662:
535:
348:
206:
616:
488:
300:
152:
104:
138:
802:
their activation threshold so they remain open even at resting potential. As a result, sodium concentrations within the cell rise, leading to increased nerve and muscle excitability. These biochemical channels cause muscle contractions, repetitive firing of the nerves and an irregular heart rhythm caused by stimulation of vagal nerves which control the parasympathetic functions of the heart, lungs and digestive tract.
474:
286:
272:
521:
90:
602:
334:
258:
648:
192:
588:
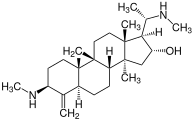
327:(see figure), which may serve as the primary alkaloid depending on the species, although it may not be present in some other organisms at all. Samandarin possesses a distinctive oxazolidine structure within the A ring. Besides samandarin, there are several other steroid alkaloids in Salamandra organisms such as samandaridin, samandarone, and cycloneosamandione.
22:
237:). This plant primarily thrives in southern and central Europe. These alkaloids are characterized by an amino group attached to the 3rd and/or 20th carbon atom. Methylation of the amino groups can be partial, complete, or absent. Buxus steroid alkaloids constitute a substantial group of bases, most of which can be categorized into three distinct groups.
534:
509:
Another category of solanum alkaloids is based on the spirosolane skeleton. In these compounds, the E-ring is a tetrahydrofuran to which a piperidine is directly attached via a spiro compound. An example of such a steroid alkaloid is tomatidenol, which is prevalent across various species within the
801:
Veratrum alkaloid compounds act by attaching to voltage-gated sodium ion channels, altering their permeability. Veratrum alkaloids cause affected sodium channels to reactivate 1000x slower than unaffected channels. Furthermore, veratrum alkaloids block inactivation of sodium channels and lower
450:
activity, they can be used as poisons against the plants' predators. They can be used as starting materials for steroidal drugs. There are various tests for identifying these alkaloids. The characteristic test involves dissolving the compound in hot amyl alcohol or ethanol and watching for the
75:
can be categorized based on their backbone structure, which may include the 5α-pregnane, Δ-pregnane, or conanine backbone. Typically, these alkaloids feature an amino group or an oxygen compound at the 3rd carbon atom. An illustrative example is latifolinin, which is derived from the conanine
641:), which also belongs to the Liliaceae family. The veracevin is based on the cevan skeleton, in which the C-ring is a five-membered instead of a six-membered ring and the D-ring is a six-membered ring. Furthermore, the high number of hydroxy groups is still remarkable.
1071:
Yoshiaki Kamano, Hiroshi
Yamamoto, Yoshihiro Tanaka, Manki Komatsu (1968), "The Isolation and Structure of new Bufadienolides, 3-(Hydrogen suberates) of Resibufogenin, Cinobufagin and Bufalin – the Structure of the so-called "Bufotoxins"",
240:
Another subgroup of Buxus steroid alkaloids possesses a tetracyclic structure. In these compounds, a bond exists between the 9th and 19th carbon atoms, forming a seven-membered ring (ring B). Buxamine E serves as an example of this group.
661:
83:. Additionally, the leaves of this plant contain two other compounds, namely funtumin and funtumidin. These compounds belong to the Apocynaceae steroid alkaloids family and share the 5α-pregnan backbone structure.
1104:
Kazutake
Shimada, Kazuo Ohishi, Hiroko Fukunaga, Jai Seup Ro, Toshio Nambara (1985), "Structure-activity relationship of bufotoxins and related compounds for the inhibition of Na+, K+ -adenosine triphosphatase",
487:
459:
Steroidal alkaloids with a solanidan backbone exhibit a distinctive bicyclic structure, which replaces the cholesterol side chain on the D-ring. A notable example is solanocapsin, as discovered in the
347:
615:
247:
The largest group consists of pentacyclic Buxus steroid alkaloids, featuring a core structure based on 4,4,14-trimethyl-9,19-cyclopregnan. Cyclobuxin D is a representative of this particular group.
244:
The third major group is distinguished by the absence of additional carbon atoms bonded to the 4th carbon atom of the A ring. Buxandonin L is an illustrative member of this category.
574:, respectively). These plants belong to the Liliaceae family. Among them, procevin is a special case, as it features a nitrogen atom from piperidine connected to the 18th carbon.
205:
1420:
Ohyama K, Okawa A, Moriuchi Y, Fujimoto Y (May 2013). "Biosynthesis of steroidal alkaloids in
Solanaceae plants: involvement of an aldehyde intermediate during C-26 amination".
706:. Because of their actions on the cardiovascular, neuromuscular, and respiratory systems, Veratrum alkaloids have been used for the treatment of various conditions like
1468:
1343:
T. Lüddecke, S. Schulz, S. Steinfartz (2018), "A salamander's toxic arsenal: review of skin poison diversity and function in true salamanders, genus
Salamandra",
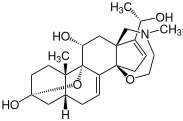
131:, consisting of 21 carbon atoms, and are distinctive for the amino group attached to the 18th carbon atom, exemplified by batrachotoxin A (see image).
103:
76:
backbone. This distinctive structure is characterized by a five-membered ring formed by an amino group bonded to both the 18th and 20th carbon atoms.
1639:
Helmut
Ripperger Klaus Schreiber (1969), "Solanum-Alkaloide, LXXXIX. Synthese des Steroidalkaloids Leptinidin und weiterer 23β-Hydroxy-solanidane",
1998:
Jiang QW, Chen MW, Cheng KJ, Yu PZ, Wei X, Shi Z (January 2016). "Therapeutic
Potential of Steroidal Alkaloids in Cancer and Other Diseases".
1853:
1775:
1746:
1711:
1601:
1251:
1219:
1189:
1154:
1056:
1001:
943:
919:
299:
40:
skeleton with nitrogen-based functional groups attached to the skeleton. More specifically, they are distinguished by their tetracyclic
1466:
Lacchini E, Goossens A (2020-10-06). "Combinatorial
Control of Plant Specialized Metabolism: Mechanisms, Functions, and Consequences".
250:
The Buxus steroid alkaloids buxamine E, buxandoline L, and cyclobuxin D are found in the leaves of common boxwood (buxus sempervirens).
1877:
1305:
798:
However, in addition to their therapeutic benefits, steroidal alkaloids, specifically veratrum alkaloids, are potentially deadly.
41:
1107:
1273:
1821:
1798:
1679:
1624:
1328:
1024:
966:
884:
151:
48:. They fall in two major categories: Solanum alkaloids and Veratrum alkaloids. A Steroidal alkaloid has also been found in
2105:
1838:
Chemotaxonomie der
Pflanzen: Eine Übersicht über die Verbreitung und die systematische Bedeutung der Pflanzenstoffe
1696:
Chemotaxonomie der
Pflanzen: Eine Übersicht über die Verbreitung und die systematische Bedeutung der Pflanzenstoffe
1541:
1477:
904:
Chemotaxonomie der
Pflanzen: Eine Übersicht über die Verbreitung und die systematische Bedeutung der Pflanzenstoffe
783:, for example, reduces interleukin-2 and -8 production whereas tomatidine inhibits specific nuclear translocation,
1532:
427:
446:
in potato. Typically they are used in plants as a protection mechanism against animals. Due to the typical anti-
752:
669:
137:
1281:
546:). This plant species belongs to the nightshade genus. Tomatidenol forms the main alkamin in the species of
398:
Starting with cholesterol, the biosynthesis of these compounds follow a similar general mechanism including
319:
occur naturally in organisms classified within the genus Salamandra. These alkaloids are derived from 3-aza-
791:
synthase. Lastly, nine steroidal alkaloids have significant antiestrogenic activity whereas seven inhibit
758:
495:
316:
1207:
1345:
772:
72:
143:
1429:
1350:
750:
antimicrobial bioactivity is accomplished by interfering with the synthesis of genetic substances in
50:
1396:
230:
1074:
743:
473:
285:
271:
479:
291:
277:
2023:
1641:
1571:
1505:
1374:
828:
726:
Steroidal alkaloids have been investigated for a wide range of potential bioactivities including
526:
460:
95:
607:
520:
263:
177:
shown here is a sterane derivative with an α-pyranone at the 17th carbon atom and an esterified
89:
653:
2081:
2015:
1977:
1944:
Dey P, Kundu A, Chakraborty HJ, Kar B, Choi WS, Lee BM, Bhakta T, Atanasov AG, Kim HS (2018).
1926:
1873:
1849:
1817:
1794:
1771:
1742:
1707:
1675:
1654:
1620:
1597:
1594:
Alkaloide: Betäubungsmittel, Halluzinogene und andere Wirkstoffe, Leitstrukturen aus der Natur
1563:
1555:
1497:
1489:
1445:
1366:
1324:
1301:
1247:
1215:
1212:
Alkaloide: Betäubungsmittel, Halluzinogene und andere Wirkstoffe, Leitstrukturen aus der Natur
1185:
1176:, vol. 3 (5 ed.), Berlin: Springer-Verlag Berlin Heidelberg GmbH, pp. 589–590,
1150:
1120:
1087:
1052:
1020:
997:
962:
939:
915:
880:
854:
792:
731:
707:
637:
Veracevin is a member of the veratrum alkaloids group. However, this occurs in the sabadilla (
601:
593:
542:
333:
1645:, vol. 102, no. 12, Weinheim: WILEY-VCH Verlag GmbH & Co., pp. 4080–4088,
577:
Veratramine is an example of veratrum steroid alkaloids, characterized by a 22,26-epimino-14-
257:
2073:
2007:
1967:
1957:
1946:"Therapeutic value of steroidal alkaloids in cancer: Current trends and future perspectives"
1916:
1908:
1841:
1734:
1699:
1646:
1545:
1481:
1437:
1358:
1239:
1177:
1142:
1112:
1079:
1044:
989:
907:
647:
191:
157:
120:
1485:
587:
1550:
1527:
1433:
1354:
1972:
1945:
1921:
1896:
1870:
Cell Culture and Somatic Cell Genetics of Plants: Phytochemicals in Plant Cell Cultures
767:
623:
540:
Tomatidenol is found, among other things, in the leaves of the bittersweet nightshade (
447:
55:
2077:
1083:
2099:
2027:
1509:
788:
727:
443:
439:
406:, and transamination before differentiating. Alkaloids found in these plants include
399:
178:
119:
Batrachotoxins are neurotoxins that are naturally occurring on the dermal surface of
1575:
1441:
1378:
353:
Salamander alkaloids, such as samandarin, occur on the skin of animals of the genus
859:
739:
735:
493:
Solanocapsin is found, among other things, in the fruits of the Jerusalem cherry (
566:
The veratrum alkaloids derive their name from the white and green germer plants (
1214:(3 ed.), Wiesbaden: Vieweg+Teubner – GWV Fachverlage GmbH, pp. 97–98,
711:
699:
695:
1267:
173:. The α-pyranones at the 17th carbon atom are specific for the bufotoxins. The
1845:
1738:
1703:
1362:
1243:
1181:
1048:
911:
780:
747:
423:
415:
395:
354:
339:
324:
54:(Frangipani vine), 'chonemorphine' was used to treat intestinal infections in
26:
1650:
1559:
1493:
1596:(3 ed.), Wiesbaden: Vieweg+Teubner – GWV Fachverlage GmbH, p. 90,
1349:, vol. 105, no. 56, Springer Berlin Heidelberg, pp. 208–216,
1146:
993:
715:
407:
403:
372:
212:
197:
174:
2019:
1981:
1930:
1567:
1501:
1449:
1370:
1174:
Hagers Handbuch der Pharmazeutischen Praxis: Chemikalien und Drogen (Am-Ch)
1116:
2085:
1658:
1298:
Studies in Natural Products in Chemistry: Structure and Chemistry (Part C)
1124:
1091:
1912:
938:, vol. 1 (10 ed.), Stuttgart: Georg Thieme Verlag, p. 50,
779:
Antiinflammation is similarly accomplished with a variety of mechanisms.
684:
419:
411:
182:
128:
1731:
Grundlagen der Arzneimittelforschung und der synthetischen Arzneimittel
703:
691:
376:
37:
21:
2011:
1962:
1238:, vol. 6, Berlin: Springer-Verlag Berlin Heidelberg, p. 45,
1043:, vol. 6, Berlin: Springer-Verlag Berlin Heidelberg, p. 45,
621:
Procevin and veratramin occur, among other things, in white veratrum (
391:
387:
45:
79:
Latifolinin is a compound that is naturally present in the bark of
20:
1770:(10 ed.), Stuttgart: Georg Thieme Verlag, pp. 680–681,
855:
Medicinal Plants Of The Asia-pacific: Drugs For The Future (2006)
742:
activity. These bioactivities are the result of a wide array of
683:
True to their name, Veratrum alkaloids come from plants of the
934:
Burkhard Fugmann, ed. (1997), "Apocynaceen-Steroidalkaloide",
784:
2048:
Clinical Neurotoxicology: Syndromes, Substances, Environments
1234:
O. Gessner, G. Barger (1938), W. Heubner, J. Schüller (ed.),
1039:
O. Gessner, G. Barger (1938), W. Heubner, J. Schüller (ed.),
1766:
Burkhard Fugmann, ed. (1997), "Veratrum–Steroidalkaloide",
906:, vol. 3, Basel: Springer Basel AG, pp. 127–129,
766:. On the other hand, tomatidine synergistically works with
58:. (Chatterjee DK et al (1987) Parasitol Res 74, 1, 30-33).
1461:
1459:
229:
Buxus steroid alkaloids are present in the leaves of the
451:
formation of a jelly-like product as the mixture cools.
371:
These compounds generally appear as their corresponding
1872:, vol. 5, San Diego: Academic Press, p. 538,
879:, London: Chapman & Hall, 1991, pp. XXV–XXVI,
109:
Funtumia africana (heterosynonym: Funtumia latifolia)]]
1840:, vol. 7, Basel: Springer Basel AG, p. 711,
1698:, vol. 7, Basel: Springer Basel AG, p. 427,
1521:
1519:
1141:, vol. 2, New York: Academid Press, p. 546,
988:, vol. 2, New York: Academid Press, p. 261,
211:
Bufotoxin is found on the skin of toads of the genus
2064:
Heilpern KL (February 1995). "Zigadenus poisoning".
1526:
Nützmann HW, Scazzocchio C, Osbourn A (2018-11-23).
823:
821:
819:
817:
815:
127:. Batrachotoxins share a structural foundation with
1137:Wolfgang Bücherl, Eleanor E. Buckley, eds. (1971),
984:Wolfgang Bücherl, Eleanor E. Buckley, eds. (1971),
1868:Friedrich Constabel, Indra K. Vasil, eds. (1984),
1300:, vol. 15, Amsterdam: Elsevier, p. 337,
44:skeleton that marks their close relationship with
1816:, London: Chapman & Hall, 1991, p. 943,
1793:, London: Chapman & Hall, 1991, p. 759,
1674:, London: Chapman & Hall, 1991, p. 858,
1619:, London: Chapman & Hall, 1991, p. 801,
1323:, London: Chapman & Hall, 1991, p. 769,
961:, London: Chapman & Hall, 1991, p. 644,
2059:
2057:
1111:, vol. 8, no. 12, pp. 1054–1059,
1078:, vol. 9, no. 54, pp. 5673–5676,
1019:, London: Chapman & Hall, 1991, p. 86,
1890:
1888:
1469:Annual Review of Cell and Developmental Biology
1401:Cornell University Department of Animal Science
2041:
2039:
2037:
1993:
1991:
1277:. Georg Thieme Verlag, retrieved {{{Datum}}}.
8:
1733:, Basel: Springer Basel AG, pp. 45–46,
1280:Error in template * unknown parameter name (
323:-homo-5β-androstane. One notable example is
169:The bufotoxins are named after the genus of
426:. The Itkin group has found several of the
1391:
1389:
1387:
1236:Handbuch der experimentellen Pharmakologie
1041:Handbuch der experimentellen Pharmakologie
762:. Solasodine inhibits growth signaling in
1971:
1961:
1920:
1549:
746:across different compounds. For example,
562:Veratrum alkaloids of white/green chervil
581:-cholestane ring system as their basis.
29:, a steroidal alkaloid found in potatoes
1724:
1722:
1587:
1585:
1528:"Metabolic Gene Clusters in Eukaryotes"
811:
690:. Alkaloids are found in the roots and
643:
583:
516:
469:
329:
253:
187:
133:
85:
2046:Furbee B (2009). "Neurotoxic plants".
1761:
1759:
1757:
1202:
1200:
1172:P. H. List, L. Hörhammer, ed. (1972),
1167:
1165:
979:
977:
897:
895:
871:
869:
867:
1486:10.1146/annurev-cellbio-011620-031429
7:
1551:10.1146/annurev-genet-120417-031237
667:Veracevin occurs in the Sabadilla (
305:Common boxwood (Buxus sempervirens)
14:
1139:Venomous Animals and Their Venoms
986:Venomous Animals and Their Venoms
660:
646:
614:
600:
586:
533:
519:
486:
472:
438:2013 they find several BSGs for
346:
332:
298:
284:
270:
256:
204:
190:
150:
136:
102:
88:
42:cyclopentanoperhydrophenanthrene
1950:International Journal of Cancer
1442:10.1016/j.phytochem.2013.01.010
1108:Journal of Pharmacobio-Dynamics
787:activation, as well as induce
694:of these plants. They include
633:Veratrum alkaloids of sabadill
1:
2078:10.1016/S0196-0644(95)70336-5
1084:10.1016/S0040-4039(00)70748-9
550:, which are native to Europe.
67:Apocynaceae steroid alkaloids
2066:Annals of Emergency Medicine
1592:Eberhard Breitmaier (2008),
1296:Atta-ur-Rahman, ed. (1995),
181:at the 3rd carbon atom with
624:Veratrum album grandiflorum
16:Class of chemical compounds
2122:
2000:Medicinal Research Reviews
428:biosynthetic gene clusters
1846:10.1007/978-3-0348-9314-5
1768:Römpp Lexikon Naturstoffe
1739:10.1007/978-3-0348-4019-4
1704:10.1007/978-3-0348-9314-5
1533:Annual Review of Genetics
1363:10.1007/s00114-018-1579-4
1244:10.1007/978-3-662-32921-4
1182:10.1007/978-3-642-80562-2
1049:10.1007/978-3-662-32921-4
936:Römpp Lexikon Naturstoffe
912:10.1007/978-3-0348-9385-5
1651:10.1002/cber.19691021215
753:Saccharomyces cerevisiae
670:Schoenocaulon officinale
639:Schoenocaulon officinale
1147:10.1016/C2013-0-10436-9
994:10.1016/C2013-0-10436-9
770:as antibiotics against
225:Buxus steroid alkaloids
1895:Hollman A (May 1991).
1814:Dictionary of steroids
1791:Dictionary of steroids
1672:Dictionary of steroids
1617:Dictionary of steroids
1321:Dictionary of steroids
1117:10.1248/bpb1978.8.1054
1017:Dictionary of steroids
959:Dictionary of steroids
877:Dictionary of steroids
759:Prototheca wickerhamii
496:Solanum pseudocapsicum
465:Solanum pseudocapsicum
125:Phyllobates terribilis
123:. The photo shows the
30:
25:Chemical structure of
1901:British Heart Journal
1346:The Science of Nature
829:"Steroidal Alkaloids"
386:includes plants like
73:apocynaceae alkaloids
24:
1913:10.1136/hrt.65.5.286
1897:"Veratrum alkaloids"
1836:R. Hegnauer (1986),
1729:Jakob Büchi (1963),
1694:R. Hegnauer (1986),
1284:): "Abruf"
1282:Template:RömppOnline
1268:Salamander-Alkaloide
902:R. Hegnauer (1964),
430:for these. In Itkin
317:salamander alkaloids
311:Salamander alkaloids
51:Chonemorpha fragrans
2106:Steroidal alkaloids
1434:2013PChem..89...26O
1397:"Steroid Alkaloids"
1355:2018SciNa.105...56L
1208:Eberhard Breitmaier
1075:Tetrahedron Letters
764:Geim original algal
505:Spirosolan backbone
34:Steroidal alkaloids
1642:Chemische Berichte
679:Veratrum alkaloids
557:Veratrum alkaloids
455:Solanidan skeleton
235:Buxus sempervirens
81:Funtumia latifolia
31:
2012:10.1002/med.21346
1963:10.1002/ijc.31965
1855:978-3-0348-9991-8
1777:978-3-132-00061-2
1748:978-3-0348-4020-0
1713:978-3-0348-9991-8
1603:978-3-8348-0531-7
1253:978-3-662-32094-5
1221:978-3-8348-0531-7
1191:978-3-642-80563-9
1156:978-0-12-138902-4
1058:978-3-662-32094-5
1003:978-0-12-138902-4
945:978-3-131-99961-0
921:978-3-0348-9386-2
852:Wiart Christophe
793:estrone sulfatase
732:anti-inflammatory
708:myasthenia gravis
548:Solanum dulcarama
543:Solanum dulcamara
375:in plants of the
144:Batrachotoxinin A
121:poison dart frogs
2113:
2090:
2089:
2061:
2052:
2051:
2043:
2032:
2031:
1995:
1986:
1985:
1975:
1965:
1956:(7): 1731–1744.
1941:
1935:
1934:
1924:
1892:
1883:
1882:
1865:
1859:
1858:
1833:
1827:
1826:
1810:
1804:
1803:
1787:
1781:
1780:
1763:
1752:
1751:
1726:
1717:
1716:
1691:
1685:
1684:
1668:
1662:
1661:
1636:
1630:
1629:
1613:
1607:
1606:
1589:
1580:
1579:
1553:
1523:
1514:
1513:
1463:
1454:
1453:
1417:
1411:
1410:
1408:
1407:
1393:
1382:
1381:
1340:
1334:
1333:
1317:
1311:
1310:
1293:
1287:
1286:
1285:
1263:
1257:
1256:
1231:
1225:
1224:
1204:
1195:
1194:
1169:
1160:
1159:
1134:
1128:
1127:
1101:
1095:
1094:
1068:
1062:
1061:
1036:
1030:
1029:
1013:
1007:
1006:
981:
972:
971:
955:
949:
948:
931:
925:
924:
899:
890:
889:
873:
862:
850:
844:
843:
841:
840:
825:
740:chemotherapeutic
664:
650:
618:
604:
590:
537:
523:
490:
476:
350:
336:
302:
288:
274:
260:
208:
194:
158:poison dart frog
154:
140:
106:
92:
2121:
2120:
2116:
2115:
2114:
2112:
2111:
2110:
2096:
2095:
2094:
2093:
2063:
2062:
2055:
2050:. Elsevier Inc.
2045:
2044:
2035:
1997:
1996:
1989:
1943:
1942:
1938:
1894:
1893:
1886:
1880:
1867:
1866:
1862:
1856:
1835:
1834:
1830:
1824:
1812:
1811:
1807:
1801:
1789:
1788:
1784:
1778:
1765:
1764:
1755:
1749:
1728:
1727:
1720:
1714:
1693:
1692:
1688:
1682:
1670:
1669:
1665:
1638:
1637:
1633:
1627:
1615:
1614:
1610:
1604:
1591:
1590:
1583:
1525:
1524:
1517:
1465:
1464:
1457:
1419:
1418:
1414:
1405:
1403:
1395:
1394:
1385:
1342:
1341:
1337:
1331:
1319:
1318:
1314:
1308:
1295:
1294:
1290:
1279:
1278:
1264:
1260:
1254:
1233:
1232:
1228:
1222:
1206:
1205:
1198:
1192:
1171:
1170:
1163:
1157:
1136:
1135:
1131:
1103:
1102:
1098:
1070:
1069:
1065:
1059:
1038:
1037:
1033:
1027:
1015:
1014:
1010:
1004:
983:
982:
975:
969:
957:
956:
952:
946:
933:
932:
928:
922:
901:
900:
893:
887:
875:
874:
865:
851:
847:
838:
836:
827:
826:
813:
808:
768:aminoglycosides
736:anti-estrogenic
724:
681:
674:
665:
656:
651:
635:
628:
619:
610:
605:
596:
591:
572:Veratrum viride
564:
559:
552:
551:
538:
529:
524:
507:
500:
491:
482:
477:
457:
434:2011 and Itkin
369:
359:
358:
351:
342:
337:
313:
306:
303:
294:
289:
280:
275:
266:
261:
251:
227:
220:
209:
200:
195:
167:
160:
155:
146:
141:
117:
110:
107:
98:
93:
69:
64:
36:have the basic
17:
12:
11:
5:
2119:
2117:
2109:
2108:
2098:
2097:
2092:
2091:
2053:
2033:
1987:
1936:
1884:
1878:
1860:
1854:
1828:
1822:
1805:
1799:
1782:
1776:
1753:
1747:
1718:
1712:
1686:
1680:
1663:
1631:
1625:
1608:
1602:
1581:
1542:Annual Reviews
1515:
1478:Annual Reviews
1455:
1422:Phytochemistry
1412:
1383:
1335:
1329:
1312:
1306:
1288:
1258:
1252:
1226:
1220:
1196:
1190:
1161:
1155:
1129:
1096:
1063:
1057:
1031:
1025:
1008:
1002:
973:
967:
950:
944:
926:
920:
891:
885:
863:
845:
810:
809:
807:
804:
723:
720:
680:
677:
676:
675:
666:
659:
657:
652:
645:
634:
631:
630:
629:
620:
613:
611:
606:
599:
597:
592:
585:
568:Veratrum album
563:
560:
558:
555:
554:
553:
539:
532:
530:
525:
518:
506:
503:
502:
501:
492:
485:
483:
478:
471:
456:
453:
448:cholinesterase
442:in tomato and
418:, tomatidine,
394:, and various
368:
362:
361:
360:
352:
345:
343:
338:
331:
312:
309:
308:
307:
304:
297:
295:
290:
283:
281:
276:
269:
267:
262:
255:
231:common boxwood
226:
223:
222:
221:
210:
203:
201:
196:
189:
166:
163:
162:
161:
156:
149:
147:
142:
135:
116:
115:Batrachotoxins
113:
112:
111:
108:
101:
99:
94:
87:
71:The family of
68:
65:
63:
60:
15:
13:
10:
9:
6:
4:
3:
2:
2118:
2107:
2104:
2103:
2101:
2087:
2083:
2079:
2075:
2072:(2): 259–62.
2071:
2067:
2060:
2058:
2054:
2049:
2042:
2040:
2038:
2034:
2029:
2025:
2021:
2017:
2013:
2009:
2006:(1): 119–43.
2005:
2001:
1994:
1992:
1988:
1983:
1979:
1974:
1969:
1964:
1959:
1955:
1951:
1947:
1940:
1937:
1932:
1928:
1923:
1918:
1914:
1910:
1906:
1902:
1898:
1891:
1889:
1885:
1881:
1879:0-12-715005-6
1875:
1871:
1864:
1861:
1857:
1851:
1847:
1843:
1839:
1832:
1829:
1825:
1819:
1815:
1809:
1806:
1802:
1796:
1792:
1786:
1783:
1779:
1773:
1769:
1762:
1760:
1758:
1754:
1750:
1744:
1740:
1736:
1732:
1725:
1723:
1719:
1715:
1709:
1705:
1701:
1697:
1690:
1687:
1683:
1677:
1673:
1667:
1664:
1660:
1656:
1652:
1648:
1644:
1643:
1635:
1632:
1628:
1622:
1618:
1612:
1609:
1605:
1599:
1595:
1588:
1586:
1582:
1577:
1573:
1569:
1565:
1561:
1557:
1552:
1547:
1543:
1539:
1535:
1534:
1529:
1522:
1520:
1516:
1511:
1507:
1503:
1499:
1495:
1491:
1487:
1483:
1479:
1475:
1471:
1470:
1462:
1460:
1456:
1451:
1447:
1443:
1439:
1435:
1431:
1428:(17): 26–31.
1427:
1423:
1416:
1413:
1402:
1398:
1392:
1390:
1388:
1384:
1380:
1376:
1372:
1368:
1364:
1360:
1356:
1352:
1348:
1347:
1339:
1336:
1332:
1326:
1322:
1316:
1313:
1309:
1307:0-444-82083-3
1303:
1299:
1292:
1289:
1283:
1276:
1275:
1270:
1269:
1262:
1259:
1255:
1249:
1245:
1241:
1237:
1230:
1227:
1223:
1217:
1213:
1209:
1203:
1201:
1197:
1193:
1187:
1183:
1179:
1175:
1168:
1166:
1162:
1158:
1152:
1148:
1144:
1140:
1133:
1130:
1126:
1122:
1118:
1114:
1110:
1109:
1100:
1097:
1093:
1089:
1085:
1081:
1077:
1076:
1067:
1064:
1060:
1054:
1050:
1046:
1042:
1035:
1032:
1028:
1022:
1018:
1012:
1009:
1005:
999:
995:
991:
987:
980:
978:
974:
970:
964:
960:
954:
951:
947:
941:
937:
930:
927:
923:
917:
913:
909:
905:
898:
896:
892:
888:
882:
878:
872:
870:
868:
864:
861:
858:, p. 454, at
857:
856:
849:
846:
834:
833:Pharmacognosy
830:
824:
822:
820:
818:
816:
812:
805:
803:
799:
796:
794:
790:
789:nitrous oxide
786:
782:
777:
775:
774:
769:
765:
761:
760:
755:
754:
749:
745:
741:
737:
733:
729:
728:antimicrobial
721:
719:
717:
713:
709:
705:
701:
697:
693:
689:
688:
678:
672:
671:
663:
658:
655:
649:
644:
642:
640:
632:
626:
625:
617:
612:
609:
603:
598:
595:
589:
584:
582:
580:
575:
573:
569:
561:
556:
549:
545:
544:
536:
531:
528:
522:
517:
515:
513:
504:
498:
497:
489:
484:
481:
475:
470:
468:
466:
462:
454:
452:
449:
445:
441:
437:
433:
429:
425:
421:
417:
413:
409:
405:
401:
400:hydroxylation
397:
393:
389:
385:
381:
380:
374:
366:
363:
356:
349:
344:
341:
335:
330:
328:
326:
322:
318:
310:
301:
296:
293:
287:
282:
279:
273:
268:
265:
259:
254:
252:
248:
245:
242:
238:
236:
232:
224:
218:
214:
207:
202:
199:
193:
188:
186:
184:
180:
179:succinic acid
176:
172:
164:
159:
153:
148:
145:
139:
134:
132:
130:
126:
122:
114:
105:
100:
97:
91:
86:
84:
82:
77:
74:
66:
61:
59:
57:
53:
52:
47:
43:
39:
35:
28:
23:
19:
2069:
2065:
2047:
2003:
1999:
1953:
1949:
1939:
1904:
1900:
1869:
1863:
1837:
1831:
1813:
1808:
1790:
1785:
1767:
1730:
1695:
1689:
1671:
1666:
1640:
1634:
1616:
1611:
1593:
1537:
1531:
1473:
1467:
1425:
1421:
1415:
1404:. Retrieved
1400:
1344:
1338:
1320:
1315:
1297:
1291:
1274:Römpp Online
1272:
1266:
1261:
1235:
1229:
1211:
1173:
1138:
1132:
1106:
1099:
1073:
1066:
1040:
1034:
1016:
1011:
985:
958:
953:
935:
929:
903:
876:
860:Google Books
853:
848:
837:. Retrieved
832:
800:
797:
778:
771:
763:
757:
751:
725:
686:
682:
668:
638:
636:
622:
578:
576:
571:
567:
565:
547:
541:
511:
508:
494:
480:Solanocapsin
464:
458:
435:
431:
383:
378:
370:
364:
320:
314:
292:Cyclobuxin D
278:Buxandonin L
249:
246:
243:
239:
234:
228:
216:
170:
168:
124:
118:
80:
78:
70:
49:
33:
32:
18:
1544:: 159–183.
1480:: 291–313.
835:. July 2012
722:Bioactivity
712:hypotension
700:cyclopamine
696:veratridine
527:Tomatidenol
461:coral shrub
396:nightshades
96:Latifolinin
56:Wistar rats
1907:(5): 286.
1823:0412270609
1800:0412270609
1681:0412270609
1626:0412270609
1406:2018-05-05
1330:0412270609
1026:0412270609
968:0412270609
886:0412270609
839:2018-05-05
806:References
781:Solasodine
748:solasodine
744:mechanisms
608:Veratramin
444:α-solanine
440:α-tomatine
424:solanidine
416:solasodine
355:Salamandra
340:Samandarin
325:samandarin
315:The toxic
264:Buxamine E
185:attached.
165:Bufotoxins
27:solanidine
2028:206251649
1560:0066-4197
1510:219947907
1494:1081-0706
1265:Entry on
773:S. aureus
716:eclampsia
654:Veracevin
408:chaconine
404:oxidation
373:glycoside
367:alkaloids
213:true toad
198:Bufotoxin
175:bufotoxin
129:pregnanes
38:steroidal
2100:Category
2020:25820039
1982:30387881
1931:18610390
1576:52161448
1568:30183405
1502:32559387
1450:23473422
1379:52924816
1371:30291447
1210:(2008),
692:rhizomes
687:Veratrum
594:Procevin
420:tomatine
412:solanine
392:tomatoes
388:potatoes
183:arginine
62:Examples
2086:7832360
1973:6767045
1922:1024632
1659:5367544
1430:Bibcode
1351:Bibcode
1125:3009774
1092:5748700
704:jervine
512:Solanum
384:Solanum
379:Solanum
365:Solanum
46:sterols
2084:
2026:
2018:
1980:
1970:
1929:
1919:
1876:
1852:
1820:
1797:
1774:
1745:
1710:
1678:
1657:
1623:
1600:
1574:
1566:
1558:
1508:
1500:
1492:
1448:
1377:
1369:
1327:
1304:
1271:. at:
1250:
1218:
1188:
1153:
1123:
1090:
1055:
1023:
1000:
965:
942:
918:
883:
738:, and
714:, and
702:, and
685:genus
510:genus
436:et al.
432:et al.
422:, and
377:genus
2024:S2CID
1572:S2CID
1540:(1).
1506:S2CID
1476:(1).
1375:S2CID
2082:PMID
2016:PMID
1978:PMID
1927:PMID
1874:ISBN
1850:ISBN
1818:ISBN
1795:ISBN
1772:ISBN
1743:ISBN
1708:ISBN
1676:ISBN
1655:PMID
1621:ISBN
1598:ISBN
1564:PMID
1556:ISSN
1498:PMID
1490:ISSN
1446:PMID
1367:PMID
1325:ISBN
1302:ISBN
1248:ISBN
1216:ISBN
1186:ISBN
1151:ISBN
1121:PMID
1088:PMID
1053:ISBN
1021:ISBN
998:ISBN
963:ISBN
940:ISBN
916:ISBN
881:ISBN
756:and
579:abeo
570:and
217:Bufo
171:Bufo
2074:doi
2008:doi
1968:PMC
1958:doi
1954:145
1917:PMC
1909:doi
1842:doi
1735:doi
1700:doi
1647:doi
1546:doi
1482:doi
1438:doi
1359:doi
1240:doi
1178:doi
1143:doi
1113:doi
1080:doi
1045:doi
990:doi
908:doi
785:JNK
467:).
2102::
2080:.
2070:25
2068:.
2056:^
2036:^
2022:.
2014:.
2004:36
2002:.
1990:^
1976:.
1966:.
1952:.
1948:.
1925:.
1915:.
1905:65
1903:.
1899:.
1887:^
1848:,
1756:^
1741:,
1721:^
1706:,
1653:,
1584:^
1570:.
1562:.
1554:.
1538:52
1536:.
1530:.
1518:^
1504:.
1496:.
1488:.
1474:36
1472:.
1458:^
1444:.
1436:.
1426:89
1424:.
1399:.
1386:^
1373:,
1365:,
1357:,
1246:,
1199:^
1184:,
1164:^
1149:,
1119:,
1086:,
1051:,
996:,
976:^
914:,
894:^
866:^
831:.
814:^
795:.
776:.
734:,
730:,
718:.
710:,
698:,
673:).
627:).
514:.
499:).
414:,
410:,
402:,
390:,
382:.
219:).
2088:.
2076::
2030:.
2010::
1984:.
1960::
1933:.
1911::
1844::
1737::
1702::
1649::
1578:.
1548::
1512:.
1484::
1452:.
1440::
1432::
1409:.
1361::
1353::
1242::
1180::
1145::
1115::
1082::
1047::
992::
910::
842:.
463:(
357:.
321:A
233:(
215:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.

![Funtumia africana (heterosynonym: Funtumia latifolia)]]](https://upload.wikimedia.org/wikipedia/commons/thumb/a/a7/Funtumia_africana-1906.jpg/85px-Funtumia_africana-1906.jpg)