:
778:
776:
772:
769:
767:
764:
761:
760:
758:
757:
753:
751:
745:
738:
735:
733:
730:
727:
723:
720:
719:
717:
716:
712:
710:
708:
700:
693:
689:
686:
684:
681:
679:
678:Anthracimycin
676:
674:
671:
669:
666:
664:
661:
659:
655:
651:
648:
644:
641:
638:
634:
630:
626:
623:
619:
618:
616:
612:
609:
607:
604:
602:
599:
597:
594:
592:
589:
588:
587:
584:
580:
576:
575:
574:
573:Tetracyclines
571:
567:
564:
560:
559:
558:
555:
551:
547:
543:
540:
536:
535:
534:
531:
527:
524:
520:
517:
513:
509:
505:
504:
503:
500:
496:
493:
489:
487:
483:
479:
476:
472:
469:
466:
465:
464:
461:
460:
456:
449:
445:
439:
433:
427:
423:
419:
415:
412:
408:
405:
401:
398:
394:
393:
390:
385:
378:
376:
374:
370:
366:
365:coccidiostats
362:
358:
354:
350:
346:
338:
336:
334:
329:
327:
323:
317:
315:
311:
307:
303:
299:
291:
289:
287:
286:gene clusters
283:
279:
278:monooxygenase
275:
267:
265:
262:
257:
255:
251:
247:
246:ketoreductase
242:
238:
234:
229:
227:
223:
219:
215:
211:
206:
202:
201:propionyl-CoA
198:
194:
186:
180:
176:
169:
167:
165:
164:genome mining
161:
157:
153:
144:
142:
139:
138:
133:
125:
120:
116:
113:
109:
105:
97:
95:
93:
89:
83:
65:
61:
53:
49:
45:
41:
37:
33:
19:
2265:(17): 3846.
2262:
2258:
2248:
2221:
2215:
2182:
2178:
2172:
2127:
2123:
2113:
2096:
2090:
2084:
2051:
2045:
2039:
2011:
2004:
1969:
1965:
1955:
1920:
1916:
1906:
1861:
1857:
1854:sp. CB09001"
1852:Streptomyces
1851:
1843:
1816:
1812:
1788:. Retrieved
1786:. 2017-05-09
1783:
1774:
1739:
1735:
1697:
1693:
1687:
1662:
1658:
1652:
1607:
1603:
1593:
1560:
1556:
1550:
1513:
1509:
1504:Streptomyces
1503:
1495:
1467:
1414:
1410:
1400:
1363:
1359:
1349:
1327:(1): 70–99.
1324:
1320:
1314:
1289:
1285:
1279:
1254:
1199:
1195:
1147:
1143:
1133:
1108:
1104:
1098:
1063:
1059:
1049:
1024:
1020:
1010:
975:
971:
931:
927:
917:
892:
888:
878:
857:
849:
809:
802:
749:
704:
701:Agricultural
542:amphotericin
512:geldanamycin
486:azithromycin
448:carcinogenic
444:Aflatoxin B1
438:Erythromycin
426:Geldanamycin
388:
382:
373:insecticides
371:and natural
342:
339:Applications
330:
318:
295:
271:
261:thioesterase
258:
241:ketosynthase
230:
190:
173:
170:Biosynthesis
160:metagenomics
148:
135:
129:
101:
81:
35:
29:
2311:Polyketides
2185:: 299–307.
1417:(1): 2325.
1105:MedChemComm
895:: 122–128.
864:Polyketides
777:Flavonoids
762:azaphilones
736:polynactins
726:insecticide
718:Pesticides
692:cannabinoid
683:Anthramycin
654:cholesterol
649:inhibitors)
629:calcineurin
586:Acetogenins
579:doxycycline
539:antifungals
475:antibiotics
432:Doxycycline
389:Polyketides
353:cytostatics
349:antifungals
345:antibiotics
343:Polyketide
250:dehydratase
214:isobutyrate
205:malonyl-CoA
110:by heating
36:polyketides
18:Polyketides
2305:Categories
2092:Chem. Ber.
1790:2021-07-05
1516:(5): 124.
1366:(1): 110.
841:References
746:Industrial
739:tetramycin
732:avermectin
707:pesticides
673:Usnic acid
658:lovastatin
639:inhibitor)
601:molvizarin
591:bullatacin
563:antibiotic
557:Polyethers
523:antibiotic
502:Ansamycins
495:ivermectin
468:Pikromycin
463:Macrolides
450:compound.
302:macrolides
197:acetyl-CoA
73:) groups:
2259:Molecules
2207:235378697
2076:205496204
1487:985609285
1125:2040-2511
1041:1445-0038
948:0368-1645
909:0368-1645
786:silymarin
759:Pigments
694:pathways)
668:Aflatoxin
643:Radicicol
633:sirolimus
611:annonacin
596:squamocin
550:pimaricin
526:rifamycin
508:antitumor
379:Medicinal
310:polyether
298:aromatics
210:coezyme A
145:Discovery
64:methylene
2291:32847100
2199:34102376
2164:22802619
2068:16322741
1996:25287333
1947:19374124
1898:30013965
1835:25250879
1766:31838842
1714:12714063
1679:11851471
1644:27388157
1604:PLOS ONE
1542:31064143
1449:30787330
1392:32448179
1341:11902441
1306:11548049
1253:(2013).
1251:Pratt CW
1226:24688489
1174:11238987
1090:21170424
1002:17898897
824:Esterase
818:See also
791:daidzein
781:curcumin
754:Examples
722:spinosad
713:Examples
606:uvaricin
566:monensin
546:nystatin
533:Polyenes
516:macbecin
457:Examples
314:polyenes
226:benzoate
222:malonate
212:include
2282:7504053
2155:3411943
2132:Bibcode
1987:4266379
1938:2766543
1889:6036704
1866:Bibcode
1864:: 254.
1813:Mycoses
1757:6935866
1635:4936691
1612:Bibcode
1585:1678529
1565:Bibcode
1533:6560455
1440:6382882
1419:Bibcode
1383:7247197
1247:Voet JG
1217:3958165
1081:3101795
993:2263081
617:Others
510:agents
322:enzymes
306:decalin
282:cyclase
108:orcinol
98:History
56:>C=O
2289:
2279:
2236:
2205:
2197:
2162:
2152:
2074:
2066:
2027:
1994:
1984:
1945:
1935:
1896:
1886:
1833:
1764:
1754:
1712:
1677:
1642:
1632:
1583:
1540:
1530:
1485:
1475:
1447:
1437:
1390:
1380:
1339:
1304:
1267:
1243:Voet D
1224:
1214:
1172:
1162:
1123:
1088:
1078:
1039:
1000:
990:
946:
907:
836:(2010)
484:, and
446:known
312:, and
224:, and
68:>CH
62:) and
52:ketone
2203:S2CID
2072:S2CID
1165:99020
854:IUPAC
647:HSP90
58:, or
48:chain
2287:PMID
2234:ISBN
2195:PMID
2160:PMID
2064:PMID
2025:ISBN
1992:PMID
1943:PMID
1894:PMID
1831:PMID
1762:PMID
1710:PMID
1675:PMID
1640:PMID
1581:PMID
1538:PMID
1483:OCLC
1473:ISBN
1445:PMID
1388:PMID
1337:PMID
1302:PMID
1265:ISBN
1222:PMID
1170:PMID
1121:ISSN
1086:PMID
1037:ISSN
998:PMID
944:ISSN
905:ISSN
652:The
637:mTOR
620:The
561:The
548:and
537:The
521:The
514:and
506:The
490:The
473:The
239:and
162:and
2277:PMC
2267:doi
2226:doi
2187:doi
2150:PMC
2140:doi
2128:109
2101:doi
2056:doi
2017:doi
1982:PMC
1974:doi
1933:PMC
1925:doi
1884:PMC
1874:doi
1821:doi
1752:PMC
1744:doi
1740:119
1702:doi
1667:doi
1630:PMC
1620:doi
1573:doi
1561:332
1528:PMC
1518:doi
1435:PMC
1427:doi
1378:PMC
1368:doi
1329:doi
1294:doi
1212:PMC
1204:doi
1160:PMC
1152:doi
1113:doi
1076:PMC
1068:doi
1029:doi
988:PMC
980:doi
936:doi
897:doi
868:doi
866:".
627:(a
256:).
199:or
134:in
30:In
2307::
2285:.
2275:.
2263:25
2261:.
2257:.
2232:.
2201:.
2193:.
2183:69
2158:.
2148:.
2138:.
2126:.
2122:.
2097:84
2070:.
2062:.
2050:.
2023:.
1990:.
1980:.
1970:53
1968:.
1964:.
1941:.
1931:.
1921:26
1919:.
1915:.
1892:.
1882:.
1872:.
1860:.
1856:.
1829:.
1817:57
1815:.
1811:.
1799:^
1782:.
1760:.
1750:.
1738:.
1734:.
1722:^
1708:.
1696:.
1673:.
1663:97
1661:.
1638:.
1628:.
1618:.
1608:11
1606:.
1602:.
1579:.
1571:.
1559:.
1536:.
1526:.
1512:.
1508:.
1481:.
1457:^
1443:.
1433:.
1425:.
1413:.
1409:.
1386:.
1376:.
1364:19
1362:.
1358:.
1335:.
1325:19
1323:.
1300:.
1290:18
1288:.
1249:,
1245:,
1234:^
1220:.
1210:.
1200:44
1198:.
1194:.
1182:^
1168:.
1158:.
1148:65
1146:.
1142:.
1119:.
1107:.
1084:.
1074:.
1064:28
1062:.
1058:.
1035:.
1023:.
1019:.
996:.
986:.
976:24
974:.
970:.
956:^
942:.
932:91
930:.
926:.
903:.
893:63
891:.
887:.
856:,
709:.
544:,
480:,
367:,
363:,
359:,
355:,
351:,
347:,
316:.
304:,
228:.
220:,
216:,
79:−]
34:,
2293:.
2269::
2242:.
2228::
2209:.
2189::
2166:.
2142::
2134::
2107:.
2103::
2078:.
2058::
2052:3
2033:.
2019::
1998:.
1976::
1949:.
1927::
1900:.
1876::
1868::
1862:6
1837:.
1823::
1793:.
1768:.
1746::
1716:.
1704::
1698:7
1681:.
1669::
1646:.
1622::
1614::
1587:.
1575::
1567::
1544:.
1520::
1514:7
1506:"
1489:.
1451:.
1429::
1421::
1415:9
1394:.
1370::
1343:.
1331::
1308:.
1296::
1273:.
1228:.
1206::
1176:.
1154::
1127:.
1115::
1109:3
1092:.
1070::
1043:.
1031::
1025:8
1004:.
982::
950:.
938::
911:.
899::
870::
728:)
518:,
82:n
77:2
70:2
66:(
54:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.