707:
other optical polymers, that is, they have refractive indices of between 1.5 and 1.7 and provide good propagation of light between approximately 400 nm and 1700 nm. Close refractive index (RI) matching between materials is important for proper propagation of light through them. Because of the ease of RI matching, PPEs are used in many optical devices as optical fluids. Extreme resistance to ionizing radiation gives PPEs an added advantage in the manufacture of solar cells and solid-state UV/blue emitters and telecommunication equipment made from high-index glasses and semiconductors.
689:
avoided, such as in certain electronic devices. A thin film of polyphenyl ether on a surface is not a thin contiguous film as one would envision, but rather comprises tiny droplets. This PPE property tends to keep the film stationary, or at least to cause it to remain in the area where the lubrication is needed, rather than migrating away by spreading and forming a new surface. As a result, contamination of other components and equipment, which do not require a lubricant, is avoided. The high surface tension of PPEs, therefore, makes them useful in lubricating electronic contacts.
724:
Due to the low volatility and excellent high-temperature thermo-oxidative stability, PPEs have also found use as a lubricant for chains used in and around kilns, metal fabrication plants, and glass molding and manufacturing equipment. In these high-temperature applications, PPEs do not form any sludge and hard deposits. The low soft-carbon residue that is left behind is removed easily by wiping. PPEs' low volatility, low flammability, and good thermodynamic properties make them ideally suited for use as heat transfer fluids and in heat sink applications as well.
720:
also used as base fluids for radiation-resistant greases used in nuclear power plant mechanisms. PPEs and their derivatives have also found use as vapor phase lubricants in gas turbines and custom bearings, and wherever extreme environmental conditions exist. Vapor phase lubrication is achieved by heating the liquid lubricant above its boiling point. The resultant vapors are then transported to the hot bearing surface. If the temperatures of the bearing surface are kept below the lubricant’s boiling point, the vapors re-condense to provide liquid lubrication.
109:
82:
1081:
28:
224:. PPEs have the disadvantage of having somewhat high pour points. For example, PPEs that contain two and three benzene rings are actually solids at room temperatures. The melting points of the ordinarily solid PPEs are lowered if they contain more m-phenylene rings, alkyl groups, or are mixtures of isomers. PPEs that contain only o- and p-substituted rings have the highest melting points.
145:(DPE), also called diphenyl oxide, the structure of which is provided in Figure 4. Low molecular weight polyphenyl ethers and thioethers are used in a variety of applications, and include high-vacuum devices, optics, electronics, and in high-temperature and radiation-resistant fluids and greases. Figure 5 shows the structure of the sulfur analogue of 3-R polyphenyl ether shown in Figure 3.
98:
20:
1087:
746:. See Figure 2 for the PPO structure. PPO polymers can be classified as plastic resins. They and their composites with polystyrene, glass, and nylon are used as high-strength, moisture-resistant engineering plastics in a number of industries, including computer, telecommunication, and automotive parts. PPOs are marketed by
706:
Polyphenyl ethers (PPEs) possess good optical clarity, a high refractive index, and other beneficial optical properties. Because of these, PPEs have the ability to meet the rigorous performance demands of signal processing in advanced photonics systems. Optical clarity of PPEs resembles that of the
419:
10 ergs/gram of radiation at 99 °C (210 °F) was compared with synthetic ester, synthetic hydrocarbon, and silicone fluids. PPE showed a viscosity increase of only 35%, while all other fluids showed a viscosity increase of 1700% and gelled. Further tests have shown PPEs to be resistant
719:
PPEs were developed for use in jet engines that involved high speed-related frictional temperatures of as high as 320 °C (608 °F). While the use of PPEs in lubricating jet engines has somewhat subsided due to their higher cost, they are still used in some aerospace applications. PPEs are
801:
Bolt, R. O., "Radiation
Effects on Lubricants," CRC Handbook of Lubrication, Vol. I, Theory and Practice of Tribology: Applications and Maintenance, pp. 3–44 , Richard E. Booser Editor, CRC Press, Boca Raton, 1983. Carroll, J. G. and Bolt. R. O., Radiation Effects on Organic Materials, Bolt. R. O.
387:
PPEs have excellent high temperature properties and good oxidation stability. With respect to volatilities, p-derivatives have the lowest volatilities, and the o-derivatives have the highest volatilities. The opposite is true for flash points and fire points. Spontaneous ignition temperatures of
723:
Polyphenyl ether technology can also provide superior fire safety and fatigue life, depending on the specific bearing design. In this application, PPEs have the advantage of providing lubrication both as a liquid at low temperatures and as a vapor at temperatures above 315 °C (599 °F).
388:
polyphenyl ethers lie between 550 and 595 °C (1,022 and 1,103 °F), alkyl substitution reduces this value by ~50 °C (122 °F). PPEs are compatible with most metals and elastomers that are commonly used in high-temperature applications. They typically swell common seal materials.
692:
Polyphenyl ether lubricants have a 30-year history of commercial service for connectors with precious and base metal contacts in telecom, automotive, aerospace, instrumentation and general-purpose applications. In addition to maintaining the current flow and providing long-term lubrication, PPEs
688:
5R4E PPE has a surface tension of 49.9 dynes/cm, which is amongst the highest in pure organic liquids. Because of this, this PPE and the other PPEs do not effectively wet metal surfaces. This property is useful when migration of a lubricant from one part of the equipment to another part must be
137:
equal to 1 is identified as pmp5P4E, indicating para, meta, para substitution of the three middle rings, a total of 5 rings, and 4 ether linkages. Meta substitution of the aryl rings in these materials is most common and often desired. Longer chain analogues with up to 10 benzene rings are also
72:
s). The phenoxy groups in the former class of polymers do not contain any substituents whereas those in the latter class contain 2 to 4 alkyl groups on the phenyl ring. The structure of an oxygen-containing PPE is provided in Figure 1 and that of a 2, 6-xylenol derived PPO is shown in Figure 2.
470:
10 torr at 25 °C. Such high vacuums are necessary in equipment such as electron microscopes, mass spectrometers and that used for various surface physics studies. Vacuum pumps are also used in the production of electric lamps, vacuum tubes, and cathode ray tubes (CRTs), semiconductor
432:
PPEs have high surface tension; hence these fluids have a lower tendency to wet metal surfaces. The surface tension of the commercially available 5R4E is 49.9 dynes/cm, one of the highest in pure organic liquids. This property is useful in applications where migration of the lubricant into the
403:
Ionizing radiation affects all organic compounds, causing a change in their properties because radiation disrupts covalent bonds that are most prevalent in organic compounds. One result of ionization is that the organic molecules disproportionate to form smaller hydrocarbon molecules as well as
216:
Typical physical properties of polyphenyl ethers are provided in Table 2. Physical properties of a particular PPE depend upon the number of aromatic rings, their substitution pattern, and whether it is an ether or a thioether. In the case of products of mixed structures, properties are hard to
715:
PPEs, being of excellent thermo-oxidative stability and radiation resistance, have found extensive use in high temperature applications that also require radiation resistance. In addition, PPEs demonstrate better wear control and load-carrying ability than mineral oils, especially when used in
462:
in combination with a fore pump are amongst the most popular. Diffusion pumps use a high boiling liquid of low vapor pressure to create a high-speed jet that strikes the gaseous molecules in the system to be evacuated and direct them into space that is being evacuated by the fore pump. A good
132:
PPEs of up to 6 phenyl rings, both oxy and thio ethers, are commercially available. See Table 1. They are characterized by indicating the substitution pattern of each ring, followed by the number of phenyl rings and the number of ether linkages. Thus, the structure in Figure 1 with
441:
While originally PPEs were developed for use in extreme environments that were experienced in aerospace applications, they are now used in other applications requiring low volatility and excellent thermo-oxidative and ionizing radiation stability. Such applications include use as
463:
diffusion fluid must therefore reflect low vapor pressure, high flash point, high thermal and oxidative stability and chemical resistance. If the diffusion pump is operating in the proximity of ionizing radiation source, good radiation stability is also desired.
693:
offer protection to connectors against aggressive acidic and oxidative environments. By providing a protective surface film, polyphenyl ethers not only protect connectors against corrosion but also against vibration-related wear and abrasion that leads to
415:) the polyphenyl ethers are the most radiation resistant. Excellent radiation stability of PPEs can be ascribed to the limited number of ionizable carbon-carbon and carbon-hydrogen bonds. In one study, the performance of PPE under the influence of 1
446:
fluids; high vacuum fluids; and in formulating jet engine lubricants, high-temperature hydraulic lubricants and greases, and heat transfer fluids. In addition, because of excellent optical properties these fluids have found use in optical devices.
697:
wear. The devices that benefit from the specialized properties of PPEs include cell phones, printers, and a variety of other electronic appliances. The protection lasts for decades or for the life of the equipment.
1688:
404:
larger hydrocarbons molecules. This is reflected by increased evaporation loss, lowering of the flash and fire points, and increased viscosity. Other chemical reactions caused by radiation include oxidation and
116:
The proper name for a phenyl ether polymer is poly(phenyl ether) or polyphenyl polyether, but the name polyphenyl ether is widely accepted. Polyphenyl ethers (PPEs) are obtained by repeated application of the
866:
Hamid, S. and Burian, S. A., "Polyphenyl Ether
Lubricants," published in Synthetics, Mineral Oils, and Bio-based Lubricants: Chemistry and Technology, Leslie R. Rudnick Editor, pp. 175–199, Taylor and Francis
847:
Using
Lubricants to Avoid Failures in Medical Electronic Connectors," by Sibtain Hamid in Medical Electronics Manufacturing, Spring 2004 and SANTOLUBES Brochure on Stationary lubricants prevent connector
877:
2002grc087 High Heat PPO.: 13C and 31P NMR Methods for
Characterizing End Groups and Chain Structures in Poly(2,6-dimethyl-1,4-phenylene oxide)/Poly(2,3,6-trimethyl-1,4-phenylene oxide) Copolymers
792:"Synthetic Lubricants," Chapter 6, pp. 96–153, Lubricants and Related Products: Synthesis, Properties, Applications, International Standards by Dieter Klamann, Verlag Chemie Gmbh publisher (1984)
783:
Joaquim, M., "Polyphenyl Ether
Lubricants" Synthetic Lubricants and High-performance Functional Fluids", R. L. Rudnick and R. L. Shubkin, Eds., p. 239, Marcel Dekker, Inc., NY, 1999
391:
Oxidation stability of un-substituted PPEs is quite good, partly because they lack easily oxidizable carbon-hydrogen bonds. Thermal decomposition temperature, as measured by the
1412:
466:
Data presented in Table 3 demonstrates polyphenyl ether to be superior to other fluids that are commonly used in diffusion pumps. PPEs help achieve the highest vacuum of 4
738:
These polymers are made through oxidative coupling of substituted phenol in the presence of oxygen and copper and amine containing catalysts, such as
1405:
1288:
1747:
1698:
1667:
1398:
905:
408:. The former leads to increased acidity, corrosivity, and coke formation; the latter causes a change in viscosity and volatility.
930:
1683:
1662:
1559:
1313:
1737:
1657:
411:
PPEs have extremely high radiation resistance. Of all classes of synthetic lubricants (with the possible exception of
1730:
1652:
1468:
1065:
995:
970:
1770:
1504:
1390:
1303:
1128:
945:
935:
811:
Joaquim, M. E. and J. F. Herber, "Lubrication of
Electronic Connectors and Equipment in Radiation Environments,
220:
The important attributes of PPEs include their thermal and oxidative stability and stability in the presence of
1574:
1564:
1426:
1113:
1060:
950:
823:
The
Surface Tension of Pure Liquid Compounds, Joseph J. Jasper, J. Phys. Chem. Ref. Data, Vol. 1, No. 4, 1972;
765:"The Ullmann Ether Condensation," by A A Moroz and Mark S Shvartsberg, 1974, Russ. Chem. Rev. 43 (8), 679-689
1164:
1055:
1040:
1020:
733:
64:
1579:
1457:
1212:
940:
898:
1759:
1463:
1108:
876:
1479:
1474:
1447:
1365:
812:
118:
86:
1799:
1794:
1609:
1370:
1293:
1207:
1159:
108:
1624:
1542:
1283:
1257:
1227:
1187:
1169:
1118:
1070:
1050:
412:
221:
1789:
1754:
1720:
1640:
1452:
1274:
1192:
891:
217:
predict from only the structural features; hence, they must be determined via measurement.
1645:
1340:
1242:
1217:
1149:
1010:
955:
81:
1509:
1495:
1360:
1247:
1237:
1005:
739:
1080:
1538:
1350:
1252:
1123:
459:
443:
142:
102:
73:
Either class can have the oxygen atoms attached at various positions around the rings.
1783:
1725:
1532:
1318:
1308:
1298:
1202:
1103:
1025:
975:
458:
are devices that remove gases from an enclosed space to greatly reduce pressure. Oil
405:
1345:
1335:
1197:
1045:
990:
965:
392:
27:
836:
1528:
1500:
1435:
1355:
1154:
1035:
1030:
824:
455:
126:
19:
1604:
1232:
1222:
1086:
47:
1693:
1599:
1569:
1439:
1000:
985:
960:
97:
54:
linkages. Commercial phenyl ether polymers belong to two chemical classes:
1614:
743:
694:
1742:
1619:
1591:
1520:
1422:
1144:
914:
857:
SANTOLUBES Brochure on
Stationary lubricants prevent connector failures
835:"Inside a Vacuum Diffusion Pump," by Manuel E. Joaquim and Bill Foley;
122:
39:
43:
813:
http://www.chemassociates.com/products/findett/PPEs_Radiation2.pdf
747:
51:
395:
procedure, is between 440 and 465 °C (824 and 869 °F).
980:
1394:
887:
424:
10 erg/g at temperatures up to 315 °C (599 °F).
31:
Figure 2: Representative
Structure of Polyphenylene Oxide (PPO)
186:
Three- and four-ring oxy- and thioethers; trade name: MCS-293
23:
Figure 1: Representative
Structure of Polyphenyl Ether (PPE)
883:
194:
Three-ring polyphenyl ether (3P2E); trade name: MCS-2167
802:
and Carroll, J. G., Eds., Academic Press, New York, 1963
420:
to gamma and associated neutron radiation dosages of 1
178:
Four-ring polyphenyl ether (4P3E); trade name: MCS-210
170:
Five-ring polyphenyl ether (5P4E); trade name: OS-124
162:
Six-ring polyphenyl ether (6P5E); trade name: OS-138
149:
Table 1: commercial polyphenyl ether products (PPEs)
1713:
1676:
1633:
1590:
1552:
1519:
1488:
1434:
1383:
1328:
1273:
1266:
1178:
1137:
1094:
921:
711:
High-temperature and radiation-resistant lubricants
228:Table 2: physical properties of polyphenyl ethers
141:The simplest member of the phenyl ether family is
112:Figure 5: structure of 3R2TE polyphenyl thioether
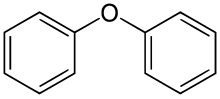
205:Diphenyl ether, diphenyl oxide, phenoxybenzene
837:http://www.xtronix.ch/pdf/Diffusion%20Pump.pdf
1406:
899:
825:https://www.nist.gov/srd/PDFfiles/jpcrd13.pdf
475:Table 3: diffusion fluid property comparison
93:-diphenoxybenzene), a simple polyphenyl ether
8:
612:Refractive index at 25 °C, 589 nm
1413:
1399:
1391:
1270:
906:
892:
884:
433:surrounding environment must be avoided.
473:
226:
189:Thiobis and bis (phenylmercapto)benzene
147:
107:
96:
80:
26:
18:
758:
996:Polyethylene terephthalate (PET, PETE)
936:Cross-linked polyethylene (PEX, XLPE)
931:Acrylonitrile butadiene styrene (ABS)
750:under the trademarked name of Noryl.
7:
1748:List of environmental health hazards
1668:List of environmental health hazards
471:processing, and vacuum engineering.
500:Vapor pressure, Torr at 25 °C
1553:Miscellaneous additives incl. PHCs
14:
1085:
1079:
971:Polybutylene terephthalate (PBT)
946:Poly(methyl methacrylate) (PMMA)
50:group as the repeating group in
951:Poly(ethyl methacrylate) (PEMA)
774:SANTOLUBES LLC Product Brochure
684:Electronic connector lubricants
584:Viscosity (cSt) at 100 °C
202:Two-ring diphenyl ether (2P1E)
173:m-Bis(m-phenoxyphenoxy)benzene
1314:Category:Plastics applications
1061:Styrene maleic anhydride (SMA)
1056:Polyvinylidene chloride (PVDC)
1041:Polytetrafluoroethylene (PTFE)
570:Viscosity (cSt) at 25 °C
556:Boiling point at 1.3 mbar, °C
121:: reaction of an alkali-metal
1:
1021:Poly(p-phenylene oxide) (PPO)
129:benzene catalyzed by copper.
1738:Persistent organic pollutant
1699:Toxic Substances Control Act
1658:Persistent organic pollutant
941:Ethylene vinyl acetate (EVA)
256:at 210 °F (99 °C)
251:at 100 °F (38 °C)
181:Bis (m-phenoxyphenyl) ether
101:Figure 4: Structure of 2R1E
1731:Great Pacific garbage patch
1653:Great Pacific garbage patch
1066:Styrene-acrylonitrile (SAN)
981:Polyetheretherketone (PEEK)
728:Polyphenylene oxides (PPOs)
1816:
1694:Japan Toxic Substances Law
1489:Miscellaneous plasticizers
731:
598:Surface tension, dynes/cm
383:Thermo-oxidative stability
1768:
1689:European REACH regulation
1684:California Proposition 65
1427:polyhalogenated compounds
1304:High-performance plastics
1129:High-performance plastics
1077:
748:SABIC Innovative Plastics
1114:Fibre-reinforced plastic
1051:Polyvinyl chloride (PVC)
451:Ultra-high-vacuum fluids
1165:Biodegradable additives
734:Poly(p-phenylene oxide)
119:Ullmann Ether Synthesis
87:Ullmann Ether Synthesis
77:Structure and synthesis
1580:Perfluorooctanoic acid
1016:Polyphenyl ether (PPE)
1011:Polyoxymethylene (POM)
956:Polyacrylic acid (PAA)
528:Density at 25 °C
321:3- and 4-ring oxythio
154:Common and trade name
113:
105:
94:
32:
24:
1760:Biodegradable plastic
1109:Thermosetting polymer
1006:Polylactic acid (PLA)
668:Radiation resistance
640:Oxidation resistance
111:
100:
84:
36:Phenyl ether polymers
30:
22:
1771:Identification codes
1371:Foam food containers
1294:Engineering plastics
654:Chemical resistance
65:polyphenylene oxides
1610:Endocrine disruptor
1208:Compression molding
1160:Polymer stabilizers
476:
413:perfluoropolyethers
399:Radiation stability
229:
212:Physical properties
197:m-Diphenoxybenzene
150:
1625:Polymer fume fever
1284:Commodity plastics
1258:Rotational molding
1228:Fiberglass molding
1188:Injection moulding
1170:Filler (materials)
1119:Corrugated plastic
1071:Tritan copolyester
1026:Polypropylene (PP)
976:Polycarbonate (PC)
626:Thermal stability
474:
244:Thermal stability
227:
222:ionizing radiation
148:
114:
106:
95:
33:
25:
1777:
1776:
1755:Plastic recycling
1721:Plastic pollution
1707:
1706:
1641:Plastic pollution
1421:Health issues of
1379:
1378:
1275:Plastics industry
1193:Plastic extrusion
1046:Polyurethane (PU)
1036:Polysulfone (PES)
991:Polyethylene (PE)
966:Polybutylene (PB)
681:
680:
514:Molecular weight
483:Polyphenyl ether
380:
379:
254:Viscosity (cSt),
249:Viscosity (cSt),
233:Polyphenyl ether
209:
208:
56:polyphenyl ethers
16:Class of polymers
1807:
1646:Rubber pollution
1496:Organophosphates
1415:
1408:
1401:
1392:
1271:
1243:Filament winding
1218:Transfer molding
1145:Polymer additive
1089:
1083:
1031:Polystyrene (PS)
908:
901:
894:
885:
879:
874:
868:
864:
858:
855:
849:
845:
839:
833:
827:
821:
815:
809:
803:
799:
793:
790:
784:
781:
775:
772:
766:
763:
542:Flash point, °C
493:Hydrocarbon oil
477:
469:
423:
418:
230:
151:
1815:
1814:
1810:
1809:
1808:
1806:
1805:
1804:
1780:
1779:
1778:
1773:
1764:
1709:
1708:
1703:
1672:
1629:
1586:
1548:
1515:
1484:
1430:
1419:
1385:
1375:
1324:
1262:
1248:Solvent bonding
1238:Plastic welding
1180:
1174:
1133:
1096:
1090:
1084:
1075:
986:Polyester (PEs)
923:
917:
912:
882:
875:
871:
865:
861:
856:
852:
846:
842:
834:
830:
822:
818:
810:
806:
800:
796:
791:
787:
782:
778:
773:
769:
764:
760:
756:
740:cuprous bromide
736:
730:
713:
704:
686:
494:
489:
484:
480:Fluid property
467:
460:diffusion pumps
453:
439:
430:
428:Surface tension
421:
416:
401:
385:
255:
250:
245:
240:
214:
79:
42:that contain a
38:are a class of
17:
12:
11:
5:
1813:
1811:
1803:
1802:
1797:
1792:
1782:
1781:
1775:
1774:
1769:
1766:
1765:
1763:
1762:
1757:
1752:
1751:
1750:
1745:
1740:
1735:
1734:
1733:
1717:
1715:
1711:
1710:
1705:
1704:
1702:
1701:
1696:
1691:
1686:
1680:
1678:
1674:
1673:
1671:
1670:
1665:
1660:
1655:
1650:
1649:
1648:
1637:
1635:
1631:
1630:
1628:
1627:
1622:
1617:
1612:
1607:
1602:
1596:
1594:
1588:
1587:
1585:
1584:
1583:
1582:
1572:
1567:
1562:
1556:
1554:
1550:
1549:
1547:
1546:
1539:Vinyl chloride
1536:
1533:Polycarbonates
1525:
1523:
1517:
1516:
1514:
1513:
1507:
1498:
1492:
1490:
1486:
1485:
1483:
1482:
1477:
1472:
1466:
1461:
1455:
1450:
1444:
1442:
1432:
1431:
1420:
1418:
1417:
1410:
1403:
1395:
1389:
1387:
1381:
1380:
1377:
1376:
1374:
1373:
1368:
1363:
1358:
1353:
1348:
1346:Packaging film
1343:
1338:
1332:
1330:
1329:Specific goods
1326:
1325:
1323:
1322:
1316:
1311:
1306:
1301:
1296:
1291:
1286:
1280:
1278:
1268:
1264:
1263:
1261:
1260:
1255:
1253:Vacuum forming
1250:
1245:
1240:
1235:
1230:
1225:
1220:
1215:
1210:
1205:
1200:
1195:
1190:
1184:
1182:
1176:
1175:
1173:
1172:
1167:
1162:
1157:
1152:
1147:
1141:
1139:
1135:
1134:
1132:
1131:
1126:
1124:Polymeric foam
1121:
1116:
1111:
1106:
1100:
1098:
1092:
1091:
1078:
1076:
1074:
1073:
1068:
1063:
1058:
1053:
1048:
1043:
1038:
1033:
1028:
1023:
1018:
1013:
1008:
1003:
1001:Polyimide (PI)
998:
993:
988:
983:
978:
973:
968:
963:
961:Polyamide (PA)
958:
953:
948:
943:
938:
933:
927:
925:
919:
918:
913:
911:
910:
903:
896:
888:
881:
880:
869:
859:
850:
840:
828:
816:
804:
794:
785:
776:
767:
757:
755:
752:
732:Main article:
729:
726:
712:
709:
703:
700:
685:
682:
679:
678:
675:
672:
669:
665:
664:
661:
658:
655:
651:
650:
647:
644:
641:
637:
636:
633:
630:
627:
623:
622:
619:
616:
613:
609:
608:
605:
602:
599:
595:
594:
591:
588:
585:
581:
580:
577:
574:
571:
567:
566:
563:
560:
557:
553:
552:
549:
546:
543:
539:
538:
535:
532:
529:
525:
524:
521:
518:
515:
511:
510:
507:
504:
501:
497:
496:
491:
486:
481:
452:
449:
444:diffusion pump
438:
435:
429:
426:
400:
397:
384:
381:
378:
377:
374:
371:
368:
365:
362:
358:
357:
354:
351:
348:
345:
342:
338:
337:
334:
331:
328:
325:
322:
318:
317:
314:
311:
308:
305:
302:
298:
297:
294:
291:
288:
285:
282:
278:
277:
274:
271:
268:
265:
262:
258:
257:
252:
247:
242:
237:
234:
213:
210:
207:
206:
203:
199:
198:
195:
191:
190:
187:
183:
182:
179:
175:
174:
171:
167:
166:
163:
159:
158:
157:Chemical name
155:
143:diphenyl ether
103:diphenyl ether
78:
75:
15:
13:
10:
9:
6:
4:
3:
2:
1812:
1801:
1798:
1796:
1793:
1791:
1788:
1787:
1785:
1772:
1767:
1761:
1758:
1756:
1753:
1749:
1746:
1744:
1741:
1739:
1736:
1732:
1729:
1728:
1727:
1726:Garbage patch
1724:
1723:
1722:
1719:
1718:
1716:
1712:
1700:
1697:
1695:
1692:
1690:
1687:
1685:
1682:
1681:
1679:
1675:
1669:
1666:
1664:
1661:
1659:
1656:
1654:
1651:
1647:
1644:
1643:
1642:
1639:
1638:
1636:
1632:
1626:
1623:
1621:
1618:
1616:
1613:
1611:
1608:
1606:
1603:
1601:
1598:
1597:
1595:
1593:
1592:Health issues
1589:
1581:
1578:
1577:
1576:
1573:
1571:
1568:
1566:
1563:
1561:
1558:
1557:
1555:
1551:
1544:
1540:
1537:
1534:
1530:
1527:
1526:
1524:
1522:
1518:
1511:
1508:
1506:
1502:
1499:
1497:
1494:
1493:
1491:
1487:
1481:
1478:
1476:
1473:
1470:
1467:
1465:
1462:
1459:
1456:
1454:
1451:
1449:
1446:
1445:
1443:
1441:
1437:
1433:
1428:
1424:
1416:
1411:
1409:
1404:
1402:
1397:
1396:
1393:
1388:
1382:
1372:
1369:
1367:
1366:Shopping bags
1364:
1362:
1359:
1357:
1354:
1352:
1349:
1347:
1344:
1342:
1339:
1337:
1334:
1333:
1331:
1327:
1321:(Agriculture)
1320:
1319:Plasticulture
1317:
1315:
1312:
1310:
1307:
1305:
1302:
1300:
1299:Geosynthetics
1297:
1295:
1292:
1290:
1287:
1285:
1282:
1281:
1279:
1276:
1272:
1269:
1265:
1259:
1256:
1254:
1251:
1249:
1246:
1244:
1241:
1239:
1236:
1234:
1231:
1229:
1226:
1224:
1221:
1219:
1216:
1214:
1211:
1209:
1206:
1204:
1203:Thermoforming
1201:
1199:
1196:
1194:
1191:
1189:
1186:
1185:
1183:
1177:
1171:
1168:
1166:
1163:
1161:
1158:
1156:
1153:
1151:
1148:
1146:
1143:
1142:
1140:
1136:
1130:
1127:
1125:
1122:
1120:
1117:
1115:
1112:
1110:
1107:
1105:
1104:Thermoplastic
1102:
1101:
1099:
1093:
1088:
1082:
1072:
1069:
1067:
1064:
1062:
1059:
1057:
1054:
1052:
1049:
1047:
1044:
1042:
1039:
1037:
1034:
1032:
1029:
1027:
1024:
1022:
1019:
1017:
1014:
1012:
1009:
1007:
1004:
1002:
999:
997:
994:
992:
989:
987:
984:
982:
979:
977:
974:
972:
969:
967:
964:
962:
959:
957:
954:
952:
949:
947:
944:
942:
939:
937:
934:
932:
929:
928:
926:
920:
916:
909:
904:
902:
897:
895:
890:
889:
886:
878:
873:
870:
863:
860:
854:
851:
844:
841:
838:
832:
829:
826:
820:
817:
814:
808:
805:
798:
795:
789:
786:
780:
777:
771:
768:
762:
759:
753:
751:
749:
745:
741:
735:
727:
725:
721:
717:
710:
708:
701:
699:
696:
690:
683:
676:
673:
670:
667:
666:
662:
659:
656:
653:
652:
648:
645:
642:
639:
638:
634:
631:
628:
625:
624:
620:
617:
614:
611:
610:
606:
603:
600:
597:
596:
592:
589:
586:
583:
582:
578:
575:
572:
569:
568:
564:
561:
558:
555:
554:
550:
547:
544:
541:
540:
536:
533:
530:
527:
526:
522:
519:
516:
513:
512:
508:
505:
502:
499:
498:
492:
487:
482:
479:
478:
472:
464:
461:
457:
450:
448:
445:
436:
434:
427:
425:
414:
409:
407:
406:isomerization
398:
396:
394:
389:
382:
375:
372:
370:316; >600
369:
366:
363:
360:
359:
355:
352:
349:
346:
343:
340:
339:
335:
332:
329:
326:
323:
320:
319:
315:
312:
309:
306:
304:Clear liquid
303:
300:
299:
295:
292:
289:
286:
284:Clear liquid
283:
280:
279:
275:
272:
269:
266:
264:Clear liquid
263:
260:
259:
253:
248:
243:
238:
235:
232:
231:
225:
223:
218:
211:
204:
201:
200:
196:
193:
192:
188:
185:
184:
180:
177:
176:
172:
169:
168:
164:
161:
160:
156:
153:
152:
146:
144:
139:
136:
130:
128:
124:
120:
110:
104:
99:
92:
88:
83:
76:
74:
71:
67:
66:
61:
57:
53:
49:
45:
41:
37:
29:
21:
1436:Plasticizers
1384:Environment
1336:Blister pack
1289:Construction
1198:Blow molding
1015:
872:
862:
853:
843:
831:
819:
807:
797:
788:
779:
770:
761:
737:
722:
718:
714:
705:
691:
687:
490:Dow Corning
465:
456:Vacuum pumps
454:
440:
437:Applications
431:
410:
402:
393:isoteniscope
390:
386:
361:2-ring 2P1E
341:3-ring 3P2E
324:Hazy liquid
301:4-ring 4P3E
281:5-ring 5P4E
261:6-ring 6P5E
219:
215:
140:
134:
131:
115:
90:
69:
63:
59:
55:
35:
34:
1677:Regulations
1529:Bisphenol A
1213:Calendering
1155:Plasticizer
1095:Mechanical
485:SANTOVAC 5
239:Pour point
236:Appearance
127:halogenated
48:thiophenoxy
1800:Lubricants
1795:Polyethers
1784:Categories
1605:Carcinogen
1570:Organotins
1440:Phthalates
1386:and health
1233:Pultrusion
1223:Laminating
1181:processing
754:References
716:bearings.
671:Excellent
657:Excellent
649:Poor-fair
646:Excellent
643:Excellent
629:Excellent
165:Bis ether
85:Figure 3:
1634:Pollution
1600:Teratogen
1531:(BPA, in
1179:Plastics
1150:Colorants
1138:Additives
922:Chemical
867:Publisher
488:Silicone
350:427; 800
330:367; 693
327:−29; −20
310:441; 825
290:453; 847
270:447; 836
246:(°C; °F)
241:(°C; °F)
89:of 4R2E (
1790:Plastics
1615:Diabetes
1521:Monomers
1501:Adipates
1423:plastics
1277:segments
1267:Products
915:Plastics
848:failures
744:pyridine
695:fretting
495:Apiezon
307:−12; 10
287:4.5; 40
40:polymers
1743:Dioxins
1663:Dioxins
1620:Obesity
1361:Cutlery
1351:Bottles
267:10; 50
138:known.
125:with a
123:phenate
62:s) and
44:phenoxy
1460:(BBzP)
1429:(PHCs)
1341:Chairs
1309:Nurdle
702:Optics
364:Solid
344:Solid
1714:Waste
1560:PBDEs
1471:(DOP)
1097:types
924:types
677:Fair
674:Good
663:Poor
660:Good
635:Poor
632:Good
621:1.48
618:1.56
615:1.67
607:30.5
604:30.5
601:49.9
587:12.0
573:1000
537:0.87
534:1.07
531:1.20
509:5×10
506:2×10
503:4×10
273:2000
52:ether
46:or a
1575:PFCs
1565:PCBs
1541:(in
1505:DEHA
1480:DINP
1475:DIDP
1469:DEHP
1464:DIHP
1448:DIBP
1425:and
1356:Bags
742:and
593:7.0
590:4.3
579:135
565:220
562:223
559:295
551:243
548:221
545:288
523:420
520:484
517:446
376:1.6
373:2.4
293:360
1543:PVC
1510:DOA
1458:BBP
1453:DBP
576:40
353:12
333:25
313:70
296:13
276:25
70:PPO
60:PPE
1786::
1438::
367:-
356:3
347:-
336:4
316:6
1545:)
1535:)
1512:)
1503:(
1414:e
1407:t
1400:v
907:e
900:t
893:v
468:×
422:×
417:×
135:n
91:p
68:(
58:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.