189:
24:
437:
Antifungal macrocyclic bis(bibenzyls) from the
Chinese liverwort Ptagiochasm intermedlum L. Chun-Feng Xie, Jian-Bo Qu, Xiu-Zhen Wu, Na Liu, Mei Ji and Hong-Xiang Lou, Natural Product Research: Formerly Natural Product Letters, 2010, Volume 24, Issue 6, pages 515-520,
407:
Seasonal
Dynamics of Riccardin C Accumulation in Primula macrocalyx Bge. Yu. S. Kosenkova, M.P. Polovinka, N.I. Komarova, D.V. Korchagina, N. Yu. Kurochkina, V.A. Cheremushkina and N.F. Salakhutdinov, Chemistry for Sustainable Development, 2009, 17, pages 507-511
390:
Riccardin C, a bisbibenzyl compound from
Primula macrocalyx. Yu. S. Kosenkova, M. P. Polovinka, N. I. Komarova, D. V. Korchagina, N. Yu. Kurochkina, V. A. Cheremushkina and N. F. Salakhutdinov, Chemistry Of Natural Compounds, Volume 43, Number 6, pages 712-713,
497:
Kostiuk, S. L., Woodcock, T., Dudin, L. F., Howes, P. D. and
Harrowven, D. C. (2011), Unified Syntheses of Cavicularin and Riccardin C: Addressing the Synthesis of an Arene Adopting a Boat Configuration. Chemistry - A European Journal, 17: 10906–10915.
90:
421:
RiccardinC, a novel cyclic bibenzyl derivative from
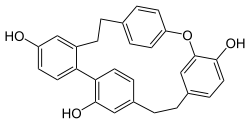
Reboulia hemisphaerica. Yoshinori Asakawa, Reiko Matsuda, Phytochemistry, Volume 21, Issue 8, 1982, Pages 2143–2144,
720:
228:
533:
212:
InChI=1S/C28H24O4/c29-22-9-13-24-21(17-22)8-3-18-4-10-23(11-5-18)32-28-16-20(7-14-26(28)30)2-1-19-6-12-25(24)27(31)15-19/h4-7,9-17,29-31H,1-3,8H2
456:
375:
203:
526:
320:
627:
725:
457:"Total Synthesis of Cavicularin and Riccardin C: Addressing the Synthesis of an Arene That Adopts a Boat Configuration"
705:
167:
519:
292:
332:
326:
484:
110:
40:
607:
603:
599:
715:
710:
641:
315:
184:
680:
623:
579:
574:
559:
311:
56:
44:
14-oxapentacyclononacosa-1(24),2(7),3,5,10(29),11,13(28),15,17,19(27),22,25-dodecaene-5,16,24-triol
663:
659:
646:
636:
632:
668:
651:
569:
409:
551:
542:
476:
371:
130:
594:
499:
468:
439:
422:
392:
251:
66:
511:
340:
188:
584:
565:
286:
699:
426:
155:
365:
684:
344:
443:
396:
308:
274:
121:
589:
364:
Fugmann, Burkhard; Lang-Fugmann, Susanne; Steglich, Wolfgang (14 May 2014).
503:
480:
472:
23:
142:
101:
285:
Except where otherwise noted, data are given for materials in their
236:
C1CC2=C(C=CC(=C2)O)C3=C(C=C(CCC4=CC(=C(C=C4)O)OC5=CC=C1C=C5)C=C3)O
89:
79:
515:
455:
David C. Harrowven; Timothy
Woodcock; Peter D. Howes (2005).
172:
367:
RÖMPP Encyclopedia
Natural Products, 1st Edition, 2000
616:
550:
154:
65:
318:isolated from the Siberian cowslip subspecies
527:
8:
534:
520:
512:
187:
129:
15:
29:Riccardin C, flat molecule representation
356:
233:
208:
183:
339:In 2005, the compound was prepared by
215:Key: JMKSVONWZFVEAI-UHFFFAOYSA-N
109:
7:
370:. Georg Thieme Verlag. p. 553.
343:together with the strained compound
721:Heterocyclic compounds with 5 rings
145:
562:(3,3′-dihydroxy-5-methoxybibenzyl)
14:
628:13,13'-O-Isoproylidenericcardin D
22:
321:Primula veris subsp. macrocalyx
289:(at 25 °C , 100 kPa).
1:
330:and in the Chinese liverwort
427:10.1016/0031-9422(82)83073-2
742:
658:Macrocyclic bis(benzyls):
279:424.49 g/mol
444:10.1080/14786410802271587
397:10.1007/s10600-007-0241-8
283:
244:
224:
199:
49:
39:
34:
21:
333:Plagiochasma intermedium
504:10.1002/chem.201101550
473:10.1002/anie.200500466
327:Reboulia hemisphaerica
545:and their glycosides
316:secondary metabolite
726:Oxygen heterocycles
681:dihydrophenanthrene
580:Isonotholaenic acid
575:Dihydro-resveratrol
18:
706:Dihydrostilbenoids
570:combretastatin B-1
552:Dihydrostilbenoids
543:Dihydrostilbenoids
293:Infobox references
16:
693:
692:
467:(25): 3899–3901.
461:Angewandte Chemie
377:978-3-13-179311-9
301:Chemical compound
299:
298:
168:CompTox Dashboard
91:Interactive image
733:
679:Cyclic bibenzyl-
617:Oligomeric forms
600:Tyrolobibenzyl A
595:Notholaenic acid
536:
529:
522:
513:
506:
495:
489:
488:
483:. Archived from
452:
446:
435:
429:
419:
413:
405:
399:
388:
382:
381:
361:
252:Chemical formula
192:
191:
176:
174:
158:
147:
133:
113:
93:
69:
26:
19:
741:
740:
736:
735:
734:
732:
731:
730:
696:
695:
694:
689:
642:Neomarchantin A
612:
546:
540:
510:
509:
496:
492:
454:
453:
449:
436:
432:
420:
416:
406:
402:
389:
385:
378:
363:
362:
358:
353:
341:total synthesis
302:
295:
290:
268:
264:
260:
254:
240:
237:
232:
231:
220:
217:
216:
213:
207:
206:
195:
185:DTXSID101030291
177:
170:
161:
148:
136:
116:
96:
83:
72:
59:
45:
30:
27:
12:
11:
5:
739:
737:
729:
728:
723:
718:
713:
708:
698:
697:
691:
690:
688:
687:
676:
675:
666:
655:
654:
649:
644:
639:
630:
624:Bis(bibenzyls)
620:
618:
614:
613:
611:
610:
597:
592:
587:
585:Lunularic acid
582:
577:
572:
566:Combretastatin
563:
556:
554:
548:
547:
541:
539:
538:
531:
524:
516:
508:
507:
490:
487:on 2012-12-10.
447:
430:
414:
400:
383:
376:
355:
354:
352:
349:
300:
297:
296:
291:
287:standard state
284:
281:
280:
277:
271:
270:
266:
262:
258:
255:
250:
247:
246:
242:
241:
239:
238:
235:
227:
226:
225:
222:
221:
219:
218:
214:
211:
210:
202:
201:
200:
197:
196:
194:
193:
180:
178:
166:
163:
162:
160:
159:
151:
149:
141:
138:
137:
135:
134:
126:
124:
118:
117:
115:
114:
106:
104:
98:
97:
95:
94:
86:
84:
77:
74:
73:
71:
70:
62:
60:
55:
52:
51:
47:
46:
43:
37:
36:
32:
31:
28:
13:
10:
9:
6:
4:
3:
2:
738:
727:
724:
722:
719:
717:
714:
712:
709:
707:
704:
703:
701:
686:
682:
678:
677:
674:
670:
667:
665:
661:
657:
656:
653:
650:
648:
645:
643:
640:
638:
634:
631:
629:
625:
622:
621:
619:
615:
609:
605:
601:
598:
596:
593:
591:
588:
586:
583:
581:
578:
576:
573:
571:
567:
564:
561:
560:Batatasin-III
558:
557:
555:
553:
549:
544:
537:
532:
530:
525:
523:
518:
517:
514:
505:
501:
494:
491:
486:
482:
478:
474:
470:
466:
462:
458:
451:
448:
445:
441:
434:
431:
428:
424:
418:
415:
411:
404:
401:
398:
394:
387:
384:
379:
373:
369:
368:
360:
357:
350:
348:
346:
342:
337:
335:
334:
329:
328:
323:
322:
317:
313:
312:bis(bibenzyl)
310:
306:
294:
288:
282:
278:
276:
273:
272:
256:
253:
249:
248:
243:
234:
230:
223:
209:
205:
198:
190:
186:
182:
181:
179:
169:
165:
164:
157:
153:
152:
150:
144:
140:
139:
132:
128:
127:
125:
123:
120:
119:
112:
108:
107:
105:
103:
100:
99:
92:
88:
87:
85:
81:
76:
75:
68:
64:
63:
61:
58:
54:
53:
48:
42:
38:
33:
25:
20:
683:derivative:
672:
660:Marchantin A
647:Plagiochin E
633:Marchantin B
493:
485:the original
464:
460:
450:
433:
417:
403:
386:
366:
359:
338:
331:
325:
319:
304:
303:
111:ChEMBL411317
50:Identifiers
17:Riccardin C
716:Cyclophanes
711:Macrocycles
685:Cavicularin
669:Riccardin B
652:Riccardin H
345:cavicularin
309:macrocyclic
305:Riccardin C
245:Properties
700:Categories
351:References
314:. It is a
275:Molar mass
122:ChemSpider
78:3D model (
67:84575-08-6
57:CAS Number
41:IUPAC name
590:Lunularin
481:15900530
156:10070992
410:article
269:
143:PubChem
131:8246532
479:
374:
229:SMILES
102:ChEMBL
35:Names
324:, in
307:is a
204:InChI
80:JSmol
671:and
662:and
635:and
606:and
568:and
477:PMID
372:ISBN
500:doi
469:doi
440:doi
423:doi
393:doi
173:EPA
146:CID
702::
626::
602:,
475:.
465:44
463:.
459:.
347:.
336:.
263:24
259:28
673:C
664:C
637:E
608:C
604:B
535:e
528:t
521:v
502::
471::
442::
425::
412:)
408:(
395::
380:.
267:4
265:O
261:H
257:C
175:)
171:(
82:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.