222:
potassium, there is no net charge imbalance across the membrane. If the membrane were to become permeable to a type of ion that is more concentrated on one side of the membrane, then that ion would contribute to membrane voltage because the permeant ions would move across the membrane with net movement of that ion type down the concentration gradient. There would be net movement from the side of the membrane with a higher concentration of the ion to the side with lower concentration. Such a movement of one ion across the membrane would result in a net imbalance of charge across the membrane and a membrane potential. This is a common mechanism by which many cells establish a membrane potential.
1158:, of 100mM. For chloride ions (Cl) the sign of the constant must be reversed (−61.54 mV). If calculating the equilibrium potential for calcium (Ca) the 2+ charge halves the simplified constant to 30.77 mV. If working at room temperature, about 21 °C, the calculated constants are approximately 58 mV for K and Na, −58 mV for Cl and 29 mV for Ca. At physiological temperature, about 29.5 °C, and physiological concentrations (which vary for each ion), the calculated potentials are approximately 67 mV for Na, −90 mV for K, −86 mV for Cl and 123 mV for Ca.
31:
204:
226:
potassium (K) ions move out of the cell. Note that potassium ions can move across the membrane in both directions but by the purely statistical process that arises from the higher concentration of potassium ions inside the cell, there will be more potassium ions moving out of the cell. Because there is a higher concentration of potassium ions inside the cells, their random molecular motion is more likely to encounter the permeability pore (
2225:). For such cells there is never any "rest" and the "resting potential" is a theoretical concept. Other cells with little in the way of membrane transport functions that change with time have a resting membrane potential that can be measured by inserting an electrode into the cell. Transmembrane potentials can also be measured optically with dyes that change their optical properties according to the membrane potential.
758:) will have different amounts of various ion transport proteins. Typically, the amount of certain potassium channels is most important for control of the resting potential (see below). Some ion pumps such as the Na+/K+-ATPase are electrogenic, that is, they produce charge imbalance across the cell membrane and can also contribute directly to the membrane potential. Most pumps use metabolic energy (ATP) to function.
250:. Put another way, the tendency of potassium to leave the cell by running down its concentration gradient is now matched by the tendency of the membrane voltage to pull potassium ions back into the cell. K continues to move across the membrane, but the rate at which it enters and leaves the cell are the same, thus, there is no
162:(sodium-potassium pump) which transports 2 potassium ions inside and 3 sodium ions outside at the cost of 1 ATP molecule. In other cases, for example, a membrane potential may be established by acidification of the inside of a membranous compartment (such as the proton pump that generates membrane potential across
774:
of potassium ions, the concentration of potassium is higher inside cells than outside. Most cells have potassium-selective ion channel proteins that remain open all the time. There will be net movement of positively charged potassium ions through these potassium channels with a resulting accumulation
241:
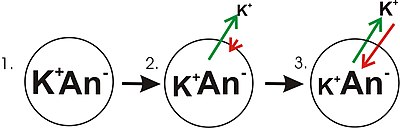
the membrane potential. As potassium continues to leave the cell, separating more charges, the membrane potential will continue to grow. The length of the arrows (green indicating concentration gradient, red indicating voltage), represents the magnitude of potassium ion movement due to each form of
1170:
as mentioned above) for its maintenance. It is a dynamic diffusion potential that takes this mechanism into account—wholly unlike the pillows equilibrium potential, which is true no matter the nature of the system under consideration. The resting membrane potential is dominated by the ionic
362:
Note that even though the membrane potential at 0 mV is stable, it is not an equilibrium condition because neither of the contributing ions is in equilibrium. Ions diffuse down their electrochemical gradients through ion channels, but the membrane potential is upheld by continual K influx and
225:
In panel 2 of the diagram, the cell membrane has been made permeable to potassium ions, but not the anions (An) inside the cell. These anions are mostly contributed by protein. There is energy stored in the potassium ion concentration gradient that can be converted into an electrical gradient when
215:
arrows indicate net movement of K due to the membrane potential. The diagram is misleading in that while the concentration of potassium ions outside of the cell increases, only a small amount of K needs to cross the membrane in order to produce a membrane potential with a magnitude large enough to
2208:
Although the GHK voltage equation and
Millman's equation are related, they are not equivalent. The critical difference is that Millman's equation assumes the current-voltage relationship to be ohmic, whereas the GHK voltage equation takes into consideration the small, instantaneous rectifications
1025:
For common usage the Nernst equation is often given in a simplified form by assuming typical human body temperature (37 °C), reducing the constants and switching to Log base 10. (The units used for concentration are unimportant as they will cancel out into a ratio). For
Potassium at normal
1021:
Potassium equilibrium potentials of around −80 millivolts (inside negative) are common. Differences are observed in different species, different tissues within the same animal, and the same tissues under different environmental conditions. Applying the Nernst
Equation above, one may account for
221:
Panel 1 of the diagram shows a diagrammatic representation of a simple cell where a concentration gradient has already been established. This panel is drawn as if the membrane has no permeability to any ion. There is no membrane potential because despite there being a concentration gradient for
199:
Cell membranes are typically permeable to only a subset of ions. These usually include potassium ions, chloride ions, bicarbonate ions, and others. To simplify the description of the ionic basis of the resting membrane potential, it is most useful to consider only one ionic species at first, and
1538:
311:
The resting potential of a cell can be most thoroughly understood by thinking of it in terms of equilibrium potentials. In the example diagram here, the model cell was given only one permeant ion (potassium). In this case, the resting potential of this cell would be the same as the equilibrium
371:
energy to pump the ions back. Because no real cell can afford such equal and large ionic permeabilities at rest, resting potential of animal cells is determined by predominant high permeability to potassium and adjusted to the required value by modulating sodium and chloride permeabilities and
182:
is assumed; that is, that there is no measurable charge excess on either side of the membrane. So, although there is an electric potential across the membrane due to charge separation, there is no actual measurable difference in the global concentration of positive and negative ions across the
315:
However, a real cell is more complicated, having permeabilities to many ions, each of which contributes to the resting potential. To understand better, consider a cell with only two permeant ions, potassium, and sodium. Consider a case where these two ions have equal concentration gradients
138:
Because the membrane permeability for potassium is much higher than that for other ions, and because of the strong chemical gradient for potassium, potassium ions flow from the cytosol out to the extracellular space carrying out positive charge, until their movement is balanced by build-up of
2071:
639:
1578:
is the concentration of ion s in compartment Y as above. Another way to view the membrane potential, considering instead the conductance of the ion channels rather than the permeability of the membrane, is using the
Millman equation (also called the Chord Conductance Equation):
1175:
across the membrane. For most cells this is potassium. As potassium is also the ion with the most negative equilibrium potential, usually the resting potential can be no more negative than the potassium equilibrium potential. The resting potential can be calculated with the
1828:
242:
energy. The direction of the arrow indicates the direction in which that particular force is applied. Thus, the building membrane voltage is an increasing force that acts counter to the tendency for net movement of potassium ions down the potassium concentration gradient.
234:
doing work by dissipating the concentration gradient. As potassium leaves the cell, it is leaving behind the anions. Therefore, a charge separation is developing as K leaves the cell. This charge separation creates a transmembrane voltage. This transmembrane voltage
779:) and continues until enough excess negative charge accumulates inside the cell to form a membrane potential which can balance the difference in concentration of potassium between inside and outside the cell. "Balance" means that the electrical force (
245:
In Panel 3, the membrane voltage has grown to the extent that its "strength" now matches the concentration gradients. Since these forces (which are applied to K) are now the same strength and oriented in opposite directions, the system is now in
1547:
has been inserted into the equation, causing the intracellular and extracellular concentrations of Cl to be reversed relative to K and Na, as chloride's negative charge is handled by inverting the fraction inside the logarithmic term.
1198:
107:), membrane voltage in the majority of non-excitable cells can also undergo changes in response to environmental or intracellular stimuli. The resting potential exists due to the differences in membrane permeabilities for
1839:
367:. Such situation with similar permeabilities for counter-acting ions, like potassium and sodium in animal cells, can be extremely costly for the cell if these permeabilities are relatively large, as it takes a lot of
931:
2607:
Cheng, K; Haspel, HC; Vallano, ML; Osotimehin, B; Sonenberg, M (1980). "Measurement of membrane potentials (psi) of erythrocytes and white adipocytes by the accumulation of triphenylmethylphosphonium cation".
434:
1145:
230:) that is the case for the potassium ions that are outside and at a lower concentration. An internal K is simply "more likely" to leave the cell than an extracellular K is to enter it. It is a matter of
1585:
2433:, 2nd edition, by Dale Purves, George J. Augustine, David Fitzpatrick, Lawrence C. Katz, Anthony-Samuel LaMantia, James O. McNamara, S. Mark Williams. Sunderland (MA): Sinauer Associates, Inc.; 2001.
290:
render the membrane voltage in plants and fungi much more negative than in the more extensively investigated animal cells, where the resting voltage is mainly determined by selective ion channels.
797:) of K is zero. A good approximation for the equilibrium potential of a given ion only needs the concentrations on either side of the membrane and the temperature. It can be calculated using the
787:, and which impedes outward diffusion, increases until it is equal in magnitude but opposite in direction to the tendency for outward diffusive movement of potassium. This balance point is an
428:
of each contributing ion's equilibrium potential. The size of each weight is the relative conductance of each ion. In the normal case, where three ions contribute to the membrane potential:
139:
negative charge on the inner surface of the membrane. Again, because of the high relative permeability for potassium, the resulting membrane potential is almost always close to the potassium
1150:
Likewise the equilibrium potential for sodium (Na) at normal human body temperature is calculated using the same simplified constant. You can calculate E assuming an outside concentration,
2398:
2429:
experiment to demonstrate the importance of K for the resting potential. The dependence of the resting potential on the extracellular concentration of K is shown in Figure 2.6 of
2213:
caused by the concentration gradient of ions. Thus, a more accurate estimate of membrane potential can be calculated using the GHK equation than with
Millman's equation.
2197:
stops the heart by shifting the resting potential to a more positive value, which depolarizes and contracts the cardiac cells permanently, not allowing the heart to
134:, and exchangers. Conventionally, resting membrane potential can be defined as a relatively stable, ground value of transmembrane voltage in animal and plant cells.
2103:). During the action potential, these weights change. If the conductances of Na and Cl are zero, the membrane potential reduces to the Nernst potential for K (as
316:
directed in opposite directions, and that the membrane permeabilities to both ions are equal. K leaving the cell will tend to drag the membrane potential toward
308:
that are in the cell membrane. How the concentrations of ions and the membrane transport proteins influence the value of the resting potential is outlined below.
200:
consider the others later. Since trans-plasma-membrane potentials are almost always determined primarily by potassium permeability, that is where to start.
1533:{\displaystyle E_{m}={\frac {RT}{F}}\ln {\left({\frac {P_{Na^{+}}_{o}+P_{K^{+}}_{o}+P_{Cl^{-}}_{i}}{P_{Na^{+}}_{i}+P_{K^{+}}_{i}+P_{Cl^{-}}_{o}}}\right)}}
293:
In most neurons the resting potential has a value of approximately −70 mV. The resting potential is mostly determined by the concentrations of the
330:. Since the permeabilities to both ions were set to be equal, the membrane potential will, at the end of the Na/K tug-of-war, end up halfway between
2066:{\displaystyle E_{m}={\frac {g_{K^{+}}}{g_{tot}}}E_{eq,K^{+}}+{\frac {g_{Na^{+}}}{g_{tot}}}E_{eq,Na^{+}}+{\frac {g_{Cl^{-}}}{g_{tot}}}E_{eq,Cl^{-}}}
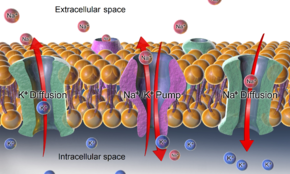
56:, as well as effects of diffusion of the involved ions, are major mechanisms to maintain the resting potential across the membranes of animal cells.
2465:
2083:
is the combined conductance of all ionic species, again in arbitrary units. The latter equation portrays the resting membrane potential as a
191:
is hugely greater than the effect of concentration so an undetectable change in concentration creates a great change in electric potential.
807:
775:
of excess negative charge inside of the cell. The outward movement of positively charged potassium ions is due to random molecular motion (
634:{\displaystyle E_{m}={\frac {g_{K^{+}}}{g_{tot}}}E_{K^{+}}+{\frac {g_{Na^{+}}}{g_{tot}}}E_{Na^{+}}+{\frac {g_{Cl^{-}}}{g_{tot}}}E_{Cl^{-}}}
2770:
1823:{\displaystyle E_{m}={\frac {g_{K^{+}}E_{eq,K^{+}}+g_{Na^{+}}E_{eq,Na^{+}}+g_{Cl^{-}}E_{eq,Cl^{-}}}{g_{K^{+}}+g_{Na^{+}}+g_{Cl^{-}}}}}
2728:
2705:
1032:
143:. But in order for this process to occur, a concentration gradient of potassium ions must first be set up. This work is done by the
2711:
2354:
in 1902 where he proposed a "Membrane Theory" that explained the resting potential of nerve and muscle as a diffusion potential.
207:
A diagram showing the progression in the development of a membrane potential from a concentration gradient (for potassium).
2556:
Ashmore, J. F.; Meech, R. W. (1986-07-24). "Ionic basis of membrane potential in outer hair cells of guinea pig cochlea".
2499:
Lewis, Rebecca; Asplin, Katie E.; Bruce, Gareth; Dart, Caroline; Mobasheri, Ali; Barrett-Jolley, Richard (2011-11-01).
1166:
The resting membrane potential is not an equilibrium potential as it relies on the constant expenditure of energy (for
2373:
187:), that is, there is no actual measurable charge excess on either side. That occurs because the effect of charge on
2797:
188:
375:
In a healthy animal cell Na permeability is about 5% of the K permeability or even less, whereas the respective
274:) in the plasma membrane, steadily operating in parallel, whereby each ion-translocator has its characteristic
2792:
2089:
of the reversal potentials of the system, where the weights are the relative conductances of each ion species (
358:
were equal but of opposite signs, halfway in between is zero, meaning that the membrane will rest at 0 mV.
2651:
Seyfarth, Ernst-August (2006-01-01). "Julius
Bernstein (1839-1917): pioneer neurobiologist and biophysicist".
722:
For determination of membrane potentials, the two most important types of membrane ion transport proteins are
2422:
282:= 'equilibrium voltage'), depending on the particular substrate concentrations inside and outside (internal
1181:
1172:
368:
323:. Na entering the cell will tend to drag the membrane potential toward the reversal potential for sodium
283:
148:
2473:
2246:
789:
734:
without direct expenditure of metabolic energy. They have selectivity for certain ions, thus, there are
35:
30:
2565:
2289:
970:
1180:
using the concentrations of ions as for the equilibrium potential while also including the relative
2802:
275:
203:
2759:
2684:
2633:
2589:
2378:
2299:
2276:
794:
743:
376:
302:
279:
254:
potassium current. Because the K is at equilibrium, membrane potential is stable, or "resting" (E
247:
140:
92:
77:
61:
2751:
2724:
2676:
2668:
2625:
2581:
2538:
2520:
2426:
2222:
2210:
984:
735:
2743:
2660:
2617:
2573:
2528:
2512:
2451:
An illustrated example of measuring membrane potentials with electrodes is in Figure 2.1 of
2363:
2351:
2194:
2190:
2182:
1177:
994:
784:
771:
739:
175:
163:
73:
1184:
of each ionic species. Under normal conditions, it is safe to assume that only potassium,
730:. Ion channel proteins create paths across cell membranes through which ions can passively
1167:
798:
755:
727:
364:
271:
159:
144:
131:
72:(or resting voltage), as opposed to the specific dynamic electrochemical phenomena called
17:
1543:
This equation resembles the Nernst equation, but has a term for each permeant ion. Also,
2569:
1022:
these differences by changes in relative K concentration or differences in temperature.
2776:
2533:
2500:
2368:
2198:
2186:
780:
690:
2786:
2085:
425:
298:
267:
155:
65:
2637:
184:
2763:
2717:
2688:
2593:
2160:
2153:
956:
216:
counter the tendency of the potassium ions to move down the concentration gradient.
174:
In most quantitative treatments of membrane potential, such as the derivation of
2334:
2324:
1008:
723:
227:
127:
1026:
body temperature one may calculate the equilibrium potential in millivolts as:
2664:
2236:
263:
2747:
2672:
2524:
2309:
2256:
776:
767:
751:
731:
231:
108:
2755:
2680:
2542:
2629:
2585:
2202:
1189:
747:
120:
262:
The resting voltage is the result of several ion-translocating enzymes (
2621:
2313:
998:
689:
is the total conductance of all permeant ions in arbitrary units (e.g.
407:
by an amount of approximately 5% of the 140 mV difference between
305:
116:
2516:
926:{\displaystyle E_{eq,K^{+}}={\frac {RT}{zF}}\ln {\frac {_{o}}{_{i}}},}
770:
ions (K) are the most important for the resting potential. Due to the
154:
In the case of the resting membrane potential across an animal cell's
2577:
2501:"The role of the membrane potential in chondrocyte volume regulation"
2266:
1185:
974:
287:
112:
100:
96:
2286:
2221:
In some cells, the membrane potential is always changing (such as
2157:
960:
104:
29:
211:
arrows indicate net movement of K down a concentration gradient.
947:
421:. Thus, the cell's resting potential will be about −73 mV.
2734:
Wright, SH (2004). "Generation of resting membrane potential".
2442:
Hille, Bertil (2001) Ion
Channels of Excitable Membranes, 3 ed.
2229:
Summary of resting potential values in different types of cells
1140:{\displaystyle E_{eq,K^{+}}=61.54mV\log {\frac {_{o}}{_{i}}},}
294:
123:
1007:
is the extracellular concentration of potassium, measured in
680:
is the relative conductance of ion X, which is dimensionless
126:, which in turn result from functional activity of various
2714:
Molecular, Cellular, and
Medical Aspects by Siegel, et al.
2350:
Resting currents in nerves were measured and described by
158:, potassium (and sodium) gradients are established by the
424:
In a more formal notation, the membrane potential is the
2773:- Online lecture notes on the resting membrane potential
1017:
is likewise the intracellular concentration of potassium
946:
is the equilibrium potential for potassium, measured in
746:. Different cells and even different parts of one cell (
2723:, 3rd ed., Sinauer Associates, Sunderland, MA (2001).
1192:(Cl) ions play large roles for the resting potential:
1842:
1588:
1201:
1035:
810:
664:
is the equilibrium potential for ion X, also in volts
437:
393:). Thus the membrane potential will not be right at
2399:"Resting Membrane Potential - Nernst - Generation"
2167:) is changed are very dangerous since they offset
2065:
1822:
1532:
1139:
925:
633:
2455:by Dale Purves, et al. (see reference #1, above).
2193:injection of potassium chloride in executions by
987:of the ion in question involved in the reaction
1555:is the membrane potential, measured in volts *
147:and/or exchangers and generally is powered by
2131:are not zero, but they are much smaller than
286:included in case of some pumps). H exporting
8:
2777:The Origin of the Resting Membrane Potential
1171:species in the system that has the greatest
655:is the membrane potential, measured in volts
783:) that results from the build-up of ionic
693:for electrical conductance), in this case
91:Apart from the latter two, which occur in
2532:
2055:
2038:
2020:
2008:
2000:
1994:
1983:
1966:
1948:
1936:
1928:
1922:
1911:
1897:
1879:
1867:
1862:
1856:
1847:
1841:
1809:
1801:
1786:
1778:
1763:
1758:
1744:
1727:
1715:
1707:
1692:
1675:
1663:
1655:
1640:
1626:
1614:
1609:
1602:
1593:
1587:
1516:
1506:
1488:
1480:
1467:
1457:
1442:
1437:
1424:
1414:
1396:
1388:
1376:
1366:
1348:
1340:
1327:
1317:
1302:
1297:
1284:
1274:
1256:
1248:
1241:
1236:
1215:
1206:
1200:
1125:
1115:
1100:
1090:
1080:
1054:
1040:
1034:
911:
901:
886:
876:
866:
840:
829:
815:
809:
623:
615:
597:
585:
577:
571:
560:
552:
534:
522:
514:
508:
497:
492:
474:
462:
457:
451:
442:
436:
2232:
202:
2390:
2117:). Normally, under resting conditions
1574:is the relative permeability of ion s *
1154:, of 10mM and an inside concentration,
1178:Goldman-Hodgkin-Katz voltage equation
7:
2494:
2492:
2490:
27:Static membrane potential in biology
2721:Ion channels of excitable membranes
2708:- online textbook by Purves, et al.
297:in the fluids on both sides of the
195:Generation of the resting potential
793:as the net transmembrane flux (or
25:
977:(= K = degrees Celsius + 273.15)
386:)and −80 mV for potassium (
2505:Journal of Cellular Physiology
2152:. Medical conditions such as
1513:
1496:
1464:
1450:
1421:
1404:
1373:
1356:
1324:
1310:
1281:
1264:
1122:
1108:
1097:
1083:
908:
894:
883:
869:
400:, but rather depolarized from
103:, and some secretory cells in
1:
2779:- Online interactive tutorial
744:sodium-selective ion channels
183:membrane (as it is estimated
84:has a value of approximately
2472:. 2015-01-24. Archived from
2217:Measuring resting potentials
379:are +60 mV for sodium (
2374:Hyperpolarization (biology)
2205:to be refilled with blood.
718:Membrane transport proteins
2819:
2771:Resting Membrane Potential
82:resting membrane potential
70:resting membrane potential
18:Resting membrane potential
2665:10.1007/s00422-005-0031-y
2163:potassium (which governs
312:potential for potassium.
189:electrochemical potential
2748:10.1152/advan.00029.2004
2223:cardiac pacemaker cells
2653:Biological Cybernetics
2067:
1824:
1534:
1141:
927:
766:For most animal cells
762:Equilibrium potentials
635:
217:
145:ion pumps/transporters
60:The relatively static
57:
2247:Skeletal muscle cells
2068:
1825:
1535:
1142:
928:
790:equilibrium potential
636:
206:
33:
2712:Basic Neurochemistry
2427:electrophysiological
2290:Smooth muscle tissue
1840:
1586:
1199:
1033:
971:absolute temperature
808:
435:
68:cells is called the
2570:1986Natur.322..368A
2300:Photoreceptor cells
2277:Smooth muscle cells
2271:-60 to -70 mV
2261:-80 to -90 mV
377:reversal potentials
276:electromotive force
2622:10.1007/bf01869476
2379:Membrane potential
2241:Resting potential
2181:. This may cause
2063:
1820:
1530:
1162:Resting potentials
1137:
997:, equal to 96,485
985:elementary charges
923:
631:
280:reversal potential
218:
141:reversal potential
78:membrane potential
62:membrane potential
58:
2564:(6077): 368–371.
2517:10.1002/jcp.22646
2511:(11): 2979–2986.
2403:TeachMePhysiology
2343:
2342:
2211:GHK flux equation
2209:predicted by the
2174:, thus affecting
2032:
1960:
1891:
1818:
1523:
1228:
1132:
983:is the number of
959:, equal to 8.314
955:is the universal
918:
858:
609:
546:
486:
180:electroneutrality
170:Electroneutrality
16:(Redirected from
2810:
2798:Membrane biology
2767:
2736:Adv Physiol Educ
2693:
2692:
2648:
2642:
2641:
2604:
2598:
2597:
2578:10.1038/322368a0
2553:
2547:
2546:
2536:
2496:
2485:
2484:
2482:
2481:
2462:
2456:
2449:
2443:
2440:
2434:
2419:
2413:
2412:
2410:
2409:
2395:
2364:Action potential
2352:Julius Bernstein
2233:
2195:lethal injection
2189:. The use of a
2138:, which renders
2086:weighted average
2072:
2070:
2069:
2064:
2062:
2061:
2060:
2059:
2033:
2031:
2030:
2015:
2014:
2013:
2012:
1995:
1990:
1989:
1988:
1987:
1961:
1959:
1958:
1943:
1942:
1941:
1940:
1923:
1918:
1917:
1916:
1915:
1892:
1890:
1889:
1874:
1873:
1872:
1871:
1857:
1852:
1851:
1833:or reformulated
1829:
1827:
1826:
1821:
1819:
1817:
1816:
1815:
1814:
1813:
1793:
1792:
1791:
1790:
1770:
1769:
1768:
1767:
1752:
1751:
1750:
1749:
1748:
1722:
1721:
1720:
1719:
1699:
1698:
1697:
1696:
1670:
1669:
1668:
1667:
1647:
1646:
1645:
1644:
1621:
1620:
1619:
1618:
1603:
1598:
1597:
1539:
1537:
1536:
1531:
1529:
1528:
1524:
1522:
1521:
1520:
1511:
1510:
1495:
1494:
1493:
1492:
1472:
1471:
1462:
1461:
1449:
1448:
1447:
1446:
1429:
1428:
1419:
1418:
1403:
1402:
1401:
1400:
1382:
1381:
1380:
1371:
1370:
1355:
1354:
1353:
1352:
1332:
1331:
1322:
1321:
1309:
1308:
1307:
1306:
1289:
1288:
1279:
1278:
1263:
1262:
1261:
1260:
1242:
1229:
1224:
1216:
1211:
1210:
1146:
1144:
1143:
1138:
1133:
1131:
1130:
1129:
1120:
1119:
1106:
1105:
1104:
1095:
1094:
1081:
1061:
1060:
1059:
1058:
995:Faraday constant
932:
930:
929:
924:
919:
917:
916:
915:
906:
905:
892:
891:
890:
881:
880:
867:
859:
857:
849:
841:
836:
835:
834:
833:
772:active transport
756:nodes of Ranvier
728:ion transporters
640:
638:
637:
632:
630:
629:
628:
627:
610:
608:
607:
592:
591:
590:
589:
572:
567:
566:
565:
564:
547:
545:
544:
529:
528:
527:
526:
509:
504:
503:
502:
501:
487:
485:
484:
469:
468:
467:
466:
452:
447:
446:
426:weighted average
365:ion transporters
214:
210:
176:Goldman equation
164:synaptic vesicle
132:ion transporters
74:action potential
53:
52:
51:
44:
43:
21:
2818:
2817:
2813:
2812:
2811:
2809:
2808:
2807:
2793:Neurophysiology
2783:
2782:
2742:(1–4): 139–42.
2733:
2702:
2697:
2696:
2650:
2649:
2645:
2606:
2605:
2601:
2555:
2554:
2550:
2498:
2497:
2488:
2479:
2477:
2464:
2463:
2459:
2450:
2446:
2441:
2437:
2420:
2416:
2407:
2405:
2397:
2396:
2392:
2387:
2360:
2348:
2231:
2219:
2201:and thus enter
2180:
2173:
2166:
2151:
2144:
2137:
2130:
2123:
2116:
2109:
2102:
2095:
2082:
2051:
2034:
2016:
2004:
1996:
1979:
1962:
1944:
1932:
1924:
1907:
1893:
1875:
1863:
1858:
1843:
1838:
1837:
1805:
1797:
1782:
1774:
1759:
1754:
1753:
1740:
1723:
1711:
1703:
1688:
1671:
1659:
1651:
1636:
1622:
1610:
1605:
1604:
1589:
1584:
1583:
1577:
1573:
1554:
1512:
1502:
1484:
1476:
1463:
1453:
1438:
1433:
1420:
1410:
1392:
1384:
1383:
1372:
1362:
1344:
1336:
1323:
1313:
1298:
1293:
1280:
1270:
1252:
1244:
1243:
1237:
1217:
1202:
1197:
1196:
1164:
1157:
1153:
1121:
1111:
1107:
1096:
1086:
1082:
1050:
1036:
1031:
1030:
1016:
1006:
1001:·mol or J·V·mol
945:
907:
897:
893:
882:
872:
868:
850:
842:
825:
811:
806:
805:
799:Nernst equation
764:
720:
713:
706:
699:
688:
679:
672:
663:
654:
619:
611:
593:
581:
573:
556:
548:
530:
518:
510:
493:
488:
470:
458:
453:
438:
433:
432:
420:
413:
406:
399:
392:
385:
359:
357:
350:
343:
336:
329:
322:
257:
212:
208:
197:
172:
156:plasma membrane
135:
93:excitable cells
86:-70mV or -0.07V
50:
48:
47:
46:
42:
40:
39:
38:
36:
28:
23:
22:
15:
12:
11:
5:
2816:
2814:
2806:
2805:
2800:
2795:
2785:
2784:
2781:
2780:
2774:
2768:
2731:
2715:
2709:
2701:
2700:External links
2698:
2695:
2694:
2643:
2616:(3): 191–201.
2610:J. Membr. Biol
2599:
2548:
2486:
2457:
2444:
2435:
2414:
2389:
2388:
2386:
2383:
2382:
2381:
2376:
2371:
2369:Depolarization
2366:
2359:
2356:
2347:
2344:
2341:
2340:
2337:
2331:
2330:
2327:
2321:
2320:
2317:
2306:
2305:
2302:
2296:
2295:
2292:
2283:
2282:
2279:
2273:
2272:
2269:
2263:
2262:
2259:
2253:
2252:
2249:
2243:
2242:
2239:
2230:
2227:
2218:
2215:
2187:cardiac arrest
2178:
2171:
2164:
2149:
2142:
2135:
2128:
2121:
2114:
2107:
2100:
2093:
2080:
2074:
2073:
2058:
2054:
2050:
2047:
2044:
2041:
2037:
2029:
2026:
2023:
2019:
2011:
2007:
2003:
1999:
1993:
1986:
1982:
1978:
1975:
1972:
1969:
1965:
1957:
1954:
1951:
1947:
1939:
1935:
1931:
1927:
1921:
1914:
1910:
1906:
1903:
1900:
1896:
1888:
1885:
1882:
1878:
1870:
1866:
1861:
1855:
1850:
1846:
1831:
1830:
1812:
1808:
1804:
1800:
1796:
1789:
1785:
1781:
1777:
1773:
1766:
1762:
1757:
1747:
1743:
1739:
1736:
1733:
1730:
1726:
1718:
1714:
1710:
1706:
1702:
1695:
1691:
1687:
1684:
1681:
1678:
1674:
1666:
1662:
1658:
1654:
1650:
1643:
1639:
1635:
1632:
1629:
1625:
1617:
1613:
1608:
1601:
1596:
1592:
1575:
1571:
1567:are as above *
1552:
1541:
1540:
1527:
1519:
1515:
1509:
1505:
1501:
1498:
1491:
1487:
1483:
1479:
1475:
1470:
1466:
1460:
1456:
1452:
1445:
1441:
1436:
1432:
1427:
1423:
1417:
1413:
1409:
1406:
1399:
1395:
1391:
1387:
1379:
1375:
1369:
1365:
1361:
1358:
1351:
1347:
1343:
1339:
1335:
1330:
1326:
1320:
1316:
1312:
1305:
1301:
1296:
1292:
1287:
1283:
1277:
1273:
1269:
1266:
1259:
1255:
1251:
1247:
1240:
1235:
1232:
1227:
1223:
1220:
1214:
1209:
1205:
1182:permeabilities
1163:
1160:
1155:
1151:
1148:
1147:
1136:
1128:
1124:
1118:
1114:
1110:
1103:
1099:
1093:
1089:
1085:
1079:
1076:
1073:
1070:
1067:
1064:
1057:
1053:
1049:
1046:
1043:
1039:
1019:
1018:
1014:
1012:
1004:
1002:
988:
978:
973:, measured in
964:
950:
943:
934:
933:
922:
914:
910:
904:
900:
896:
889:
885:
879:
875:
871:
865:
862:
856:
853:
848:
845:
839:
832:
828:
824:
821:
818:
814:
763:
760:
719:
716:
715:
714:
711:
704:
697:
686:
681:
677:
670:
665:
661:
656:
652:
643:
642:
626:
622:
618:
614:
606:
603:
600:
596:
588:
584:
580:
576:
570:
563:
559:
555:
551:
543:
540:
537:
533:
525:
521:
517:
513:
507:
500:
496:
491:
483:
480:
477:
473:
465:
461:
456:
450:
445:
441:
418:
411:
404:
397:
390:
383:
363:Na efflux via
355:
348:
341:
334:
327:
320:
268:cotransporters
260:
259:
255:
243:
223:
196:
193:
171:
168:
49:
41:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2815:
2804:
2801:
2799:
2796:
2794:
2791:
2790:
2788:
2778:
2775:
2772:
2769:
2765:
2761:
2757:
2753:
2749:
2745:
2741:
2737:
2732:
2730:
2729:0-87893-321-2
2726:
2722:
2719:
2716:
2713:
2710:
2707:
2704:
2703:
2699:
2690:
2686:
2682:
2678:
2674:
2670:
2666:
2662:
2658:
2654:
2647:
2644:
2639:
2635:
2631:
2627:
2623:
2619:
2615:
2611:
2603:
2600:
2595:
2591:
2587:
2583:
2579:
2575:
2571:
2567:
2563:
2559:
2552:
2549:
2544:
2540:
2535:
2530:
2526:
2522:
2518:
2514:
2510:
2506:
2502:
2495:
2493:
2491:
2487:
2476:on 2015-11-07
2475:
2471:
2470:users.rcn.com
2467:
2461:
2458:
2454:
2448:
2445:
2439:
2436:
2432:
2428:
2424:
2418:
2415:
2404:
2400:
2394:
2391:
2384:
2380:
2377:
2375:
2372:
2370:
2367:
2365:
2362:
2361:
2357:
2355:
2353:
2345:
2338:
2336:
2333:
2332:
2329:-8.4 mV
2328:
2326:
2323:
2322:
2319:-15 to -40mV
2318:
2315:
2311:
2308:
2307:
2303:
2301:
2298:
2297:
2293:
2291:
2288:
2285:
2284:
2280:
2278:
2275:
2274:
2270:
2268:
2265:
2264:
2260:
2258:
2255:
2254:
2250:
2248:
2245:
2244:
2240:
2238:
2235:
2234:
2228:
2226:
2224:
2216:
2214:
2212:
2206:
2204:
2200:
2196:
2192:
2188:
2184:
2177:
2170:
2162:
2159:
2155:
2148:
2141:
2134:
2127:
2120:
2113:
2106:
2099:
2092:
2088:
2087:
2079:
2056:
2052:
2048:
2045:
2042:
2039:
2035:
2027:
2024:
2021:
2017:
2009:
2005:
2001:
1997:
1991:
1984:
1980:
1976:
1973:
1970:
1967:
1963:
1955:
1952:
1949:
1945:
1937:
1933:
1929:
1925:
1919:
1912:
1908:
1904:
1901:
1898:
1894:
1886:
1883:
1880:
1876:
1868:
1864:
1859:
1853:
1848:
1844:
1836:
1835:
1834:
1810:
1806:
1802:
1798:
1794:
1787:
1783:
1779:
1775:
1771:
1764:
1760:
1755:
1745:
1741:
1737:
1734:
1731:
1728:
1724:
1716:
1712:
1708:
1704:
1700:
1693:
1689:
1685:
1682:
1679:
1676:
1672:
1664:
1660:
1656:
1652:
1648:
1641:
1637:
1633:
1630:
1627:
1623:
1615:
1611:
1606:
1599:
1594:
1590:
1582:
1581:
1580:
1570:
1566:
1562:
1558:
1551:
1546:
1525:
1517:
1507:
1503:
1499:
1489:
1485:
1481:
1477:
1473:
1468:
1458:
1454:
1443:
1439:
1434:
1430:
1425:
1415:
1411:
1407:
1397:
1393:
1389:
1385:
1377:
1367:
1363:
1359:
1349:
1345:
1341:
1337:
1333:
1328:
1318:
1314:
1303:
1299:
1294:
1290:
1285:
1275:
1271:
1267:
1257:
1253:
1249:
1245:
1238:
1233:
1230:
1225:
1221:
1218:
1212:
1207:
1203:
1195:
1194:
1193:
1191:
1187:
1183:
1179:
1174:
1169:
1161:
1159:
1134:
1126:
1116:
1112:
1101:
1091:
1087:
1077:
1074:
1071:
1068:
1065:
1062:
1055:
1051:
1047:
1044:
1041:
1037:
1029:
1028:
1027:
1023:
1013:
1010:
1003:
1000:
996:
992:
989:
986:
982:
979:
976:
972:
968:
965:
962:
958:
954:
951:
949:
942:
939:
938:
937:
920:
912:
902:
898:
887:
877:
873:
863:
860:
854:
851:
846:
843:
837:
830:
826:
822:
819:
816:
812:
804:
803:
802:
800:
796:
792:
791:
786:
782:
778:
773:
769:
761:
759:
757:
753:
749:
745:
741:
737:
733:
729:
725:
717:
710:
703:
696:
692:
685:
682:
676:
669:
666:
660:
657:
651:
648:
647:
646:
624:
620:
616:
612:
604:
601:
598:
594:
586:
582:
578:
574:
568:
561:
557:
553:
549:
541:
538:
535:
531:
523:
519:
515:
511:
505:
498:
494:
489:
481:
478:
475:
471:
463:
459:
454:
448:
443:
439:
431:
430:
429:
427:
422:
417:
410:
403:
396:
389:
382:
378:
373:
370:
366:
360:
354:
347:
340:
333:
326:
319:
313:
309:
307:
304:
303:ion transport
300:
299:cell membrane
296:
291:
289:
285:
281:
277:
273:
269:
265:
253:
249:
244:
240:
239:
233:
229:
224:
220:
219:
205:
201:
194:
192:
190:
186:
181:
177:
169:
167:
165:
161:
157:
152:
150:
146:
142:
136:
133:
129:
125:
122:
118:
114:
110:
106:
102:
98:
94:
89:
87:
83:
79:
75:
71:
67:
63:
55:
32:
19:
2739:
2735:
2720:
2718:Bertil Hille
2706:Neuroscience
2656:
2652:
2646:
2613:
2609:
2602:
2561:
2557:
2551:
2508:
2504:
2478:. Retrieved
2474:the original
2469:
2460:
2453:Neuroscience
2452:
2447:
2438:
2431:Neuroscience
2430:
2417:
2406:. Retrieved
2402:
2393:
2349:
2335:Chondrocytes
2325:Erythrocytes
2304:-40 mV
2281:-60 mV
2251:-95 mV
2220:
2207:
2175:
2168:
2154:hyperkalemia
2146:
2139:
2132:
2125:
2118:
2111:
2104:
2097:
2090:
2084:
2077:
2075:
1832:
1568:
1564:
1560:
1556:
1549:
1544:
1542:
1165:
1149:
1024:
1020:
1011:·m or mmol·l
990:
980:
966:
957:gas constant
952:
940:
935:
788:
765:
724:ion channels
721:
708:
701:
694:
683:
674:
667:
658:
649:
644:
423:
415:
408:
401:
394:
387:
380:
374:
361:
352:
345:
338:
331:
324:
317:
314:
310:
292:
261:
251:
237:
236:
198:
179:
173:
166:membranes).
153:
137:
128:ion channels
90:
85:
81:
69:
59:
2183:arrhythmias
1173:conductance
1168:ionic pumps
752:cell bodies
372:gradients.
248:equilibrium
228:ion channel
160:Na/K-ATPase
76:and graded
2803:Potentials
2787:Categories
2659:(1): 2–8.
2480:2016-06-01
2408:2024-09-18
2385:References
2237:Cell types
2199:repolarize
736:potassium-
264:uniporters
2673:0340-1200
2525:1097-4652
2466:"Muscles"
2310:Hair cell
2257:Astroglia
2156:in which
2145:close to
2057:−
2010:−
1811:−
1746:−
1717:−
1508:−
1490:−
1368:−
1350:−
1234:
1188:(Na) and
1078:
864:
781:potential
777:diffusion
768:potassium
748:dendrites
740:chloride-
625:−
587:−
232:diffusion
109:potassium
66:quiescent
2756:15545342
2681:16341542
2638:19693916
2543:21328349
2358:See also
2203:diastole
1190:chloride
999:coulombs
306:proteins
301:and the
121:chloride
2764:5009629
2689:2842501
2630:6779011
2594:4371640
2586:2426595
2566:Bibcode
2534:3229839
2423:example
2346:History
2314:Cochlea
2267:Neurons
993:is the
975:kelvins
969:is the
936:where
795:current
732:diffuse
691:siemens
645:where
117:calcium
101:muscles
97:neurons
54:-ATPase
2762:
2754:
2727:
2687:
2679:
2671:
2636:
2628:
2592:
2584:
2558:Nature
2541:
2531:
2523:
2425:of an
2294:-45mV
2076:where
1563:, and
1186:sodium
963:·K·mol
961:joules
785:charge
742:, and
344:. As
288:ATPase
270:, and
119:, and
113:sodium
105:glands
80:. The
2760:S2CID
2685:S2CID
2634:S2CID
2590:S2CID
2339:-8mV
2287:Aorta
2191:bolus
2172:eq,K+
2161:serum
2158:blood
2150:eq,K+
1066:61.54
948:volts
272:pumps
209:Green
185:below
2752:PMID
2725:ISBN
2677:PMID
2669:ISSN
2626:PMID
2582:PMID
2539:PMID
2521:ISSN
2185:and
2124:and
944:eq,K
726:and
414:and
351:and
337:and
295:ions
124:ions
34:The
2744:doi
2661:doi
2618:doi
2574:doi
2562:322
2529:PMC
2513:doi
2509:226
2421:An
2129:Cl−
2122:Na+
2115:tot
2101:tot
2081:tot
1075:log
1009:mol
687:tot
678:tot
369:ATP
284:ATP
278:(=
252:net
213:Red
149:ATP
88:.
64:of
2789::
2758:.
2750:.
2740:28
2738:.
2683:.
2675:.
2667:.
2657:94
2655:.
2632:.
2624:.
2614:56
2612:.
2588:.
2580:.
2572:.
2560:.
2537:.
2527:.
2519:.
2507:.
2503:.
2489:^
2468:.
2401:.
2316:)
2136:K+
2110:=
1559:,
1231:ln
861:ln
801::
754:,
750:,
738:,
712:Cl
707:+
705:Na
700:+
419:Na
384:Na
349:Na
335:Na
328:Na
266:,
258:).
238:is
178:,
151:.
130:,
115:,
111:,
99:,
45:/K
37:Na
2766:.
2746::
2691:.
2663::
2640:.
2620::
2596:.
2576::
2568::
2545:.
2515::
2483:.
2411:.
2312:(
2179:m
2176:E
2169:E
2165:o
2147:E
2143:m
2140:E
2133:g
2126:g
2119:g
2112:g
2108:K
2105:g
2098:g
2096:/
2094:X
2091:g
2078:g
2053:l
2049:C
2046:,
2043:q
2040:e
2036:E
2028:t
2025:o
2022:t
2018:g
2006:l
2002:C
1998:g
1992:+
1985:+
1981:a
1977:N
1974:,
1971:q
1968:e
1964:E
1956:t
1953:o
1950:t
1946:g
1938:+
1934:a
1930:N
1926:g
1920:+
1913:+
1909:K
1905:,
1902:q
1899:e
1895:E
1887:t
1884:o
1881:t
1877:g
1869:+
1865:K
1860:g
1854:=
1849:m
1845:E
1807:l
1803:C
1799:g
1795:+
1788:+
1784:a
1780:N
1776:g
1772:+
1765:+
1761:K
1756:g
1742:l
1738:C
1735:,
1732:q
1729:e
1725:E
1713:l
1709:C
1705:g
1701:+
1694:+
1690:a
1686:N
1683:,
1680:q
1677:e
1673:E
1665:+
1661:a
1657:N
1653:g
1649:+
1642:+
1638:K
1634:,
1631:q
1628:e
1624:E
1616:+
1612:K
1607:g
1600:=
1595:m
1591:E
1576:Y
1572:s
1569:P
1565:F
1561:T
1557:R
1553:m
1550:E
1548:*
1545:z
1526:)
1518:o
1514:]
1504:l
1500:C
1497:[
1486:l
1482:C
1478:P
1474:+
1469:i
1465:]
1459:+
1455:K
1451:[
1444:+
1440:K
1435:P
1431:+
1426:i
1422:]
1416:+
1412:a
1408:N
1405:[
1398:+
1394:a
1390:N
1386:P
1378:i
1374:]
1364:l
1360:C
1357:[
1346:l
1342:C
1338:P
1334:+
1329:o
1325:]
1319:+
1315:K
1311:[
1304:+
1300:K
1295:P
1291:+
1286:o
1282:]
1276:+
1272:a
1268:N
1265:[
1258:+
1254:a
1250:N
1246:P
1239:(
1226:F
1222:T
1219:R
1213:=
1208:m
1204:E
1156:i
1152:o
1135:,
1127:i
1123:]
1117:+
1113:K
1109:[
1102:o
1098:]
1092:+
1088:K
1084:[
1072:V
1069:m
1063:=
1056:+
1052:K
1048:,
1045:q
1042:e
1038:E
1015:i
1005:o
991:F
981:z
967:T
953:R
941:E
921:,
913:i
909:]
903:+
899:K
895:[
888:o
884:]
878:+
874:K
870:[
855:F
852:z
847:T
844:R
838:=
831:+
827:K
823:,
820:q
817:e
813:E
709:g
702:g
698:K
695:g
684:g
675:g
673:/
671:X
668:g
662:X
659:E
653:m
650:E
641:,
621:l
617:C
613:E
605:t
602:o
599:t
595:g
583:l
579:C
575:g
569:+
562:+
558:a
554:N
550:E
542:t
539:o
536:t
532:g
524:+
520:a
516:N
512:g
506:+
499:+
495:K
490:E
482:t
479:o
476:t
472:g
464:+
460:K
455:g
449:=
444:m
440:E
416:E
412:K
409:E
405:K
402:E
398:K
395:E
391:K
388:E
381:E
356:K
353:E
346:E
342:K
339:E
332:E
325:E
321:K
318:E
256:K
95:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.