557:
the risk of progression or death observed in those treated with ridaforolimus compared to placebo (hazard ratio=0.72). Median PFS was 17.7 weeks for those treated with ridaforolimus compared to 14.6 weeks in the placebo group. Furthermore, based on the full analysis of PFS determined by investigator assessment, there was a statistically significant (p<0.0001) 31 percent reduction by ridaforolimus in the risk of progression or death compared to placebo (hazard ratio=0.69). In the investigator assessment analysis, median PFS was 22.4 weeks for those treated with ridaforolimus compared to 14.7 weeks in the placebo group
294:
271:
405:
InChI=1S/C53H84NO14P/c1-32-18-14-13-15-19-33(2)44(63-8)30-40-23-21-38(7)53(61,67-40)50(58)51(59)54-25-17-16-20-41(54)52(60)66-45(35(4)28-39-22-24-43(46(29-39)64-9)68-69(11,12)62)31-42(55)34(3)27-37(6)48(57)49(65-10)47(56)36(5)26-32/h13-15,18-19,27,32,34-36,38-41,43-46,48-49,57,61H,16-17,20-26,28-31H2,1-12H3/b15-13+,18-14+,33-19+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43-,44+,45+,46-,48-,49+,53-/m1/s1
512:& Company announced a clinical development and marketing agreement. With this agreement, Ariad received $ 125 million in upfront payments from Merck and $ 53 million in milestone payments. Future payments are triggered upon acceptance of the NDA by the FDA with another payment when the drug receives marketing approval. There are similar milestones for acceptance and approval in both Europe and
31:
3619:
363:
533:
On June 6, 2011, Ariad and Merck announced detailed results from the largest randomized study ever in the soft tissue and bone sarcoma population, the Phase III SUCCEED clinical trial. SUCCEED evaluated oral ridaforolimus, in patients with metastatic soft-tissue or bone sarcomas who previously had a
556:
Based on 552 progression-free survival (PFS) events in 711 patients, (ridaforolimus (N=347), placebo (N=364) determined by an independent radiological review committee, the study achieved its primary endpoint of improvement in PFS, with a statistically significant (p=0.0001) 28 percent reduction in
553:
study of oral ridaforolimus administered at 40 mg/day (five of seven days per week) in patients with metastatic soft-tissue or bone sarcomas who previously had a favorable response to chemotherapy. Oral ridaforolimus was granted a
Special Protocol Assessment (SPA) by the FDA for the SUCCEED
520:(NDA) for ridaforolimus to the U.S. Food and Drug Administration (FDA) and a marketing application in the European Union in 2011. After formal rejection by the FDA in June 2012 ARIAD/MSD decided to withdraw their EMA application for Ridaforolimus in November 2012.
546:
of the study. The complete study results were presented by Sant P. Chawla, M.D., director, Sarcoma
Oncology Center, Santa Monica, CA, during the 2011 American Society of Clinical Oncology (ASCO) annual meeting.
756:
3659:
43:
421:
457:, a protein that acts as a central regulator of protein synthesis, cell proliferation, cell cycle progression and cell survival, integrating signals from proteins, such as
749:
2187:
566:
377:
742:
3654:
516:. Other milestone payments are tied to revenue goals for the drug. ARIAD has opted to co-promote ridaforolimus in the U.S. Merck plans to submit a
3103:
673:
1748:
1391:
884:
716:
651:
586:
549:
The SUCCEED (Sarcoma Multi-Center
Clinical Evaluation of the Efficacy of Ridaforolimus) trial was a randomized (1:1), placebo-controlled,
385:
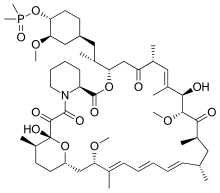
CC1CCC2CC(/C(=C/C=C/C=C/C(CC(C(=O)C(C(/C(=C/C(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)OP(=O)(C)C)C)/C)O)OC)C)C)/C)OC
1862:
3046:
2416:
397:
2180:
1282:
808:
587:"ARIAD Reports First Quarter 2009 Development Progress and Financial Results- Ridaforolimus New USAN Name to Replace Deforolimus"
3455:
76:
3609:
2252:
1628:
469:
known to be important to malignancy. Blocking mTOR creates a starvation-like effect in cancer cells by interfering with
190:
3639:
2361:
2173:
785:
608:
Mita MM, Gong J, Chawla SP (September 2013). "Ridaforolimus in advanced or metastatic soft tissue and bone sarcomas".
250:
1809:
2231:
1934:
1783:
1532:
1034:
2909:
1259:
1125:
838:
539:
3289:
1269:
795:
695:
159:
3284:
765:
289:
3089:
2515:
2196:
1637:
1120:
849:
239:
3649:
3276:
1195:
1135:
1105:
1008:
844:
734:
505:
150:
2165:
1050:
945:
782:
517:
266:
3644:
2497:
1903:
1170:
1160:
980:
105:
3446:
2547:
1558:
1200:
1190:
1175:
1095:
633:
3486:
3344:
3496:
3182:
2623:
3664:
3451:
3441:
2242:
2200:
1235:
625:
219:
179:
3247:
3150:
2813:
2709:
1075:
917:
912:
617:
543:
306:
114:
199:
96:)-4-hexatriaconta-16,24,26,28-tetraen-12-yl]propyl]-2-methoxycyclohexyl dimethylphosphinate
3623:
3546:
3431:
293:
270:
3436:
3304:
2952:
2786:
2765:
2761:
2757:
2385:
2222:
2012:
1910:
1858:
1456:
769:
509:
501:
3633:
3586:
3516:
3426:
3333:
3119:
2819:
2741:
2693:
2673:
2531:
2298:
2264:
2069:
1959:
1898:
1830:
1825:
1815:
1591:
1587:
1575:
1382:
1165:
1130:
950:
930:
474:
282:
139:
637:
3596:
3556:
3541:
3536:
3252:
3202:
3197:
3177:
3057:
3052:
2915:
2900:
2848:
2824:
2771:
2648:
2638:
2457:
2374:
2369:
2327:
2280:
2237:
2204:
2109:
2094:
2064:
2044:
1926:
1874:
1835:
1723:
1702:
1609:
1571:
1472:
1447:
1443:
1327:
1292:
1245:
1230:
1185:
1060:
1030:
955:
773:
550:
535:
488:
It has had promising results in a clinical trial for advanced soft tissue and bone
482:
56:
51:
621:
3591:
3531:
3521:
3511:
3411:
3401:
3391:
3386:
3351:
3262:
3257:
3232:
3222:
3212:
3187:
3172:
3167:
3125:
3095:
3081:
3076:
3062:
3005:
2963:
2958:
2944:
2939:
2925:
2905:
2858:
2834:
2792:
2776:
2747:
2731:
2699:
2683:
2678:
2653:
2603:
2593:
2588:
2583:
2572:
2562:
2467:
2379:
2337:
2322:
2316:
2312:
2259:
2139:
2119:
2114:
2104:
2084:
2059:
2054:
2037:
1985:
1975:
1970:
1965:
1867:
1794:
1764:
1759:
1754:
1738:
1728:
1708:
1583:
1579:
1567:
1563:
1492:
1396:
1342:
1337:
1302:
1240:
1225:
1215:
1210:
1205:
1180:
1145:
1100:
1080:
1070:
1025:
990:
970:
926:
889:
877:
872:
834:
818:
470:
466:
3581:
3576:
3561:
3501:
3491:
3476:
3471:
3466:
3416:
3406:
3396:
3361:
3339:
3325:
3237:
3227:
3217:
3207:
3192:
3135:
3130:
3109:
3024:
3019:
2977:
2971:
2872:
2829:
2725:
2715:
2668:
2658:
2633:
2628:
2618:
2613:
2608:
2598:
2557:
2542:
2537:
2521:
2462:
2442:
2391:
2351:
2332:
2274:
2154:
2149:
2124:
2089:
2074:
2049:
2032:
2022:
1980:
1951:
1916:
1882:
1799:
1789:
1769:
1733:
1718:
1713:
1683:
1617:
1613:
1595:
1543:
1522:
1482:
1477:
1416:
1347:
1322:
1297:
1220:
1155:
1115:
1110:
1090:
1085:
1065:
1055:
1045:
960:
940:
922:
830:
478:
339:
170:
22:
453:) is an investigational targeted and small-molecule inhibitor of the protein
3571:
3566:
3551:
3526:
3481:
3461:
3421:
3356:
3320:
3242:
3038:
2991:
2920:
2886:
2853:
2663:
2643:
2552:
2452:
2292:
2144:
2134:
2129:
2099:
2079:
2027:
2017:
2005:
2000:
1946:
1941:
1887:
1820:
1693:
1688:
1673:
1668:
1663:
1653:
1648:
1643:
1548:
1538:
1517:
1512:
1507:
1502:
1497:
1487:
1467:
1431:
1426:
1421:
1411:
1406:
1373:
1368:
1363:
1332:
1312:
1307:
1150:
1140:
965:
813:
629:
30:
3506:
3381:
3070:
2578:
2488:
2424:
2346:
1894:
1658:
1462:
1401:
1357:
1317:
1287:
1040:
904:
900:
825:
2999:
998:
489:
125:
3314:
3145:
230:
3013:
2866:
1878:
1604:
1438:
1387:
867:
513:
362:
353:
210:
2985:
2933:
2894:
2880:
2434:
2308:
1993:
1779:
1277:
1003:
985:
975:
935:
803:
458:
454:
2169:
738:
2842:
462:
255:
428:
3607:
538:. In this patient population, ridaforolimus improved
3374:
3302:
3275:
3160:
2803:
2505:
2496:
2487:
2480:
2433:
2415:
2406:
2360:
2291:
2221:
2212:
1850:
1627:
1268:
1258:
1018:
899:
859:
794:
781:
351:
338:
305:
300:
281:
249:
229:
209:
189:
169:
158:
149:
124:
104:
67:
42:
37:
3660:Drugs developed by Takeda Pharmaceutical Company
138:
113:
2181:
750:
8:
567:Discovery and development of mTOR inhibitors
508:. On May 5, 2010, Ariad Pharmaceuticals and
21:
2502:
2493:
2484:
2412:
2218:
2188:
2174:
2166:
1265:
791:
757:
743:
735:
292:
269:
178:
29:
198:
3614:
578:
500:Ridaforolimus is being co-developed by
402:
382:
265:
81:
610:Expert Review of Clinical Pharmacology
283:
20:
676:. Phx.corporate-ir.net. 17 March 2011
238:
218:
7:
55:
719:. Phx.corporate-ir.net. 6 June 2011
129:
3655:Drugs developed by Merck & Co.
332:
14:
3617:
323:
317:
542:(PFS) compared to placebo, the
410:Key:BUROJSBIWGDYCN-GAUTUEMISA-N
326:
311:
1:
589:. ARIAD Pharmaceuticals. 2009
2253:dihydroorotate dehydrogenase
622:10.1586/17512433.2013.827397
2232:purine synthesis inhibitors
3681:
1260:Tyrosine kinase inhibitors
301:Chemical and physical data
2417:IL-1 receptor antagonists
1126:Mirvetuximab soravtansine
540:progression-free survival
418:
393:
373:
72:
28:
3290:Anti-lymphocyte globulin
1270:Receptor tyrosine kinase
796:Receptor tyrosine kinase
3285:Anti-thymocyte globulin
2197:Immunosuppressive drugs
860:Others for solid tumors
766:Targeted cancer therapy
696:"UKMi New Drugs Online"
496:Commercial arrangements
3090:Interleukin-6 receptor
2516:Complement component 5
1121:Loncastuximab tesirine
850:Trastuzumab deruxtecan
717:"ARIAD - News release"
674:"ARIAD - News release"
654:. Phx.corporate-ir.net
652:"ARIAD - News release"
534:favorable response to
1196:Sacituzumab govitecan
1136:Moxetumomab pasudotox
1106:Inotuzumab ozogamicin
1009:Gemtuzumab ozogamicin
845:Trastuzumab emtansine
786:monoclonal antibodies
770:antineoplastic agents
506:ARIAD Pharmaceuticals
1051:Belantamab mafodotin
518:New Drug Application
449:; formerly known as
1904:Denileukin diftitox
1566:(ALK, ROS1, NTRK),
1171:Polatuzumab vedotin
1161:Oportuzumab monatox
25:
3640:Immunosuppressants
3447:Diroximel fumarate
3120:IL-2 receptor/CD25
2548:Certolizumab pegol
2201:Immunosuppressants
1590:(ROS1, TRK, ALK),
1096:Enfortumab vedotin
3605:
3604:
3452:Efgartigimod alfa
3442:Dimethyl fumarate
3370:
3369:
3298:
3297:
3271:
3270:
2476:
2475:
2402:
2401:
2243:Mycophenolic acid
2163:
2162:
1846:
1845:
1254:
1253:
1236:Tisotumab vedotin
529:Phase III SUCCEED
436:
435:
364:Interactive image
251:CompTox Dashboard
16:Chemical compound
3672:
3622:
3621:
3620:
3613:
3248:Telimomab aritox
3151:Zolimomab aritox
2972:CD62L/L-selectin
2710:Immunoglobulin E
2503:
2494:
2485:
2413:
2219:
2190:
2183:
2176:
2167:
1877:peptide against
1450:(AXL, ALK, LTK))
1266:
1076:Dinutuximab beta
792:
759:
752:
745:
736:
729:
728:
726:
724:
713:
707:
706:
704:
702:
692:
686:
685:
683:
681:
670:
664:
663:
661:
659:
648:
642:
641:
605:
599:
598:
596:
594:
583:
544:primary endpoint
432:
431:
424:
366:
346:
334:
328:
325:
319:
313:
296:
285:
274:
273:
259:
257:
242:
222:
202:
182:
162:
142:
132:
131:
117:
59:
33:
26:
24:
3680:
3679:
3675:
3674:
3673:
3671:
3670:
3669:
3630:
3629:
3628:
3618:
3616:
3608:
3606:
3601:
3547:Rozanolixizumab
3432:Deucravacitinib
3366:
3294:
3267:
3156:
2805:
2799:
2507:
2472:
2429:
2408:
2398:
2356:
2296:
2287:
2223:Antimetabolites
2214:
2208:
2194:
2164:
2159:
2013:Pi3K inhibitors
1911:mTOR inhibitors
1842:
1623:
1594:(VEGFR, FGFR),
1250:
1014:
895:
855:
777:
763:
733:
732:
722:
720:
715:
714:
710:
700:
698:
694:
693:
689:
679:
677:
672:
671:
667:
657:
655:
650:
649:
645:
607:
606:
602:
592:
590:
585:
584:
580:
575:
563:
555:
548:
531:
526:
524:Clinical trials
498:
441:(also known as
427:
425:
422:(what is this?)
419:
414:
411:
406:
401:
400:
389:
386:
381:
380:
369:
344:
331:
322:
316:
277:
267:DTXSID001025942
253:
245:
225:
205:
185:
165:
145:
128:
120:
100:
97:
80:
79:
63:
17:
12:
11:
5:
3678:
3676:
3668:
3667:
3662:
3657:
3652:
3647:
3642:
3632:
3631:
3627:
3626:
3603:
3602:
3600:
3599:
3594:
3589:
3584:
3579:
3574:
3569:
3564:
3559:
3554:
3549:
3544:
3539:
3534:
3529:
3524:
3519:
3514:
3509:
3504:
3499:
3494:
3489:
3484:
3479:
3474:
3469:
3464:
3459:
3456:+hyaluronidase
3449:
3444:
3439:
3437:Deuruxolitinib
3434:
3429:
3424:
3419:
3414:
3409:
3404:
3399:
3394:
3389:
3384:
3378:
3376:
3372:
3371:
3368:
3367:
3365:
3364:
3359:
3354:
3349:
3348:
3347:
3342:
3330:
3329:
3328:
3323:
3310:
3308:
3300:
3299:
3296:
3295:
3293:
3292:
3287:
3281:
3279:
3273:
3272:
3269:
3268:
3266:
3265:
3260:
3255:
3250:
3245:
3240:
3235:
3230:
3225:
3220:
3215:
3210:
3205:
3200:
3195:
3190:
3185:
3180:
3175:
3170:
3164:
3162:
3158:
3157:
3155:
3154:
3141:
3140:
3139:
3138:
3133:
3128:
3115:
3114:
3113:
3112:
3100:
3099:
3098:
3086:
3085:
3084:
3079:
3067:
3066:
3065:
3060:
3055:
3043:
3042:
3041:
3030:
3029:
3028:
3027:
3022:
3010:
3009:
3008:
2996:
2995:
2994:
2982:
2981:
2980:
2968:
2967:
2966:
2961:
2949:
2948:
2947:
2942:
2930:
2929:
2928:
2923:
2918:
2913:
2910:+hyaluronidase
2903:
2891:
2890:
2889:
2877:
2876:
2875:
2863:
2862:
2861:
2856:
2851:
2839:
2838:
2837:
2832:
2827:
2822:
2809:
2807:
2801:
2800:
2798:
2797:
2796:
2795:
2782:
2781:
2780:
2779:
2774:
2753:
2752:
2751:
2750:
2737:
2736:
2735:
2734:
2721:
2720:
2719:
2718:
2705:
2704:
2703:
2702:
2689:
2688:
2687:
2686:
2681:
2676:
2671:
2666:
2661:
2656:
2651:
2646:
2641:
2636:
2631:
2626:
2621:
2616:
2611:
2606:
2601:
2596:
2591:
2586:
2581:
2568:
2567:
2566:
2565:
2560:
2555:
2550:
2545:
2540:
2527:
2526:
2525:
2524:
2511:
2509:
2500:
2491:
2482:
2478:
2477:
2474:
2473:
2471:
2470:
2465:
2460:
2455:
2450:
2445:
2439:
2437:
2431:
2430:
2428:
2427:
2421:
2419:
2410:
2404:
2403:
2400:
2399:
2397:
2396:
2395:
2394:
2386:PDE4 inhibitor
2382:
2377:
2372:
2366:
2364:
2358:
2357:
2355:
2354:
2349:
2343:
2342:
2341:
2340:
2335:
2330:
2325:
2304:
2302:
2289:
2288:
2286:
2285:
2284:
2283:
2270:
2269:
2268:
2267:
2262:
2248:
2247:
2246:
2245:
2240:
2227:
2225:
2216:
2210:
2209:
2195:
2193:
2192:
2185:
2178:
2170:
2161:
2160:
2158:
2157:
2152:
2147:
2142:
2137:
2132:
2127:
2122:
2117:
2112:
2107:
2102:
2097:
2092:
2087:
2082:
2077:
2072:
2067:
2062:
2057:
2052:
2047:
2042:
2041:
2040:
2035:
2030:
2025:
2020:
2010:
2009:
2008:
2003:
1990:
1989:
1988:
1983:
1978:
1973:
1968:
1960:CDK inhibitors
1956:
1955:
1954:
1949:
1944:
1931:
1930:
1929:
1924:
1919:
1907:
1891:
1871:
1859:fusion protein
1854:
1852:
1848:
1847:
1844:
1843:
1841:
1840:
1839:
1838:
1833:
1828:
1823:
1818:
1805:
1804:
1803:
1802:
1797:
1792:
1775:
1774:
1773:
1772:
1767:
1762:
1757:
1744:
1743:
1742:
1741:
1736:
1731:
1726:
1721:
1716:
1711:
1698:
1697:
1691:
1679:
1678:
1677:
1676:
1671:
1666:
1661:
1656:
1651:
1646:
1633:
1631:
1625:
1624:
1622:
1621:
1600:
1599:
1598:(VEGFR, EGFR).
1554:
1553:
1552:
1551:
1546:
1541:
1528:
1527:
1526:
1525:
1520:
1515:
1510:
1505:
1500:
1495:
1490:
1485:
1480:
1475:
1470:
1465:
1452:
1451:
1435:
1429:
1424:
1419:
1414:
1409:
1404:
1399:
1379:
1378:
1377:
1376:
1371:
1366:
1356:HER1/EGFR and
1352:
1351:
1345:
1340:
1335:
1330:
1325:
1320:
1315:
1310:
1305:
1300:
1295:
1290:
1274:
1272:
1263:
1256:
1255:
1252:
1251:
1249:
1248:
1243:
1238:
1233:
1228:
1223:
1218:
1213:
1208:
1203:
1198:
1193:
1188:
1183:
1178:
1173:
1168:
1163:
1158:
1153:
1148:
1143:
1138:
1133:
1128:
1123:
1118:
1113:
1108:
1103:
1098:
1093:
1088:
1083:
1078:
1073:
1068:
1063:
1058:
1053:
1048:
1043:
1038:
1035:+hyaluronidase
1028:
1022:
1020:
1016:
1015:
1013:
1012:
995:
994:
968:
963:
958:
953:
948:
943:
909:
907:
897:
896:
894:
893:
881:
875:
863:
861:
857:
856:
854:
853:
847:
842:
839:+hyaluronidase
822:
816:
800:
798:
789:
779:
778:
764:
762:
761:
754:
747:
739:
731:
730:
708:
687:
665:
643:
600:
577:
576:
574:
571:
570:
569:
562:
559:
530:
527:
525:
522:
497:
494:
434:
433:
416:
415:
413:
412:
409:
407:
404:
396:
395:
394:
391:
390:
388:
387:
384:
376:
375:
374:
371:
370:
368:
367:
359:
357:
349:
348:
342:
336:
335:
329:
320:
314:
309:
303:
302:
298:
297:
287:
279:
278:
276:
275:
262:
260:
247:
246:
244:
243:
235:
233:
227:
226:
224:
223:
215:
213:
207:
206:
204:
203:
195:
193:
187:
186:
184:
183:
175:
173:
167:
166:
164:
163:
155:
153:
147:
146:
144:
143:
135:
133:
122:
121:
119:
118:
110:
108:
102:
101:
99:
98:
83:
75:
74:
73:
70:
69:
65:
64:
62:
61:
48:
46:
40:
39:
35:
34:
15:
13:
10:
9:
6:
4:
3:
2:
3677:
3666:
3663:
3661:
3658:
3656:
3653:
3651:
3648:
3646:
3643:
3641:
3638:
3637:
3635:
3625:
3615:
3611:
3598:
3595:
3593:
3590:
3588:
3587:Tildrakizumab
3585:
3583:
3580:
3578:
3575:
3573:
3570:
3568:
3565:
3563:
3560:
3558:
3555:
3553:
3550:
3548:
3545:
3543:
3540:
3538:
3535:
3533:
3530:
3528:
3525:
3523:
3520:
3518:
3517:Pegcetacoplan
3515:
3513:
3510:
3508:
3505:
3503:
3500:
3498:
3495:
3493:
3490:
3488:
3485:
3483:
3480:
3478:
3475:
3473:
3470:
3468:
3465:
3463:
3460:
3457:
3453:
3450:
3448:
3445:
3443:
3440:
3438:
3435:
3433:
3430:
3428:
3427:Darvadstrocel
3425:
3423:
3420:
3418:
3415:
3413:
3410:
3408:
3405:
3403:
3400:
3398:
3395:
3393:
3390:
3388:
3385:
3383:
3380:
3379:
3377:
3373:
3363:
3360:
3358:
3355:
3353:
3350:
3346:
3343:
3341:
3338:
3337:
3336:
3335:
3334:TNF inhibitor
3331:
3327:
3324:
3322:
3319:
3318:
3317:
3316:
3312:
3311:
3309:
3306:
3301:
3291:
3288:
3286:
3283:
3282:
3280:
3278:
3274:
3264:
3261:
3259:
3256:
3254:
3251:
3249:
3246:
3244:
3241:
3239:
3236:
3234:
3231:
3229:
3226:
3224:
3221:
3219:
3216:
3214:
3211:
3209:
3206:
3204:
3201:
3199:
3196:
3194:
3191:
3189:
3186:
3184:
3181:
3179:
3176:
3174:
3171:
3169:
3166:
3165:
3163:
3159:
3152:
3148:
3147:
3143:
3142:
3137:
3134:
3132:
3129:
3127:
3124:
3123:
3122:
3121:
3117:
3116:
3111:
3108:
3107:
3106:
3105:
3101:
3097:
3094:
3093:
3092:
3091:
3087:
3083:
3080:
3078:
3075:
3074:
3073:
3072:
3068:
3064:
3061:
3059:
3056:
3054:
3051:
3050:
3049:
3048:
3044:
3040:
3037:
3036:
3035:
3032:
3031:
3026:
3023:
3021:
3018:
3017:
3016:
3015:
3011:
3007:
3004:
3003:
3002:
3001:
3000:CD147/Basigin
2997:
2993:
2990:
2989:
2988:
2987:
2983:
2979:
2976:
2975:
2974:
2973:
2969:
2965:
2962:
2960:
2957:
2956:
2955:
2954:
2950:
2946:
2943:
2941:
2938:
2937:
2936:
2935:
2931:
2927:
2924:
2922:
2919:
2917:
2914:
2911:
2907:
2904:
2902:
2899:
2898:
2897:
2896:
2892:
2888:
2885:
2884:
2883:
2882:
2878:
2874:
2871:
2870:
2869:
2868:
2864:
2860:
2857:
2855:
2852:
2850:
2847:
2846:
2845:
2844:
2840:
2836:
2833:
2831:
2828:
2826:
2823:
2821:
2820:Muromonab-CD3
2818:
2817:
2816:
2815:
2811:
2810:
2808:
2802:
2794:
2791:
2790:
2789:
2788:
2784:
2783:
2778:
2775:
2773:
2770:
2769:
2768:
2767:
2763:
2759:
2755:
2754:
2749:
2746:
2745:
2744:
2743:
2739:
2738:
2733:
2730:
2729:
2728:
2727:
2723:
2722:
2717:
2714:
2713:
2712:
2711:
2707:
2706:
2701:
2698:
2697:
2696:
2695:
2694:Interleukin 5
2691:
2690:
2685:
2682:
2680:
2677:
2675:
2674:Tildrakizumab
2672:
2670:
2667:
2665:
2662:
2660:
2657:
2655:
2652:
2650:
2647:
2645:
2642:
2640:
2637:
2635:
2632:
2630:
2627:
2625:
2622:
2620:
2617:
2615:
2612:
2610:
2607:
2605:
2602:
2600:
2597:
2595:
2592:
2590:
2587:
2585:
2582:
2580:
2577:
2576:
2575:
2574:
2570:
2569:
2564:
2561:
2559:
2556:
2554:
2551:
2549:
2546:
2544:
2541:
2539:
2536:
2535:
2534:
2533:
2529:
2528:
2523:
2520:
2519:
2518:
2517:
2513:
2512:
2510:
2508:(noncellular)
2504:
2501:
2499:
2495:
2492:
2490:
2486:
2483:
2481:Extracellular
2479:
2469:
2466:
2464:
2461:
2459:
2456:
2454:
2451:
2449:
2448:Ridaforolimus
2446:
2444:
2441:
2440:
2438:
2436:
2432:
2426:
2423:
2422:
2420:
2418:
2414:
2411:
2407:Intracellular
2405:
2393:
2390:
2389:
2388:
2387:
2383:
2381:
2378:
2376:
2373:
2371:
2368:
2367:
2365:
2363:
2359:
2353:
2350:
2348:
2345:
2344:
2339:
2336:
2334:
2331:
2329:
2326:
2324:
2321:
2320:
2319:
2318:
2314:
2310:
2306:
2305:
2303:
2300:
2294:
2290:
2282:
2279:
2278:
2277:
2276:
2272:
2271:
2266:
2265:Teriflunomide
2263:
2261:
2258:
2257:
2255:
2254:
2250:
2249:
2244:
2241:
2239:
2236:
2235:
2234:
2233:
2229:
2228:
2226:
2224:
2220:
2217:
2213:Intracellular
2211:
2206:
2202:
2198:
2191:
2186:
2184:
2179:
2177:
2172:
2171:
2168:
2156:
2153:
2151:
2148:
2146:
2143:
2141:
2138:
2136:
2133:
2131:
2128:
2126:
2123:
2121:
2118:
2116:
2113:
2111:
2108:
2106:
2103:
2101:
2098:
2096:
2093:
2091:
2088:
2086:
2083:
2081:
2078:
2076:
2073:
2071:
2070:Larotrectinib
2068:
2066:
2063:
2061:
2058:
2056:
2053:
2051:
2048:
2046:
2043:
2039:
2036:
2034:
2031:
2029:
2026:
2024:
2021:
2019:
2016:
2015:
2014:
2011:
2007:
2004:
2002:
1999:
1998:
1997:
1995:
1991:
1987:
1984:
1982:
1979:
1977:
1974:
1972:
1969:
1967:
1964:
1963:
1962:
1961:
1957:
1953:
1950:
1948:
1945:
1943:
1940:
1939:
1938:
1936:
1932:
1928:
1925:
1923:
1922:Ridaforolimus
1920:
1918:
1915:
1914:
1913:
1912:
1908:
1905:
1901:
1900:
1896:
1892:
1889:
1885:
1884:
1880:
1876:
1872:
1869:
1865:
1864:
1860:
1856:
1855:
1853:
1849:
1837:
1834:
1832:
1831:Pirtobrutinib
1829:
1827:
1826:Orelabrutinib
1824:
1822:
1819:
1817:
1816:Acalabrutinib
1814:
1813:
1812:
1811:
1807:
1806:
1801:
1798:
1796:
1793:
1791:
1788:
1787:
1786:
1785:
1781:
1777:
1776:
1771:
1768:
1766:
1763:
1761:
1758:
1756:
1753:
1752:
1751:
1750:
1746:
1745:
1740:
1737:
1735:
1732:
1730:
1727:
1725:
1722:
1720:
1717:
1715:
1712:
1710:
1707:
1706:
1705:
1704:
1700:
1699:
1695:
1692:
1690:
1686:
1685:
1681:
1680:
1675:
1672:
1670:
1667:
1665:
1662:
1660:
1657:
1655:
1652:
1650:
1647:
1645:
1642:
1641:
1640:
1639:
1635:
1634:
1632:
1630:
1626:
1619:
1615:
1611:
1608:
1606:
1602:
1601:
1597:
1593:
1592:Selpercatinib
1589:
1588:Repotrectinib
1585:
1581:
1577:
1576:Larotrectinib
1573:
1569:
1565:
1562:
1560:
1556:
1555:
1550:
1547:
1545:
1542:
1540:
1537:
1536:
1535:
1534:
1530:
1529:
1524:
1521:
1519:
1516:
1514:
1511:
1509:
1506:
1504:
1501:
1499:
1496:
1494:
1491:
1489:
1486:
1484:
1481:
1479:
1476:
1474:
1471:
1469:
1466:
1464:
1461:
1460:
1459:
1458:
1454:
1453:
1449:
1445:
1441:
1440:
1436:
1433:
1430:
1428:
1425:
1423:
1420:
1418:
1415:
1413:
1410:
1408:
1405:
1403:
1400:
1398:
1394:
1393:
1389:
1384:
1383:RTK class III
1381:
1380:
1375:
1372:
1370:
1367:
1365:
1362:
1361:
1360:
1359:
1354:
1353:
1349:
1346:
1344:
1341:
1339:
1336:
1334:
1331:
1329:
1326:
1324:
1321:
1319:
1316:
1314:
1311:
1309:
1306:
1304:
1301:
1299:
1296:
1294:
1291:
1289:
1285:
1284:
1279:
1276:
1275:
1273:
1271:
1267:
1264:
1261:
1257:
1247:
1244:
1242:
1239:
1237:
1234:
1232:
1229:
1227:
1224:
1222:
1219:
1217:
1214:
1212:
1209:
1207:
1204:
1202:
1199:
1197:
1194:
1192:
1189:
1187:
1184:
1182:
1179:
1177:
1174:
1172:
1169:
1167:
1166:Pembrolizumab
1164:
1162:
1159:
1157:
1154:
1152:
1149:
1147:
1144:
1142:
1139:
1137:
1134:
1132:
1131:Mogamulizumab
1129:
1127:
1124:
1122:
1119:
1117:
1114:
1112:
1109:
1107:
1104:
1102:
1099:
1097:
1094:
1092:
1089:
1087:
1084:
1082:
1079:
1077:
1074:
1072:
1069:
1067:
1064:
1062:
1059:
1057:
1054:
1052:
1049:
1047:
1044:
1042:
1039:
1036:
1032:
1029:
1027:
1024:
1023:
1021:
1017:
1010:
1006:
1005:
1000:
997:
996:
992:
988:
987:
982:
978:
977:
972:
969:
967:
964:
962:
959:
957:
954:
952:
951:Mosunetuzumab
949:
947:
944:
942:
938:
937:
932:
931:Mosunetuzumab
928:
924:
920:
919:
914:
911:
910:
908:
906:
902:
898:
891:
887:
886:
882:
879:
876:
874:
870:
869:
865:
864:
862:
858:
851:
848:
846:
843:
840:
836:
832:
828:
827:
823:
820:
817:
815:
811:
810:
805:
802:
801:
799:
797:
793:
790:
787:
784:
780:
775:
771:
767:
760:
755:
753:
748:
746:
741:
740:
737:
718:
712:
709:
697:
691:
688:
675:
669:
666:
653:
647:
644:
639:
635:
631:
627:
623:
619:
616:(5): 465–82.
615:
611:
604:
601:
588:
582:
579:
572:
568:
565:
564:
560:
558:
552:
545:
541:
537:
528:
523:
521:
519:
515:
511:
507:
503:
495:
493:
491:
486:
484:
480:
476:
472:
468:
464:
460:
456:
452:
448:
444:
440:
439:Ridaforolimus
430:
423:
417:
408:
403:
399:
392:
383:
379:
372:
365:
361:
360:
358:
355:
350:
343:
341:
337:
310:
308:
304:
299:
295:
291:
288:
286:
284:ECHA InfoCard
280:
272:
268:
264:
263:
261:
252:
248:
241:
240:ChEMBL2103804
237:
236:
234:
232:
228:
221:
217:
216:
214:
212:
208:
201:
197:
196:
194:
192:
188:
181:
177:
176:
174:
172:
168:
161:
157:
156:
154:
152:
148:
141:
137:
136:
134:
127:
123:
116:
112:
111:
109:
107:
103:
95:
91:
87:
82:
78:
71:
66:
58:
53:
50:
49:
47:
45:
41:
38:Clinical data
36:
32:
27:
23:Ridaforolimus
19:
3650:Phosphinates
3597:Upadacitinib
3557:Satralizumab
3542:Ritlecitinib
3537:Risankizumab
3332:
3313:
3253:Teprotumumab
3203:Inebilizumab
3198:Fontolizumab
3178:Atorolimumab
3146:T-lymphocyte
3144:
3118:
3102:
3088:
3069:
3058:Lerdelimumab
3053:Bertilimumab
3045:
3033:
3012:
2998:
2984:
2970:
2951:
2932:
2916:Pascolizumab
2901:Obinutuzumab
2893:
2879:
2865:
2849:Clenoliximab
2841:
2825:Otelixizumab
2812:
2785:
2772:Lebrikizumab
2756:
2740:
2724:
2708:
2692:
2649:Satralizumab
2639:Risankizumab
2571:
2530:
2514:
2506:Serum target
2458:Temsirolimus
2447:
2384:
2375:Pomalidomide
2370:Lenalidomide
2328:Pimecrolimus
2307:
2281:Methotrexate
2273:
2251:
2238:Azathioprine
2230:
2215:(initiation)
2110:Pexidartinib
2095:Odronextamab
2065:Gilteritinib
2045:Cabozantinib
1992:
1958:
1933:
1927:Temsirolimus
1921:
1909:
1893:
1875:proapoptotic
1873:
1857:
1836:Zanubrutinib
1808:
1778:
1747:
1724:Lestaurtinib
1703:Janus kinase
1701:
1682:
1636:
1629:Non-receptor
1610:Cabozantinib
1603:
1572:Infigratinib
1557:
1531:
1473:Fruquintinib
1455:
1448:Gilteritinib
1444:Lestaurtinib
1437:
1386:
1355:
1328:Mobocertinib
1293:Aumolertinib
1281:
1246:Tremelimumab
1231:Tislelizumab
1186:Retifanlimab
1061:Blinatumomab
1031:Atezolizumab
1002:
984:
974:
956:Obinutuzumab
934:
916:
883:
866:
824:
807:
721:. Retrieved
711:
699:. Retrieved
690:
678:. Retrieved
668:
656:. Retrieved
646:
613:
609:
603:
591:. Retrieved
581:
551:double-blind
536:chemotherapy
532:
499:
487:
483:angiogenesis
450:
446:
442:
438:
437:
426:
420:
93:
89:
85:
18:
3592:Tofacitinib
3532:Ravulizumab
3522:Pirfenidone
3512:Peficitinib
3412:Canakinumab
3402:Briakinumab
3392:Bimekizumab
3387:Baricitinib
3352:Aflibercept
3263:Vepalimomab
3258:Vapaliximab
3233:Rovelizumab
3223:Pexelizumab
3213:Morolimumab
3188:Cedelizumab
3173:Anifrolumab
3168:Alemtuzumab
3126:Basiliximab
3096:Tocilizumab
3082:Vedolizumab
3077:Natalizumab
3063:Metelimumab
3006:Gavilimomab
2964:Toralizumab
2959:Teneliximab
2945:Lumiliximab
2940:Gomiliximab
2926:Ublituximab
2906:Ocrelizumab
2859:Zanolimumab
2835:Visilizumab
2793:Secukinumab
2777:Ustekinumab
2748:Elsilimomab
2732:Faralimomab
2700:Mepolizumab
2684:Ustekinumab
2679:Tocilizumab
2654:Secukinumab
2604:Canakinumab
2594:Briakinumab
2589:Bimekizumab
2584:Basiliximab
2573:Interleukin
2563:Nerelimomab
2468:Zotarolimus
2409:(reception)
2380:Thalidomide
2338:Voclosporin
2323:Ciclosporin
2317:Calcineurin
2313:Cyclophilin
2260:Leflunomide
2256:inhibitors
2140:Tebentafusp
2120:Regorafenib
2115:Quizartinib
2105:Pemigatinib
2085:Midostaurin
2060:Erdafitinib
2055:Entrectinib
2038:Parsaclisib
1986:Trilaciclib
1976:Palbociclib
1971:Dalpiciclib
1966:Abemaciclib
1868:Aflibercept
1795:Entrectinib
1765:Selumetinib
1760:Cobimetinib
1755:Binimetinib
1739:Ruxolitinib
1729:Momelotinib
1709:Baricitinib
1607:inhibitors:
1584:Pralsetinib
1580:Pemigatinib
1568:Futibatinib
1564:Entrectinib
1561:inhibitors:
1493:Regorafenib
1397:Avapritinib
1343:Rociletinib
1338:Osimertinib
1303:Dacomitinib
1241:Toripalimab
1226:Teclistamab
1216:Talquetamab
1211:Tafasitamab
1206:Sugemalimab
1201:Serplulimab
1191:Sabatolimab
1181:Ramucirumab
1176:Prolgolimab
1146:Necitumumab
1101:Epcoritamab
1081:Dostarlimab
1071:Daratumumab
1026:Amivantamab
991:Alemtuzumab
981:Brentuximab
971:Tositumomab
946:Ibritumomab
927:Elranatamab
890:Bevacizumab
878:Edrecolomab
873:Catumaxomab
835:Trastuzumab
819:Panitumumab
471:cell growth
451:deforolimus
347: g·mol
290:100.207.749
220:CHEBI:82677
115:572924-54-0
68:Identifiers
3645:Macrolides
3634:Categories
3582:Sutimlimab
3577:Spesolimab
3562:Siltuximab
3502:Olokizumab
3492:Ixekizumab
3487:Itacitinib
3477:Guselkumab
3472:Fingolimod
3467:Filgotinib
3417:Crovalimab
3407:Brodalumab
3397:Blisibimod
3362:Rilonacept
3345:Opinercept
3340:Etanercept
3326:Belatacept
3277:Polyclonal
3238:Siplizumab
3228:Reslizumab
3218:Ofatumumab
3208:Maslimomab
3193:Emapalumab
3136:Inolimomab
3131:Daclizumab
3110:Odulimomab
3025:Ruplizumab
3020:Frexalimab
2978:Aselizumab
2873:Efalizumab
2830:Teplizumab
2726:Interferon
2716:Omalizumab
2669:Spesolimab
2659:Siltuximab
2634:Rilonacept
2629:Olokizumab
2619:Ixekizumab
2614:Guselkumab
2609:Daclizumab
2599:Brodalumab
2558:Infliximab
2543:Afelimomab
2538:Adalimumab
2522:Eculizumab
2498:Monoclonal
2489:Antibodies
2463:Umirolimus
2443:Everolimus
2392:Apremilast
2352:Gusperimus
2333:Tacrolimus
2301:inhibitors
2293:Macrolides
2275:antifolate
2155:Venetoclax
2150:Vandetanib
2125:Ripretinib
2090:Nintedanib
2075:Lenvatinib
2050:Capmatinib
2033:Idelalisib
2023:Copanlisib
1996:inhibitors
1981:Ribociclib
1952:Vismodegib
1937:inhibitors
1917:Everolimus
1883:prohibitin
1800:Lorlatinib
1790:Crizotinib
1770:Trametinib
1734:Pacritinib
1719:Filgotinib
1714:Fedratinib
1618:Crizotinib
1614:Capmatinib
1596:Vandetanib
1544:Brigatinib
1523:Vandetanib
1483:Nintedanib
1478:Lenvatinib
1417:Ripretinib
1348:Vandetanib
1323:Lazertinib
1298:Brigatinib
1221:Tarlatamab
1156:Olaratumab
1116:Isatuximab
1111:Ipilimumab
1091:Elotuzumab
1086:Durvalumab
1066:Cemiplimab
1056:Bermekimab
1046:Axatilimab
961:Ofatumumab
941:Glofitamab
923:Glofitamab
831:Pertuzumab
573:References
479:metabolism
352:3D model (
340:Molar mass
200:48Z35KB15K
171:ChemSpider
151:IUPHAR/BPS
106:CAS Number
77:IUPAC name
3572:Sirukumab
3567:Siponimod
3552:Sarilumab
3527:Ponesimod
3497:Netakimab
3482:Iptacopan
3462:Etrasimod
3422:Danicopan
3357:Alefacept
3321:Abatacept
3243:Talizumab
3183:Begelomab
3039:Belimumab
2992:Galiximab
2921:Rituximab
2887:Erlizumab
2854:Keliximab
2664:Sirukumab
2644:Sarilumab
2624:Netakimab
2553:Golimumab
2453:Sirolimus
2145:Tepotinib
2135:Sunitinib
2130:Sorafenib
2100:Pazopanib
2080:Masitinib
2028:Duvelisib
2018:Alpelisib
2006:Sotorasib
2001:Adagrasib
1947:Sonidegib
1942:Glasdegib
1888:Adipotide
1821:Ibrutinib
1694:Dasatinib
1689:Bosutinib
1674:Radotinib
1669:Ponatinib
1664:Nilotinib
1654:Dasatinib
1649:Bosutinib
1644:Asciminib
1612:(VEGFR),
1570:(FGFR2),
1549:Ceritinib
1539:Alectinib
1518:Toceranib
1513:Tivozanib
1508:Sunitinib
1503:Sorafenib
1498:Semaxanib
1488:Pazopanib
1468:Cediranib
1432:Toceranib
1427:Sunitinib
1422:Sorafenib
1412:Pazopanib
1407:Masitinib
1374:Tucatinib
1369:Neratinib
1364:Lapatinib
1333:Olmutinib
1313:Gefitinib
1308:Erlotinib
1283:HER1/EGFR
1151:Nivolumab
1141:Naxitamab
966:Rituximab
814:Cetuximab
809:HER1/EGFR
723:7 October
680:7 October
658:7 October
3665:Polyenes
3624:Medicine
3507:Ozanimod
3382:Avacopan
3375:Unsorted
3161:Unsorted
3071:Integrin
2804:Cellular
2579:Anakinra
2425:Anakinra
2347:Abetimus
2199: /
1935:hedgehog
1897:against
1895:exotoxin
1861:against
1810:Bruton's
1659:Imatinib
1582:(FGFR),
1578:(NTRK),
1463:Axitinib
1402:Axitinib
1358:HER2/neu
1318:Icotinib
1288:Afatinib
1262:("-nib")
1041:Avelumab
913:lymphoid
905:lymphoma
901:Leukemia
826:HER2/neu
788:("-mab")
701:11 April
638:29816218
630:23971829
561:See also
554:trial.
475:division
429:(verify)
180:24597928
140:11520894
44:ATC code
3303:-cept (
1638:bcr-abl
999:myeloid
490:sarcoma
447:MK-8669
443:AP23573
345:990.222
307:Formula
126:PubChem
60:)
54: (
52:L01EG03
3610:Portal
3315:CTLA-4
3305:Fusion
2806:target
2787:IL-17A
2764:, and
2297:other
885:VEGF-A
636:
628:
481:, and
378:SMILES
231:ChEMBL
3104:LFA-1
3014:CD154
2867:CD11a
2766:IL-23
2762:IL-13
2758:IL-12
2362:IMiDs
1879:ANXA2
1851:Other
1749:MAP2K
1620:(ALK)
1605:c-MET
1457:VEGFR
1392:PDGFR
1388:C-kit
1019:Other
868:EpCAM
634:S2CID
593:7 May
514:Japan
510:Merck
502:Merck
398:InChI
354:JSmol
211:ChEBI
3034:BLyS
2986:CD80
2953:CD40
2934:CD23
2895:CD20
2881:CD18
2742:IL-6
2435:mTOR
2309:FKBP
2299:IL-2
1994:KRAS
1899:IL-2
1881:and
1863:VEGF
1780:EML4
1439:FLT3
1390:and
1278:ErbB
1004:CD33
986:CD52
976:CD30
936:CD20
804:ErbB
725:2012
703:2013
682:2012
660:2012
626:PMID
595:2009
504:and
467:PTEN
465:and
459:PI3K
455:mTOR
445:and
191:UNII
160:7884
3047:CAT
2843:CD4
2814:CD3
2532:TNF
2205:L04
1784:ALK
1684:Src
1559:RET
1533:ALK
983:),
973:),
933:),
918:CD3
774:L01
618:doi
463:AKT
256:EPA
130:CID
57:WHO
3636::
2760:,
1616:,
1586:,
1574:,
1446:,
1385::
1280::
1001::
929:,
925:,
915::
833:,
806::
783:CI
768:/
632:.
624:.
612:.
492:.
485:.
477:,
473:,
461:,
330:14
321:84
315:53
92:,4
88:,2
84:(1
3612::
3458:)
3454:(
3307:)
3153:)
3149:(
2912:)
2908:(
2315:/
2311:/
2295:/
2207:)
2203:(
2189:e
2182:t
2175:v
1906:)
1902:(
1890:)
1886:(
1870:)
1866:(
1782:-
1696:)
1687:(
1442:(
1434:)
1395:(
1350:)
1286:(
1037:)
1033:(
1011:)
1007:(
993:)
989:(
979:(
939:(
921:(
903:/
892:)
888:(
880:)
871:(
852:)
841:)
837:(
829:(
821:)
812:(
776:)
772:(
758:e
751:t
744:v
727:.
705:.
684:.
662:.
640:.
620::
614:6
597:.
356:)
333:P
327:O
324:N
318:H
312:C
258:)
254:(
94:S
90:R
86:R
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.