517:
442:
257:
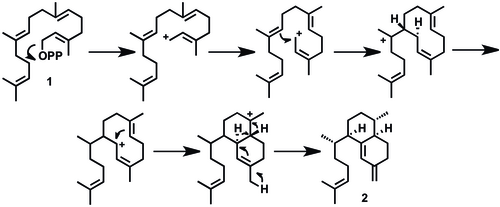
436:), is described below. Elisabethatriene synthase, a diterpene cyclase enzyme, catalyzes the transformation of the diterpene GGPP to a 10-membered carbon skeleton followed by hydride migration towards the bicyclic ring system. This cyclase enzyme has been identified as a key enzyme in forming the carbon skeleton of pseudopterosins in one step. An alternative mechanism has been proposed in which a six-membered ring is formed first, then a second ring closing for the bicyclic system.
546:
24:
138:
396:, found in the Bahamas and Florida Keys. Pseudopterosins A-D, which differ in the degree of acetylation at the sugar ring, were first isolated and reported in 1986. There are at least 25 unique diterpenes isolated from this species of marine animal. Samples of
280:
InChI=1S/C25H36O6/c1-11(2)8-15-9-13(4)16-7-6-12(3)18-20(16)19(15)14(5)21(27)24(18)31-25-23(29)22(28)17(26)10-30-25/h8,12-13,15-17,22-23,25-29H,6-7,9-10H2,1-5H3/t12-,13-,15-,16+,17+,22-,23+,25-/m0/s1
400:
from the
Bahamas are found to have higher concentrations of pseudopterosins than populations from the Florida Keys, which have a greater diversity in diterpene structures.
296:
408:
Pseudopterosins have anti-inflammatory and analgesic activity, with a mechanism of action different from the common non-steroidal anti-inflammatory drugs,
271:
499:
have been identified using radio labeling studies. An alternative mechanism has been proposed with no hydroxyquinone intermediate (
557:
The proposed synthesis of artificial anti-inflammatory metabolites is modeled after pseudopterosins and is based on the bicyclic
392:
429:
214:
235:
680:
368:
516:
158:
441:
70:
36:
252:
685:
104:
424:) has been identified as a key intermediate for the synthesis of the class of pseudopterosins and
526:
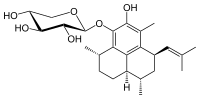
The branching point for the biosynthesis of the tricyclic pseudopterosins versus the bicyclic
412:. Commercially, pseudopterosins are found in skin creams as topical anti-inflammatory agents.
178:
428:-pseudopterosins. A proposed mechanistic pathway for the synthesis of elisabethatriene from
319:
114:
223:
451:
The biosynthesis of the pseudopterosins continues with an aromatization to erogorgiaene (
256:
362:
674:
203:
545:
538:
is oxidized once then hydroxylated followed by glycosylation to make the bicyclic
56:)-5-Hydroxy-3,6,9-trimethyl-7-(2-methylprop-1-en-1-yl)-2,3,7,8,9,9a-hexahydro-1
23:
347:
169:
507:
undergoes two subsequent oxidations at C-6 and C-7 to a structure resembling
387:
384:
190:
409:
149:
361:
Except where otherwise noted, data are given for materials in their
544:
515:
440:
137:
127:
479:). This is a plausible biosynthetic pathway, and intermediates
642:
A. Mayer, P. Jacobson, W. Fenical, R. Jabocs and K. Glaser.
240:
463:), and another oxidation to an ortho-hydroxyquinone (
603:S. Look, W. Fenical, G. Matsumoto and J. Clardy.
520:Overall biosynthetic scheme for pseudopterosin A
304:O4(Oc1c3c2c(c(c1O)C)(\C=C(/C)C)C(C)2CC3C)OC(O)4O
202:
113:
455:), two oxidations to a dihyroxyerogorgiaene (
8:
475:) and glycosylation yield Pseudopterosin A (
656:
654:
652:
586:
584:
582:
580:
578:
255:
177:
15:
222:
511:, then glycosylation to pseudopterosin.
574:
534:, the aromatized bicycle erogorgiaene.
301:
276:
251:
390:isolated from the gorgonian sea whip
283:Key: DBGVVIGAVAIWRU-GYGPFBJXSA-N
157:
7:
530:-pseudopterosins occurs at compound
445:GPP cyclization to elisabethatriene
193:
14:
660:R. Kerr, A. Kohl, and T. Ferns.
331:
22:
365:(at 25 °C , 100 kPa).
631:Arch. of Biochem. and Biophys.
337:
325:
1:
430:geranylgeranyl pyrophosphate
592:J. Ind Microbiol Biotechnol
702:
590:A. Kohl, A. Ata, R. Kerr.
561:-pseudopterosin structure
91:)-2-{oxy}oxane-3,4,5-triol
393:Antillogorgia elisabethae
359:
312:
292:
267:
97:
69:
35:
30:
21:
662:J. Ind Microbiol Biotech
471:), re-aromatization to (
554:
521:
446:
616:A. Kohl and R. Kerr.
549:General structure of
548:
519:
444:
71:Systematic IUPAC name
607:(1986) 51: 5140-5145
503:). Rather, molecule
664:(2006) 33: 532-538.
646:(1998) 62: 401-407.
633:(2004) 424: 97-104.
594:(2003) 30: 495-499.
355: g·mol
18:
629:A. Kohl, R. Kerr.
555:
542:-pseudopterosins.
522:
447:
420:Elisabethatriene (
369:Infobox references
16:
681:Phenol glycosides
467:). Ring closure (
377:Chemical compound
375:
374:
236:CompTox Dashboard
139:Interactive image
63:
60:-phenalen-4-yl β-
17:Pseudopterosin A
693:
665:
658:
647:
640:
634:
627:
621:
620:(2003) 1: 54-65.
614:
608:
601:
595:
588:
553:-pseudopterosins
381:Pseudopterosin A
354:
339:
333:
327:
320:Chemical formula
260:
259:
244:
242:
226:
206:
195:
181:
161:
141:
117:
61:
26:
19:
701:
700:
696:
695:
694:
692:
691:
690:
671:
670:
669:
668:
659:
650:
641:
637:
628:
624:
615:
611:
602:
598:
589:
576:
571:
418:
406:
378:
371:
366:
352:
342:
336:
330:
322:
308:
305:
300:
299:
288:
285:
284:
281:
275:
274:
263:
253:DTXSID301030380
245:
238:
229:
209:
196:
184:
164:
144:
131:
120:
107:
93:
92:
65:
64:-xylopyranoside
12:
11:
5:
699:
697:
689:
688:
683:
673:
672:
667:
666:
648:
635:
622:
609:
596:
573:
572:
570:
567:
524:
523:
449:
448:
417:
414:
405:
402:
398:P. elisabethae
376:
373:
372:
367:
363:standard state
360:
357:
356:
350:
344:
343:
340:
334:
328:
323:
318:
315:
314:
310:
309:
307:
306:
303:
295:
294:
293:
290:
289:
287:
286:
282:
279:
278:
270:
269:
268:
265:
264:
262:
261:
248:
246:
234:
231:
230:
228:
227:
219:
217:
211:
210:
208:
207:
199:
197:
189:
186:
185:
183:
182:
174:
172:
166:
165:
163:
162:
154:
152:
146:
145:
143:
142:
134:
132:
125:
122:
121:
119:
118:
110:
108:
103:
100:
99:
95:
94:
74:
73:
67:
66:
39:
33:
32:
28:
27:
13:
10:
9:
6:
4:
3:
2:
698:
687:
684:
682:
679:
678:
676:
663:
657:
655:
653:
649:
645:
644:Life Sciences
639:
636:
632:
626:
623:
619:
613:
610:
606:
605:J. Org. Chem.
600:
597:
593:
587:
585:
583:
581:
579:
575:
568:
566:
564:
560:
552:
547:
543:
541:
537:
533:
529:
518:
514:
513:
512:
510:
506:
502:
498:
494:
490:
486:
482:
478:
474:
470:
466:
462:
458:
454:
443:
439:
438:
437:
435:
431:
427:
423:
415:
413:
411:
403:
401:
399:
395:
394:
389:
386:
382:
370:
364:
358:
351:
349:
346:
345:
324:
321:
317:
316:
311:
302:
298:
291:
277:
273:
266:
258:
254:
250:
249:
247:
237:
233:
232:
225:
221:
220:
218:
216:
213:
212:
205:
201:
200:
198:
192:
188:
187:
180:
176:
175:
173:
171:
168:
167:
160:
156:
155:
153:
151:
148:
147:
140:
136:
135:
133:
129:
124:
123:
116:
112:
111:
109:
106:
102:
101:
96:
90:
86:
82:
78:
72:
68:
59:
55:
51:
47:
43:
38:
34:
29:
25:
20:
661:
643:
638:
630:
625:
617:
612:
604:
599:
591:
562:
558:
556:
550:
539:
535:
531:
527:
525:
508:
504:
500:
496:
492:
488:
484:
480:
476:
472:
468:
464:
460:
456:
452:
450:
433:
425:
421:
419:
416:Biosynthesis
407:
397:
391:
380:
379:
159:ChEMBL476886
98:Identifiers
88:
84:
80:
76:
57:
53:
49:
45:
41:
313:Properties
115:104855-20-1
686:Diterpenes
675:Categories
618:Mar. Drugs
569:References
348:Molar mass
224:5P6979KNB7
170:ChemSpider
126:3D model (
105:CAS Number
37:IUPAC name
388:glycoside
385:diterpene
204:11732783
459:, then
432:(GGPP,
353:432.557
191:PubChem
179:9907496
495:, and
410:NSAIDs
297:SMILES
150:ChEMBL
31:Names
383:is a
272:InChI
128:JSmol
559:seco
551:seco
540:seco
528:seco
426:seco
404:Uses
215:UNII
241:EPA
194:CID
52:,9a
677::
651:^
577:^
565:.
536:11
532:11
491:,
487:,
483:,
335:36
329:25
87:,5
83:,4
79:,3
75:(2
48:,9
44:,7
40:(3
563:6
509:8
505:3
501:6
497:8
493:7
489:6
485:3
481:2
477:9
473:8
469:7
465:6
461:5
457:4
453:3
434:1
422:2
341:6
338:O
332:H
326:C
243:)
239:(
130:)
89:R
85:S
81:R
77:S
62:D
58:H
54:R
50:S
46:R
42:S
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.