357:
325:
463:, but the barriers for these processes are typically high such that these processes do not lead to line broadening. For some compounds, dynamics occur via dissociation of a ligand, giving a pentacoordinate intermediate, which is subject to the mechanisms discussed above. Yet another mechanism, exhibited by Fe(CO)
491:
107:
and typically involves recording spectra at various temperatures. In the ideal case, low temperature spectra can be assigned to the "slow exchange limit", whereas spectra recorded at higher temperatures correspond to molecules at "fast exchange limit". Typically, high temperature spectra are simpler
497:
At temperatures near 100 °C, the 500 MHz H NMR spectrum of DMF shows only one signal for the methyl groups. Near room temperature, however, separate signals are seen for the non-equivalent methyl groups. The rate of exchange can be calculated at the temperature where the two signals are
306:
590:
is the difference in Hz between the frequencies of the exchanging sites. These frequencies are obtained from the limiting low-temperature NMR spectrum. At these lower temperatures, the dynamics continue, of course, but the contribution of the dynamics to line broadening is negligible.
130:
For processes that are too slow for traditional DNMR analysis, the technique spin saturation transfer (SST, also called EXSY for exchange spectroscopy) is applicable. This magnetization transfer technique gives rate information, provided that the rates exceed
1358:
Vancea, L.; Bennett, M. J.; Jones, C. E.; Smith, R. A.; Graham, W. A. G. (1977). "Stereochemically
Nonrigid Six-Coordinate Metal Carbonyl Complexes. 1. Polytopal Rearrangement and X-Ray Structure of Tetracarbonylbis(trimethylsilyl)iron".
108:
than those recorded at low temperatures, since at high temperatures, equivalent sites are averaged out. Prior to the advent of DNMR, kinetics of reactions were measured on non-equilibrium mixtures, monitoring the approach to equilibrium.
581:
102:
Temperature dependent changes in the NMR spectra result from dynamics associated with the fluxional molecules when those dynamics proceed at rates comparable to the frequency differences observed by NMR. The experiment is called
167:
348:
and carbon-13 NMR spectra of cyclohexane show each only singlets near room temperature. At low temperatures, the singlet in the H NMR spectrum decoalesces but the C NMR spectrum remains unchanged.
655:
1425:; Davison, A.; Faller, J. W.; Lippard, S. J.; Morehouse, S. M. (1966). "Stereochemically Nonrigid Organometallic Compounds. I. π-Cyclopentadienyliron Dicarbonyl σ-Cyclopentadiene".
376:, even at temperatures as low as −100 °C, fails to distinguish the axial from the equatorial fluorine environments. The apparent equivalency arises from the low barrier for
1083:
Casey H. Londergan; Clifford P. Kubiak (2003). "Electron
Transfer and Dynamic Infrared-Band Coalescence: It Looks Like Dynamic NMR Spectroscopy, but a Billion Times Faster".
373:
369:
340:. Carbon–hydrogen bonds that are axial in one configuration become equatorial in the other, and vice versa. At room temperature the two chair conformations rapidly
1143:
111:
Many molecular processes exhibit fluxionality that can be probed on the NMR time scale. Beyond the examples highlighted below, other classic examples include the
504:
396:: only one signal is observed in the C NMR spectrum near room temperature) whereas at low temperatures, two signals in a 2:3 ratio can be resolved. In
1048:
Jarek, R. L.; Flesher, R. J.; Shin, S. K. (1997). "Kinetics of
Internal Rotation of N,N-Dimethylacetamide: A Spin-Saturation Transfer Experiment".
1342:
356:
301:{\displaystyle k\sim \Delta \nu _{\circ }\sim 2(10\mathrm {cm} ^{-1})(300\cdot 10^{8}\mathrm {cm/s} )\sim 6\times 10^{11}\mathrm {s} ^{-1}\cdot }
311:
Clearly, processes that induce line-broadening on the IR time-scale must be much more rapid than the cases that exchange on the NMR time scale.
901:
475:
and related hydride complexes, is intramolecular scrambling of ligands over the faces of the tetrahedron defined by the four CO ligands.
66:, the term fluxional depends on the context and the method used to assess the dynamics. Often, a molecule is considered fluxional if its
455:, featuring close-packed array of six ligating atoms surrounding a central atom. Such compounds do rearrange intramolecularly via the
1460:
1386:
H. S. Gutowsky; C. H. Holm (1956). "Rate
Processes and Nuclear Magnetic Resonance Spectra. II. Hindered Internal Rotation of Amides".
372:
consists of a P-coupled doublet, indicating that the equatorial and axial fluorine centers interchange rapidly on the NMR timescale.
1478:
1127:
962:
875:
878:
694:
75:
427:
there is no rigid molecular structure; the H atoms are always in motion. More precisely, the spatial distribution of protons in
863:: The use of permutation-inversion groups for the symmetry classification of the states of fluxional (or non-rigid) molecules.
1165:-butylcyclohexane by Dynamic NMR Spectroscopy and Computational Methods. Observation of Chair and Twist-Boat Conformations".
78:) due to chemical exchange. In some cases, where the rates are slow, fluxionality is not detected spectroscopically, but by
452:
604:
58:
interchange between symmetry-equivalent positions. Because virtually all molecules are fluxional in some respects, e.g.
161:. Application of the equation for coalescence of two signals separated by 10 cm gives the following result:
365:
120:
59:
744:
ligand splits at low temperatures owing to the slow hopping of the Fe center from carbon to carbon in the η-C
451:
While nonrigidity is common for pentacoordinate species, six-coordinate species typically adopt a more rigid
1201:
Gutowsky, H. S.; McCall, D. W.; Slichter, C. P. (1953). "Nuclear
Magnetic Resonance Multiplets in Liquids".
91:
150:
1339:
498:
just merged. This "coalescence temperature" depends on the measuring field. The relevant equation is:
1395:
1256:
1211:
1057:
1022:
844:
397:
341:
761:
802:
1282:
1137:
777:
385:
112:
1456:
1317:
1274:
1247:
1183:
1123:
1100:
958:
897:
871:
792:
752:
ligand. Two mechanisms have been proposed, with the consensus favoring the 1,2 shift pathway.
484:
154:
79:
35:
886:, Wiley, New York, 1975 (reprinted by Dover 1992), describing the term "semi-rigid molecule".
1434:
1422:
1403:
1368:
1309:
1264:
1219:
1175:
1092:
1065:
1030:
997:
933:
852:
132:
63:
891:
1346:
797:
381:
1300:
Thompson, KC; Crittenden, DL; Jordan, MJ (2005). "CH5+: Chemistry's chameleon unmasked".
1399:
1260:
1215:
1061:
1026:
848:
1203:
782:
576:{\displaystyle k={\frac {\pi \Delta \nu _{\circ }}{2^{1/2}}}\sim 2\Delta \nu _{\circ }}
456:
377:
324:
1472:
1167:
720:
At 30 °C, the H NMR spectrum shows only two peaks, one typical (δ5.6) of the η-C
424:
158:
71:
67:
1286:
1237:
Asvany, O.; Kumar P, P.; Redlich, B.; Hegemann, I.; Schlemmer, S.; Marx, D. (2005).
1001:
787:
460:
17:
404:), a similar pattern is observed even though this compound has only four ligands.
333:
124:
1451:
Robert B. Jordan, Reaction
Mechanisms of Inorganic and Organometallic Systems (
925:
384:, by which the axial and equatorial fluorine atoms rapidly exchange positions.
857:
832:
766:
345:
116:
149:
Although less common, some dynamics are also observable on the time-scale of
1269:
1238:
938:
772:
658:
408:
337:
31:
1321:
1278:
1187:
1104:
1096:
51:
1438:
1372:
992:
John W. Faller "Stereochemical
Nonrigidity of Organometallic Complexes"
1407:
1313:
1223:
1179:
1069:
1034:
778:
Molecular symmetry § Molecular rotation and molecular nonrigidity
328:
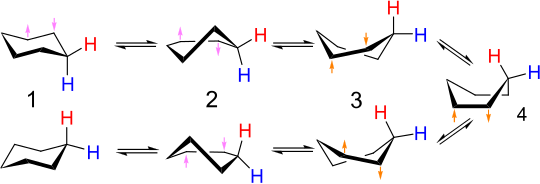
Cyclohexane chair flip (ring inversion) reaction via boat conformation
1340:
http://www.theochem.ruhr-uni-bochum.de/research/marx/topic4b.en.html
1157:
Gill, G.; Pawar, D. M.; Noe, E. A. (2005). "Conformational Study of
693:
490:
692:
323:
55:
27:
Molecules whose atoms interchange between symmetric positions
489:
355:
94:
exhibit fluxionality. Fluxionality is, however, pervasive.
827:, McGraw-Hill, New York, 1955 (reprinted by Dover 1980)
332:
The interconversion of equivalent chair conformers of
607:
507:
170:
54:
that undergo dynamics such that some or all of their
994:
Encyclopedia of
Inorganic and Bioinorganic Chemistry
650:{\displaystyle k\sim 2(500)=1000\mathrm {s} ^{-1}}
649:
575:
300:
439:is many times broader than its parent molecule CH
352:Berry pseudorotation of pentacoordinate compounds
1013:Bryant, Robert G. (1983). "The NMR time scale".
1239:"Understanding the Infrared Spectrum of Bare CH
957:(2nd ed.). Philadelphia: W. B. Saunders.
823:E. B. Wilson, J. C. Decius, and P. C. Cross,
483:A classic example of a fluxional molecule is
8:
833:"The symmetry groups of non-rigid molecules"
690:exhibits the phenomenon of "ring whizzing".
336:(and many other cyclic compounds) is called
773:Hapticity § Hapticity and fluxionality
1142:: CS1 maint: location missing publisher (
896:(2 ed.). Ottawa: NRC Research Press.
1268:
937:
856:
638:
633:
606:
567:
542:
538:
527:
514:
506:
286:
281:
274:
249:
242:
236:
211:
203:
184:
169:
818:Molecular Vibrational-Rotational Spectra
697:The structure of the ring whizzer Fe(η-C
917:
1334:For an animation of the dynamics of CH
1135:
1122:(2nd ed.). Oxford. p. 373.
890:Philip R. Bunker; Per Jensen (2006).
364:A prototypical fluxional molecule is
7:
407:A well-studied fluxional ion is the
893:Molecular Symmetry and Spectroscopy
868:Fundamentals of Molecular Symmetry
634:
560:
520:
360:Iron-pentacarbonyl-Berry-mechanism
282:
254:
246:
243:
207:
204:
177:
25:
736:. The singlet assigned to the η-C
392:) follows the pattern set for PF
76:Heisenberg uncertainty principle
1002:10.1002/9781119951438.eibc0211
831:Longuet-Higgins, H.C. (1963).
623:
617:
258:
223:
220:
196:
1:
1453:Topics in Inorganic Chemistry
1085:Chemistry: A European Journal
1050:Journal of Chemical Education
1015:Journal of Chemical Education
955:Physical Methods in Chemistry
816:D. Papoušek and M. R. Aliev,
453:octahedral molecular geometry
320:Cyclohexane and related rings
74:(beyond that dictated by the
866:P. R. Bunker and P. Jensen,
374:Fluorine-19 NMR spectroscopy
157:in a mixed-valence dimer of
996:2011, Wiley-VCH, Weinheim.
1495:
953:Drago, Russell S. (1977).
884:Molecular Rotation Spectra
728:and the other assigned η-C
858:10.1080/00268976300100501
820:Elsevier, Amsterdam, 1982
1479:Chemical bond properties
980:Dynamic NMR Spectroscopy
366:phosphorus pentafluoride
92:organometallic compounds
1270:10.1126/science.1113729
939:10.1351/goldbook.F02463
1097:10.1002/chem.200305028
769:, a fluxional molecule
717:
651:
598:= 1ppm @ 500 MHz
577:
494:
447:Six-coordinate species
361:
329:
302:
978:J. Sandström (1982).
696:
657:(ca. 0.5 millisecond
652:
578:
493:
359:
327:
303:
86:Spectroscopic studies
1421:Bennett, Jr. M. J.;
825:Molecular Vibrations
605:
505:
398:sulfur tetrafluoride
168:
1439:10.1021/ja00971a012
1400:1956JChPh..25.1228G
1373:10.1021/ic50170a035
1361:Inorganic Chemistry
1261:2005Sci...309.1219A
1255:(5738): 1219–1222.
1216:1953JChPh..21..279G
1174:(26): 10726–10731.
1118:J, Clayden (2003).
1062:1997JChEd..74..978J
1027:1983JChEd..60..933B
849:1963MolPh...6..445L
762:Pyramidal inversion
670:The compound Fe(η-C
82:and other methods.
70:signature exhibits
18:Semi-rigid molecule
1345:2007-12-24 at the
870:, CRC Press, 1998
718:
647:
594:For example, if Δν
573:
495:
386:Iron pentacarbonyl
362:
330:
298:
113:Cope rearrangement
1408:10.1063/1.1743184
1314:10.1021/ja0482280
1308:(13): 4954–4958.
1224:10.1063/1.1698874
1180:10.1021/jo051654z
1120:Organic chemistry
1070:10.1021/ed074p978
1035:10.1021/ed060p933
982:. Academic Press.
903:978-0-660-19628-2
837:Molecular Physics
793:Bartell mechanism
552:
485:dimethylformamide
479:Dimethylformamide
155:electron transfer
153:. One example is
80:isotopic labeling
64:organic compounds
36:molecular physics
16:(Redirected from
1486:
1463:
1449:
1443:
1442:
1427:J. Am. Chem. Soc
1418:
1412:
1411:
1394:(6): 1228–1234.
1383:
1377:
1376:
1355:
1349:
1332:
1326:
1325:
1302:J. Am. Chem. Soc
1297:
1291:
1290:
1272:
1234:
1228:
1227:
1198:
1192:
1191:
1154:
1148:
1147:
1141:
1133:
1115:
1109:
1108:
1080:
1074:
1073:
1045:
1039:
1038:
1010:
1004:
990:
984:
983:
975:
969:
968:
950:
944:
943:
941:
922:
907:
862:
860:
656:
654:
653:
648:
646:
645:
637:
582:
580:
579:
574:
572:
571:
553:
551:
550:
546:
533:
532:
531:
515:
438:
437:
436:
422:
421:
420:
307:
305:
304:
299:
294:
293:
285:
279:
278:
257:
253:
241:
240:
219:
218:
210:
189:
188:
98:NMR spectroscopy
21:
1494:
1493:
1489:
1488:
1487:
1485:
1484:
1483:
1469:
1468:
1467:
1466:
1450:
1446:
1420:
1419:
1415:
1385:
1384:
1380:
1357:
1356:
1352:
1347:Wayback Machine
1337:
1333:
1329:
1299:
1298:
1294:
1242:
1236:
1235:
1231:
1200:
1199:
1195:
1156:
1155:
1151:
1134:
1130:
1117:
1116:
1112:
1082:
1081:
1077:
1047:
1046:
1042:
1012:
1011:
1007:
991:
987:
977:
976:
972:
965:
952:
951:
947:
930:IUPAC Gold Book
924:
923:
919:
914:
904:
889:
830:
813:
811:Further reading
798:Berry mechanism
758:
751:
747:
743:
739:
735:
731:
727:
723:
716:
712:
708:
704:
700:
689:
685:
681:
677:
673:
668:
666:"Ring whizzing"
632:
603:
602:
597:
589:
563:
534:
523:
516:
503:
502:
481:
474:
470:
466:
449:
442:
435:
432:
431:
430:
428:
419:
416:
415:
414:
412:
403:
395:
391:
382:Berry mechanism
354:
322:
317:
280:
270:
232:
202:
180:
166:
165:
151:IR spectroscopy
147:
145:IR spectroscopy
139:
121:chair inversion
100:
88:
72:line-broadening
28:
23:
22:
15:
12:
11:
5:
1492:
1490:
1482:
1481:
1471:
1470:
1465:
1464:
1461:978-0195301007
1444:
1413:
1378:
1367:(4): 897–902.
1350:
1335:
1327:
1292:
1240:
1229:
1204:J. Chem. Phys.
1193:
1149:
1128:
1110:
1091:(24): 5969ff.
1075:
1040:
1005:
985:
970:
963:
945:
916:
915:
913:
910:
909:
908:
902:
887:
880:
864:
843:(5): 445–460.
828:
821:
812:
809:
808:
807:
806:
805:
803:Ray–Dutt twist
800:
795:
790:
783:Pseudorotation
780:
775:
770:
764:
757:
754:
749:
745:
741:
737:
733:
729:
725:
721:
714:
710:
706:
702:
698:
687:
683:
679:
675:
671:
667:
664:
663:
662:
644:
641:
636:
631:
628:
625:
622:
619:
616:
613:
610:
595:
587:
584:
583:
570:
566:
562:
559:
556:
549:
545:
541:
537:
530:
526:
522:
519:
513:
510:
480:
477:
472:
468:
464:
457:Ray-Dutt twist
448:
445:
440:
433:
417:
401:
393:
389:
378:pseudorotation
370:F NMR spectrum
353:
350:
321:
318:
316:
313:
309:
308:
297:
292:
289:
284:
277:
273:
269:
266:
263:
260:
256:
252:
248:
245:
239:
235:
231:
228:
225:
222:
217:
214:
209:
206:
201:
198:
195:
192:
187:
183:
179:
176:
173:
159:metal clusters
146:
143:
137:
99:
96:
87:
84:
60:bond rotations
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1491:
1480:
1477:
1476:
1474:
1462:
1458:
1454:
1448:
1445:
1440:
1436:
1432:
1428:
1424:
1423:Cotton, F. A.
1417:
1414:
1409:
1405:
1401:
1397:
1393:
1389:
1388:J. Chem. Phys
1382:
1379:
1374:
1370:
1366:
1362:
1354:
1351:
1348:
1344:
1341:
1331:
1328:
1323:
1319:
1315:
1311:
1307:
1303:
1296:
1293:
1288:
1284:
1280:
1276:
1271:
1266:
1262:
1258:
1254:
1250:
1249:
1244:
1233:
1230:
1225:
1221:
1217:
1213:
1209:
1206:
1205:
1197:
1194:
1189:
1185:
1181:
1177:
1173:
1170:
1169:
1168:J. Org. Chem.
1164:
1160:
1153:
1150:
1145:
1139:
1131:
1129:9780191666216
1125:
1121:
1114:
1111:
1106:
1102:
1098:
1094:
1090:
1086:
1079:
1076:
1071:
1067:
1063:
1059:
1055:
1051:
1044:
1041:
1036:
1032:
1028:
1024:
1020:
1016:
1009:
1006:
1003:
999:
995:
989:
986:
981:
974:
971:
966:
964:0-7216-3184-3
960:
956:
949:
946:
940:
935:
931:
927:
921:
918:
911:
905:
899:
895:
894:
888:
885:
882:H. W. Kroto,
881:
879:
877:
876:0-7503-0941-5
873:
869:
865:
859:
854:
850:
846:
842:
838:
834:
829:
826:
822:
819:
815:
814:
810:
804:
801:
799:
796:
794:
791:
789:
786:
785:
784:
781:
779:
776:
774:
771:
768:
765:
763:
760:
759:
755:
753:
695:
691:
665:
660:
642:
639:
629:
626:
620:
614:
611:
608:
601:
600:
599:
592:
568:
564:
557:
554:
547:
543:
539:
535:
528:
524:
517:
511:
508:
501:
500:
499:
492:
488:
486:
478:
476:
462:
458:
454:
446:
444:
426:
425:absolute zero
410:
405:
399:
387:
383:
379:
375:
371:
367:
358:
351:
349:
347:
343:
339:
338:ring flipping
335:
326:
319:
314:
312:
295:
290:
287:
275:
271:
267:
264:
261:
250:
237:
233:
229:
226:
215:
212:
199:
193:
190:
185:
181:
174:
171:
164:
163:
162:
160:
156:
152:
144:
142:
140:
136:
128:
126:
122:
118:
114:
109:
106:
97:
95:
93:
85:
83:
81:
77:
73:
69:
68:spectroscopic
65:
61:
57:
53:
49:
45:
41:
37:
33:
19:
1452:
1447:
1433:(88): 4371.
1430:
1426:
1416:
1391:
1387:
1381:
1364:
1360:
1353:
1330:
1305:
1301:
1295:
1252:
1246:
1232:
1207:
1202:
1196:
1171:
1166:
1162:
1158:
1152:
1119:
1113:
1088:
1084:
1078:
1053:
1049:
1043:
1018:
1014:
1008:
993:
988:
979:
973:
954:
948:
929:
920:
892:
883:
867:
840:
836:
824:
817:
788:Bailar twist
719:
669:
593:
585:
496:
482:
461:Bailar twist
450:
406:
363:
331:
310:
148:
134:
129:
110:
104:
101:
89:
47:
43:
39:
29:
1021:(11): 933.
926:"Fluxional"
443:, methane.
342:equilibrate
334:cyclohexane
125:cyclohexane
1210:(2): 279.
1056:(8): 978.
912:References
767:Bullvalene
423:. Even at
117:bullvalene
1455:), 2007.
1138:cite book
659:half-life
640:−
612:∼
569:∘
565:ν
561:Δ
555:∼
529:∘
525:ν
521:Δ
518:π
409:methanium
296:⋅
288:−
268:×
262:∼
230:⋅
213:−
191:∼
186:∘
182:ν
178:Δ
175:∼
52:molecules
48:molecules
44:non-rigid
40:fluxional
32:chemistry
1473:Category
1343:Archived
1322:15796561
1287:28745636
1279:15994376
1188:16355992
1161:-1,4-Di-
1105:14679508
932:. 2014.
756:See also
586:where Δν
459:and the
380:via the
315:Examples
119:and the
62:in most
1396:Bibcode
1257:Bibcode
1248:Science
1212:Bibcode
1058:Bibcode
1023:Bibcode
845:Bibcode
487:(DMF).
388:(Fe(CO)
346:proton-
1459:
1338:, see
1320:
1285:
1277:
1186:
1126:
1103:
961:
900:
874:
705:) (η-C
411:ion,
368:. Its
344:. The
1283:S2CID
713:)(CO)
686:)(CO)
678:)(η-C
467:(SiMe
90:Many
56:atoms
1457:ISBN
1431:1966
1318:PMID
1275:PMID
1184:PMID
1163:tert
1144:link
1124:ISBN
1101:PMID
959:ISBN
898:ISBN
872:ISBN
630:1000
105:DNMR
50:are
42:(or
34:and
1435:doi
1404:doi
1369:doi
1310:doi
1306:127
1265:doi
1253:309
1220:doi
1176:doi
1159:cis
1093:doi
1066:doi
1031:doi
998:doi
934:doi
853:doi
621:500
400:(SF
227:300
123:in
115:in
30:In
1475::
1429:.
1402:.
1392:25
1390:.
1365:16
1363:.
1316:.
1304:.
1281:.
1273:.
1263:.
1251:.
1245:.
1218:.
1208:21
1182:.
1172:70
1140:}}
1136:{{
1099:.
1087:.
1064:.
1054:74
1052:.
1029:.
1019:60
1017:.
928:.
851:.
839:.
835:.
429:CH
413:CH
276:11
272:10
234:10
200:10
141:.
133:1/
127:.
46:)
38:,
1441:.
1437::
1410:.
1406::
1398::
1375:.
1371::
1336:5
1324:.
1312::
1289:.
1267::
1259::
1243:"
1241:5
1226:.
1222::
1214::
1190:.
1178::
1146:)
1132:.
1107:.
1095::
1089:9
1072:.
1068::
1060::
1037:.
1033::
1025::
1000::
967:.
942:.
936::
906:.
861:.
855::
847::
841:6
750:5
748:H
746:5
742:5
740:H
738:5
734:5
732:H
730:5
726:5
724:H
722:5
715:2
711:5
709:H
707:5
703:5
701:H
699:5
688:2
684:5
682:H
680:5
676:5
674:H
672:5
661:)
643:1
635:s
627:=
624:)
618:(
615:2
609:k
596:o
588:o
558:2
548:2
544:/
540:1
536:2
512:=
509:k
473:2
471:)
469:3
465:4
441:4
434:5
418:5
402:4
394:5
390:5
291:1
283:s
265:6
259:)
255:s
251:/
247:m
244:c
238:8
224:(
221:)
216:1
208:m
205:c
197:(
194:2
172:k
138:1
135:T
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.