1902:
506:
31:
1912:
1938:
641:
and the process of using the same, US Patent 5,888,472) the recognition of the unique ion exchange properties and the potential use to remove toxins from the body were identified shortly thereafter ("process for removing toxins from bodily fluids using zirconium or titanium microporous compositions,
522:. Zirconium silicates have been extensively used in medical and dental applications because of their proven safety. 11 zirconium silicates were screened by an iterative optimization process. ZS-9 selectively captures potassium ions, presumably by mimicking the actions of physiologic
1419:
1412:
509:
Cross-sections of ZS-9 pores with three different ions (K⁺ = potassium, Na⁺ = sodium, Ca²⁺ = calcium). The specificity for potassium is thought to be caused by the diameter and composition of the pores, which resembles
1405:
211:
614:
for an increased risk of death. However, there is disagreement regarding whether a modestly elevated levels directly causes problems. One viewpoint is that mild to moderate hyperkalemia is a
721:
166:
1292:
751:
634:(FDA) in May 2016, due to issues associated with manufacturing. On 18 May 2018, the FDA approved sodium zirconium cyclosilicate for treatment of adults with hyperkalemia.
64:
1628:
924:
573:
1813:
1973:
1958:
552:
Hyperkalemia is rare among those who are otherwise healthy. Among those who are in hospital, rates are between 1% and 2.5%. Common causes include
836:
1550:
1176:
1342:"Systematic Review and Meta-Analysis of Patiromer and Sodium Zirconium Cyclosilicate: A New Armamentarium for the Treatment of Hyperkalemia"
579:
There is no universally accepted definition of what level of hyperkalemia is mild, moderate, or severe. However, if hyperkalemia causes any
115:
1492:
945:
Denry I, Kelly JR. State of the art of zirconia for dental applications. Dental
Materials. Volume 24, Issue 3, March 2008, Pages 299–307
569:
787:
711:
1683:
1668:
1288:
619:
743:
293:
196:
96:
1963:
477:
Sodium zirconium cyclosilicate was approved for medical use in the
European Union and in the United States in 2018. It was
35:
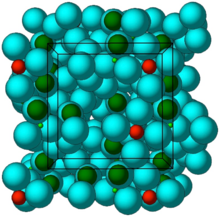
Crystal structure of ZS-9. Blue spheres = oxygen atoms, red spheres = zirconium atoms, green spheres = silicon atoms.
1856:
1928:
1968:
1318:
918:
631:
604:
350:
1397:
637:
It was first practically synthesized by UOP in the late 1990s. (reference -zirconium silicate and zirconium germate
1543:
912:
781:
1517:
1851:
152:
1915:
1887:
1803:
1798:
1693:
654:
535:
467:
1758:
1623:
1459:
828:
74:
1905:
1536:
968:
1507:
1482:
592:
580:
527:
456:
310:
159:
1978:
1088:
523:
519:
126:
650:
One review found a decrease in potassium of 0.17 mEq/L at one hour and 0.67 mEq/L at 48 hours.
22:
1643:
1497:
1433:
1371:
1269:
1215:
1172:
1140:
1080:
1045:
996:
894:
611:
584:
557:
511:
235:
223:
46:
1474:
1446:
1361:
1353:
1259:
1251:
1205:
1130:
1122:
1072:
1035:
1027:
986:
976:
884:
876:
776:
588:
478:
395:
257:
359:
319:
1942:
1773:
1613:
1487:
638:
265:
1063:
McDonald TJ, Oram RA, Vaidya B (October 2015). "Investigating hyperkalaemia in adults".
972:
1583:
1437:
1366:
1341:
1264:
1239:
1135:
1110:
1040:
1015:
991:
956:
889:
864:
595:
in the absence of ECG changes are managed aggressively. Several approaches are used to
565:
561:
553:
1392:
1952:
1638:
1593:
1573:
1092:
716:
662:
615:
88:
1882:
1828:
1818:
1748:
1658:
1608:
1603:
1429:
596:
547:
526:. ZS-9 is an inorganic cation exchanger crystalline with a high capacity to entrap
494:
441:
179:
174:
685:
1194:"Damned if you do, damned if you don't: potassium binding resins in hyperkalemia"
981:
1823:
1778:
1768:
1763:
1743:
1738:
1723:
1718:
1703:
1673:
1633:
1588:
1578:
1559:
505:
482:
82:
618:
that denotes underlying medical problems. Accordingly, these problems are both
1808:
1793:
1753:
1733:
1713:
1698:
1653:
1648:
1618:
1502:
1126:
1031:
880:
437:
1314:
957:"Characterization of structure and function of ZS-9, a K+ selective ion trap"
1861:
1788:
1688:
1678:
1663:
1512:
1454:
600:
460:
275:
68:
30:
1375:
1273:
1238:
Elliott MJ, Ronksley PE, Clase CM, Ahmed SB, Hemmelgarn BR (October 2010).
1219:
1144:
1084:
1049:
1000:
898:
1846:
1728:
1708:
1598:
1210:
1193:
658:
531:
459:. Use is likely safe in pregnancy and breastfeeding. It works by binding
445:
330:
110:
1255:
564:. A number of medications can also cause high blood potassium including
339:
1167:
Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM (2012).
1357:
1076:
630:
In the United States, regulatory approval of ZS-9 was rejected by the
1866:
379:
591:
and is treated urgently. Potassium levels greater than 6.5 to 7.0
504:
471:
452:
913:"Drug Approval Package: Lokelma (sodium zirconium cyclosilicate)"
744:"Lokelma- sodium zirconium cyclosilicate powder, for suspension"
599:. Other approved potassium binders in the United States include
463:
370:
241:
1532:
1401:
1315:"Lokelma (Sodium zirconium cyclosilicate) FDA Approval History"
229:
105:
1528:
1016:"Updates in hyperkalemia: Outcomes and therapeutic strategies"
1289:"AstraZeneca's $ 2.7B hyperkalemia drug ZS-9 rejected by FDA"
829:"Sodium Zirconium Cyclosilicate Monograph for Professionals"
955:
Stavros F, Yang A, Leon A, Nuttall M, Rasmussen HS (2014).
865:"Sodium Zirconium Cyclosilicate: A Review in Hyperkalaemia"
444:. Onset of effects occurs in one to six hours. It is taken
205:
137:
1111:"Pathogenesis, diagnosis and management of hyperkalemia"
301:
Silicic acid, sodium zirconium(4+) salt (3:2:1), hydrate
218:
1198:
Clinical
Journal of the American Society of Nephrology
1926:
1340:
Meaney CJ, Beccari MV, Yang Y, Zhao J (April 2017).
1875:
1837:
1566:
1473:
1445:
394:
389:
369:
349:
329:
309:
284:
274:
264:
256:
195:
190:
165:
151:
125:
95:
81:
63:
55:
45:
40:
1162:
1160:
1158:
1156:
1154:
1240:"Management of patients with acute hyperkalemia"
1171:(Chapter 17, page 672, 9th ed.). Elsevier.
493:Sodium zirconium cyclosilicate is used to treat
680:
678:
497:. Onset of effects occurs in one to six hours.
318:
1020:Reviews in Endocrine & Metabolic Disorders
771:
769:
1544:
1413:
1233:
1231:
1229:
1192:Watson M, Abbott KC, Yuan CM (October 2010).
712:"Summary Basis of Decision (SBD) for Lokelma"
16:Medication used to treat high blood potassium
8:
1629:Budesonide/glycopyrronium bromide/formoterol
114:
21:
610:Hyperkalemia, particularly if severe, is a
249:In general: ℞ (Prescription only)
1551:
1537:
1529:
1420:
1406:
1398:
1104:
1102:
823:
821:
819:
817:
815:
813:
811:
809:
807:
805:
29:
1365:
1263:
1209:
1134:
1039:
990:
980:
888:
858:
856:
854:
665:. Use has been studied for up to a year.
358:
574:angiotensin converting enzyme inhibitors
1933:
674:
338:
298:
87:
690:Therapeutic Goods Administration (TGA)
20:
1109:Lehnhardt A, Kemper MJ (March 2011).
73:
7:
1911:
754:from the original on 5 December 2022
653:It appears effective in people with
530:cations, specifically potassium and
178:
1493:Calcium acetate/magnesium carbonate
790:from the original on 8 January 2021
587:due to a risk of potentially fatal
378:
927:from the original on 13 April 2021
839:from the original on 1 August 2020
14:
1684:Glycopyrronium bromide/formoterol
1321:from the original on 12 June 2018
1936:
1910:
1901:
1900:
1295:from the original on 28 May 2016
724:from the original on 31 May 2022
1169:Brenner and Rector's The Kidney
1974:Drugs developed by AstraZeneca
1959:Chelating agents used as drugs
1784:Sodium zirconium cyclosilicate
1465:Sodium zirconium cyclosilicate
430:Sodium zirconium cyclosilicate
116:Sodium zirconium cyclosilicate
23:Sodium zirconium cyclosilicate
1:
1857:Cambridge Antibody Technology
620:proximate and ultimate causes
982:10.1371/journal.pone.0114686
919:Food and Drug Administration
632:Food and Drug Administration
605:sodium polystyrene sulfonate
451:Common side effects include
432:, sold under the brand name
1995:
583:change it is considered a
545:
470:which is then lost in the
390:Chemical and physical data
1896:
1287:Ben Adams (27 May 2016).
1127:10.1007/s00467-010-1699-3
1032:10.1007/s11154-016-9384-x
1014:Kovesdy CP (March 2017).
881:10.1007/s40265-018-0991-6
782:European Medicines Agency
289:
28:
1518:Sucroferric oxyhydroxide
1852:Alexion Pharmaceuticals
1428:Drugs for treatment of
863:Hoy SM (October 2018).
1804:Trastuzumab deruxtecan
1799:Tixagevimab/cilgavimab
1694:Isosorbide mononitrate
655:chronic kidney disease
642:US Patent 6,332,985).
589:abnormal heart rhythms
515:
468:gastrointestinal tract
1964:Nephrology procedures
1759:Salbutamol/budesonide
1624:Budesonide/formoterol
1460:Polystyrene sulfonate
786:. 17 September 2018.
750:. 30 September 2022.
508:
1211:10.2215/CJN.03700410
1115:Pediatric Nephrology
495:high blood potassium
442:high blood potassium
1969:Zirconium compounds
1508:Lanthanum carbonate
1483:Aluminium hydroxide
1256:10.1503/cmaj.100461
973:2014PLoSO...9k4686S
720:. 23 October 2014.
501:Mechanism of action
457:low blood potassium
214:(Prescription only)
25:
1840:acquired companies
597:treat hyperkalemia
524:potassium channels
520:zirconium silicate
516:
512:potassium channels
1924:
1923:
1644:Disufenton sodium
1526:
1525:
1498:Calcium carbonate
1475:Phosphate binders
1447:Potassium binders
1434:hyperphosphatemia
1358:10.1002/phar.1906
1291:. FierceBiotech.
1178:978-1-4160-6193-9
1077:10.1136/bmj.h4762
875:(15): 1605–1613.
585:medical emergency
558:hypoaldosteronism
427:
426:
245:
233:
221:
209:
141:
108:
1986:
1941:
1940:
1939:
1932:
1914:
1913:
1904:
1903:
1888:Louis Schweitzer
1838:Predecessors and
1553:
1546:
1539:
1530:
1422:
1415:
1408:
1399:
1380:
1379:
1369:
1337:
1331:
1330:
1328:
1326:
1311:
1305:
1304:
1302:
1300:
1284:
1278:
1277:
1267:
1235:
1224:
1223:
1213:
1189:
1183:
1182:
1164:
1149:
1148:
1138:
1106:
1097:
1096:
1060:
1054:
1053:
1043:
1011:
1005:
1004:
994:
984:
952:
946:
943:
937:
936:
934:
932:
909:
903:
902:
892:
860:
849:
848:
846:
844:
825:
800:
799:
797:
795:
773:
764:
763:
761:
759:
740:
734:
733:
731:
729:
708:
702:
701:
699:
697:
682:
639:molecular sieves
616:secondary effect
382:
362:
342:
322:
243:
240:
231:
228:
220:
217:
207:
204:
182:
139:
136:
118:
107:
104:
91:
77:
33:
26:
24:
1994:
1993:
1989:
1988:
1987:
1985:
1984:
1983:
1949:
1948:
1947:
1937:
1935:
1927:
1925:
1920:
1892:
1871:
1839:
1833:
1774:Sebelipase alfa
1614:Brompheniramine
1562:
1557:
1527:
1522:
1488:Calcium acetate
1469:
1441:
1426:
1389:
1387:Further reading
1384:
1383:
1346:Pharmacotherapy
1339:
1338:
1334:
1324:
1322:
1313:
1312:
1308:
1298:
1296:
1286:
1285:
1281:
1237:
1236:
1227:
1191:
1190:
1186:
1179:
1166:
1165:
1152:
1108:
1107:
1100:
1062:
1061:
1057:
1013:
1012:
1008:
967:(12): e114686.
954:
953:
949:
944:
940:
930:
928:
923:. 8 June 2018.
911:
910:
906:
862:
861:
852:
842:
840:
827:
826:
803:
793:
791:
775:
774:
767:
757:
755:
742:
741:
737:
727:
725:
710:
709:
705:
695:
693:
686:"Lokelma APMDS"
684:
683:
676:
671:
648:
628:
550:
544:
503:
491:
423:
419:
415:
411:
407:
403:
385:
365:
345:
325:
305:
302:
297:
296:
266:Bioavailability
258:Pharmacokinetic
252:
186:
154:
147:
128:
121:
36:
17:
12:
11:
5:
1992:
1990:
1982:
1981:
1976:
1971:
1966:
1961:
1951:
1950:
1946:
1945:
1922:
1921:
1919:
1918:
1908:
1897:
1894:
1893:
1891:
1890:
1885:
1879:
1877:
1873:
1872:
1870:
1869:
1864:
1859:
1854:
1849:
1843:
1841:
1835:
1834:
1832:
1831:
1826:
1821:
1816:
1811:
1806:
1801:
1796:
1791:
1786:
1781:
1776:
1771:
1766:
1761:
1756:
1751:
1746:
1741:
1736:
1731:
1726:
1721:
1716:
1711:
1706:
1701:
1696:
1691:
1686:
1681:
1676:
1671:
1666:
1661:
1656:
1651:
1646:
1641:
1636:
1631:
1626:
1621:
1616:
1611:
1606:
1601:
1596:
1591:
1586:
1584:Andexanet alfa
1581:
1576:
1570:
1568:
1564:
1563:
1558:
1556:
1555:
1548:
1541:
1533:
1524:
1523:
1521:
1520:
1515:
1510:
1505:
1500:
1495:
1490:
1485:
1479:
1477:
1471:
1470:
1468:
1467:
1462:
1457:
1451:
1449:
1443:
1442:
1427:
1425:
1424:
1417:
1410:
1402:
1396:
1395:
1388:
1385:
1382:
1381:
1352:(4): 401–411.
1332:
1306:
1279:
1250:(15): 1631–5.
1225:
1204:(10): 1723–6.
1184:
1177:
1150:
1098:
1055:
1006:
947:
938:
904:
850:
801:
777:"Lokelma EPAR"
765:
735:
703:
673:
672:
670:
667:
647:
644:
627:
624:
566:spironolactone
562:rhabdomyolysis
554:kidney failure
546:Main article:
543:
540:
502:
499:
490:
487:
440:used to treat
425:
424:
421:
417:
413:
409:
405:
401:
398:
392:
391:
387:
386:
384:
383:
375:
373:
367:
366:
364:
363:
355:
353:
347:
346:
344:
343:
335:
333:
327:
326:
324:
323:
315:
313:
307:
306:
304:
303:
300:
292:
291:
290:
287:
286:
282:
281:
278:
272:
271:
268:
262:
261:
254:
253:
251:
250:
247:
238:
226:
215:
201:
199:
193:
192:
188:
187:
185:
184:
171:
169:
163:
162:
157:
155:administration
149:
148:
146:
145:
143:
133:
131:
123:
122:
120:
119:
101:
99:
93:
92:
85:
79:
78:
71:
61:
60:
57:
53:
52:
49:
43:
42:
38:
37:
34:
15:
13:
10:
9:
6:
4:
3:
2:
1991:
1980:
1977:
1975:
1972:
1970:
1967:
1965:
1962:
1960:
1957:
1956:
1954:
1944:
1934:
1930:
1917:
1909:
1907:
1899:
1898:
1895:
1889:
1886:
1884:
1881:
1880:
1878:
1874:
1868:
1865:
1863:
1860:
1858:
1855:
1853:
1850:
1848:
1845:
1844:
1842:
1836:
1830:
1827:
1825:
1822:
1820:
1817:
1815:
1812:
1810:
1807:
1805:
1802:
1800:
1797:
1795:
1792:
1790:
1787:
1785:
1782:
1780:
1777:
1775:
1772:
1770:
1767:
1765:
1762:
1760:
1757:
1755:
1752:
1750:
1747:
1745:
1742:
1740:
1737:
1735:
1732:
1730:
1727:
1725:
1722:
1720:
1717:
1715:
1712:
1710:
1707:
1705:
1702:
1700:
1697:
1695:
1692:
1690:
1687:
1685:
1682:
1680:
1677:
1675:
1672:
1670:
1667:
1665:
1662:
1660:
1657:
1655:
1652:
1650:
1647:
1645:
1642:
1640:
1639:Dapagliflozin
1637:
1635:
1632:
1630:
1627:
1625:
1622:
1620:
1617:
1615:
1612:
1610:
1607:
1605:
1602:
1600:
1597:
1595:
1594:Asfotase alfa
1592:
1590:
1587:
1585:
1582:
1580:
1577:
1575:
1574:Acalabrutinib
1572:
1571:
1569:
1565:
1561:
1554:
1549:
1547:
1542:
1540:
1535:
1534:
1531:
1519:
1516:
1514:
1511:
1509:
1506:
1504:
1501:
1499:
1496:
1494:
1491:
1489:
1486:
1484:
1481:
1480:
1478:
1476:
1472:
1466:
1463:
1461:
1458:
1456:
1453:
1452:
1450:
1448:
1444:
1439:
1435:
1431:
1423:
1418:
1416:
1411:
1409:
1404:
1403:
1400:
1394:
1391:
1390:
1386:
1377:
1373:
1368:
1363:
1359:
1355:
1351:
1347:
1343:
1336:
1333:
1320:
1316:
1310:
1307:
1294:
1290:
1283:
1280:
1275:
1271:
1266:
1261:
1257:
1253:
1249:
1245:
1241:
1234:
1232:
1230:
1226:
1221:
1217:
1212:
1207:
1203:
1199:
1195:
1188:
1185:
1180:
1174:
1170:
1163:
1161:
1159:
1157:
1155:
1151:
1146:
1142:
1137:
1132:
1128:
1124:
1121:(3): 377–84.
1120:
1116:
1112:
1105:
1103:
1099:
1094:
1090:
1086:
1082:
1078:
1074:
1070:
1066:
1059:
1056:
1051:
1047:
1042:
1037:
1033:
1029:
1025:
1021:
1017:
1010:
1007:
1002:
998:
993:
988:
983:
978:
974:
970:
966:
962:
958:
951:
948:
942:
939:
926:
922:
920:
914:
908:
905:
900:
896:
891:
886:
882:
878:
874:
870:
866:
859:
857:
855:
851:
838:
834:
830:
824:
822:
820:
818:
816:
814:
812:
810:
808:
806:
802:
789:
785:
783:
778:
772:
770:
766:
753:
749:
745:
739:
736:
723:
719:
718:
717:Health Canada
713:
707:
704:
692:. 24 May 2024
691:
687:
681:
679:
675:
668:
666:
664:
663:heart failure
660:
656:
651:
645:
643:
640:
635:
633:
625:
623:
621:
617:
613:
608:
606:
602:
598:
594:
590:
586:
582:
577:
575:
571:
567:
563:
559:
555:
549:
541:
539:
537:
534:ions, in the
533:
529:
525:
521:
513:
507:
500:
498:
496:
488:
486:
484:
480:
475:
473:
469:
465:
462:
458:
454:
449:
447:
443:
439:
435:
431:
399:
397:
393:
388:
381:
377:
376:
374:
372:
368:
361:
357:
356:
354:
352:
348:
341:
337:
336:
334:
332:
328:
321:
317:
316:
314:
312:
308:
299:
295:
288:
283:
279:
277:
273:
269:
267:
263:
259:
255:
248:
246: Rx-only
239:
237:
227:
225:
216:
213:
203:
202:
200:
198:
194:
189:
181:
176:
173:
172:
170:
168:
164:
161:
158:
156:
150:
144:
135:
134:
132:
130:
124:
117:
112:
103:
102:
100:
98:
94:
90:
86:
84:
80:
76:
72:
70:
66:
62:
58:
54:
50:
48:
44:
41:Clinical data
39:
32:
27:
19:
1883:Tom McKillop
1829:Zolmitriptan
1819:Ximelagatran
1783:
1749:Rosuvastatin
1659:Esomeprazole
1609:Bicalutamide
1604:Benralizumab
1464:
1430:hyperkalemia
1393:CADTH review
1349:
1345:
1335:
1323:. Retrieved
1309:
1297:. Retrieved
1282:
1247:
1243:
1201:
1197:
1187:
1168:
1118:
1114:
1068:
1064:
1058:
1026:(1): 41–47.
1023:
1019:
1009:
964:
960:
950:
941:
929:. Retrieved
916:
907:
872:
868:
841:. Retrieved
832:
792:. Retrieved
780:
756:. Retrieved
747:
738:
726:. Retrieved
715:
706:
694:. Retrieved
689:
652:
649:
636:
629:
609:
578:
551:
548:Hyperkalemia
517:
492:
476:
450:
433:
429:
428:
270:Not absorbed
197:Legal status
191:Legal status
97:License data
18:
1824:Zafirlukast
1779:Selumetinib
1769:Saxagliptin
1764:Savolitinib
1744:Roflumilast
1739:Ravulizumab
1724:Palivizumab
1719:Osimertinib
1704:Motavizumab
1674:Fulvestrant
1634:Candesartan
1589:Anifrolumab
1579:Anastrozole
1560:AstraZeneca
758:27 February
489:Medical use
483:AstraZeneca
285:Identifiers
83:MedlinePlus
56:Other names
47:Trade names
1953:Categories
1809:Vandetanib
1794:Ticagrelor
1754:Roxadustat
1734:Quetiapine
1714:Omeprazole
1699:Metoprolol
1654:Eculizumab
1649:Durvalumab
1619:Budesonide
1503:Colestilan
843:11 October
794:11 October
669:References
622:of death,
542:Background
528:monovalent
518:ZS-9 is a
438:medication
360:D652ZWF066
320:17141-74-1
311:CAS Number
294:IUPAC name
1979:Potassium
1862:MedImmune
1814:Vaxzevria
1789:Tamoxifen
1689:Goserelin
1679:Gefitinib
1664:Exenatide
1513:Sevelamer
1455:Patiromer
1093:206907572
1071:: h4762.
833:Drugs.com
601:patiromer
479:developed
461:potassium
276:Excretion
153:Routes of
127:Pregnancy
75:Monograph
69:Drugs.com
1943:Medicine
1906:Category
1847:Astra AB
1729:Propofol
1709:Olaparib
1599:Atenolol
1567:Products
1376:28122118
1319:Archived
1293:Archived
1274:20855477
1220:20798253
1145:21181208
1085:26487322
1050:27600582
1001:25531770
961:PLOS ONE
925:Archived
899:30306338
837:Archived
788:Archived
752:Archived
748:DailyMed
722:Archived
659:diabetes
646:Research
536:GI tract
532:ammonium
453:swelling
446:by mouth
331:DrugBank
167:ATC code
160:By mouth
142: B1
129:category
111:DailyMed
1916:Commons
1669:FluMist
1367:5388568
1325:11 June
1265:2952010
1136:3061004
1041:5339065
992:4273971
969:Bibcode
890:6433811
696:10 June
626:History
466:in the
436:, is a
434:Lokelma
396:Formula
340:DB14048
222::
183:)
177: (
175:V03AE10
113::
89:a618035
51:Lokelma
1929:Portal
1876:People
1867:Zeneca
1374:
1364:
1299:27 May
1272:
1262:
1218:
1175:
1143:
1133:
1091:
1083:
1048:
1038:
999:
989:
897:
887:
728:29 May
661:, and
612:marker
593:mmol/L
572:, and
570:NSAIDs
560:, and
400:(2Na·H
380:D10727
236:℞-only
234:
224:℞-only
210:
109:
1438:V03AE
1089:S2CID
931:7 May
921:(FDA)
917:U.S.
869:Drugs
784:(EMA)
472:stool
280:Feces
1432:and
1372:PMID
1327:2018
1301:2016
1270:PMID
1244:CMAJ
1216:PMID
1173:ISBN
1141:PMID
1081:PMID
1046:PMID
997:PMID
933:2020
895:PMID
845:2019
796:2019
760:2023
730:2022
698:2024
603:and
464:ions
455:and
404:O·3H
371:KEGG
351:UNII
260:data
65:AHFS
59:ZS-9
1362:PMC
1354:doi
1260:PMC
1252:doi
1248:182
1206:doi
1131:PMC
1123:doi
1073:doi
1069:351
1065:BMJ
1036:PMC
1028:doi
987:PMC
977:doi
885:PMC
877:doi
581:ECG
481:by
416:ZrO
408:SiO
180:WHO
1955::
1370:.
1360:.
1350:37
1348:.
1344:.
1317:.
1268:.
1258:.
1246:.
1242:.
1228:^
1214:.
1200:.
1196:.
1153:^
1139:.
1129:.
1119:26
1117:.
1113:.
1101:^
1087:.
1079:.
1067:.
1044:.
1034:.
1024:18
1022:.
1018:.
995:.
985:.
975:.
963:.
959:.
915:.
893:.
883:.
873:78
871:.
867:.
853:^
835:.
831:.
804:^
779:.
768:^
746:.
714:.
688:.
677:^
657:,
607:.
576:.
568:,
556:,
538:.
485:.
474:.
448:.
412:·H
242:EU
230:US
219:CA
212:S4
206:AU
138:AU
106:US
1931::
1552:e
1545:t
1538:v
1440:)
1436:(
1421:e
1414:t
1407:v
1378:.
1356::
1329:.
1303:.
1276:.
1254::
1222:.
1208::
1202:5
1181:.
1147:.
1125::
1095:.
1075::
1052:.
1030::
1003:.
979::
971::
965:9
935:.
901:.
879::
847:.
798:.
762:.
732:.
700:.
514:.
422:n
420:)
418:6
414:4
410:4
406:4
402:2
244::
232::
208::
140::
67:/
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.