656:. Indeed, by introducing a SSE in the battery architecture there's the possibility to use metallic lithium as anode material, with the possibility to achieve a high energy density battery thanks to its high specific capacity of 3860 mAh g. The use of a lithium metal anode(LMA) is prevented in a liquid electrolyte above all because of the dendritic growth of a pure Li electrode that easily cause short circuits after few cycles; other related issues are volume expansions, solid-electrolyte-interface (SEI) reactivity and 'dead' lithium. The usage of a SSE guarantees a homogeneous contact with the metallic lithium electrode and possess the mechanical properties to impede the uncontrolled deposition of Li ions during the charging phase. At the same time, a SSE finds very promising application in
85:, is the first step in the realization of a lighter, thinner and cheaper rechargeable battery. Moreover, this allows the reach of gravimetric and volumetric energy densities, high enough to achieve 500 miles per single charge in an electric vehicle. Despite the promising advantages, there are still many limitations that are hindering the transition of SSEs from academia research to large-scale production, depending mainly on the poor ionic conductivity compared to that of liquid counterparts. However, many car
519:
422:), electrochemical compatibility with most common electrode materials, a low degree of crystallinity, mechanical stability, low temperature sensitivity are all characteristics for the ideal SPE candidate. In general though the ionic conductivity is lower than the ISEs and their rate capability is restricted, limiting fast charging. PEO-based SPE is the first solid-state polymer in which ionic conductivity was demonstrated both through inter and intra molecular through
631:(EC)) to create a gel, whose properties can be modified based on the matrix loading. Matrix content ranging from 10 to 40 wt% can shift the mechanical properties of the electrolyte from a soft paste into a hard gel. However, a tradeoff between mechanical strength and ionic conductivity as one goes up with changing matrix content the other suffers. Despite this, matrix content in these materials can have added benefits including enhanced lithium
20:
644:
265:
polymer electrolyte (CPE). On the other hand, a QSSE, also called gel polymer electrolyte (GPE), is a freestanding membrane that contains a certain amount of liquid component immobilized inside the solid matrix. In general the nomenclatures SPE and GPE are used interchangeably but they have a substantially different
418:, making them greatly compatible with large-scale manufacturing processes. Moreover, they possess higher elasticity and plasticity giving stability at the interface, flexibility and improved resistance to volume changes during operation. A good dissolution of Li salts, low glass transition temperature (T
556:
acts to increase the ionic conductivity of the electrolyte as well as soften the electrolyte for improved interfacial contact. The matrix of GPEs consist of a polymer network swollen in a solvent that contains the active ions (e.g., Li, Na, Mg, etc.). This allows for the composite to contain both the
1396:
Agostini, Marco; Lim, Du Hyun; Sadd, Matthew; Fasciani, Chiara; Navarra, Maria
Assunta; Panero, Stefania; Brutti, Sergio; Matic, Aleksandar; Scrosati, Bruno (11 September 2017). "Stabilizing the Performance of High-Capacity Sulfur Composite Electrodes by a New Gel Polymer Electrolyte Configuration".
538:
while the solid matrix adds mechanical stability to the material as a whole. As the name suggests, QSSEs can have a range of mechanical properties from strong solid-like materials to those in a paste form. QSSEs can be subdivided into a number of categories including gel polymer electrolytes (GPEs),
299:(of the order of GPa) and high transfer number compared to other classes of SSEs. They are generally brittle and with this comes a low compatibility and stability towards the electrode, with a rapidly increasing interfacial resistance and a complicated scale-up from academic to industry. They can be
2909:
Bouchet, Renaud; Maria, Sébastien; Meziane, Rachid; Aboulaich, Abdelmaula; Lienafa, Livie; Bonnet, Jean-Pierre; Phan, Trang N. T.; Bertin, Denis; Gigmes, Didier; Devaux, Didier; Denoyel, Renaud; Armand, Michel (31 March 2013). "Single-ion BAB triblock copolymers as highly efficient electrolytes for
543:
electrolytes, and gel electrolytes (also known as "soggy sand" electrolytes). The most common QSSE, GPEs have a substantially different ionic conduction mechanism than SPEs, which conduct ions through the interaction with the substitutional groups of the polymer chains. Meanwhile, GPEs conduct ions
243:
The SE must be compatible with the electrode materials used in batteries as there is already a high chance of increased resistance in SSBs due to limited contact area between electrolyte and electrode materials. It should also be stable in contact with
Lithium metal. It should be lighter so that it
470:
are also gaining a lot of interest as standalone SPEs or blended with other polymers, on one side for their environmentally friendliness and on the other for their high complexation capability on the salts. Furthermore, different strategies are considered to increase the ionic conductivity of SPEs
277:
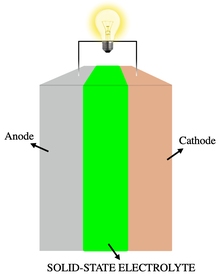
All-solid-state electrolytes are divided into inorganic solid electrolyte (ISE), solid polymer electrolyte (SPE) and composite polymer electrolyte (CPE). They are solid at room temperature and the ionic movement occurs at the solid-state. Their main advantage is the complete removal of any liquid
264:
SSEs have the same role of a traditional liquid electrolyte and they are classified into all-solid-state electrolyte and quasi-solid-state electrolyte (QSSE). All-solid-state electrolytes are furthermore divided into inorganic solid electrolyte (ISE), solid polymer electrolyte (SPE) and composite
2840:
Keller, Marlou; Appetecchi, Giovanni
Battista; Kim, Guk-Tae; Sharova, Varvara; Schneider, Meike; Schuhmacher, Jörg; Roters, Andreas; Passerini, Stefano (June 2017). "Electrochemical performance of a solvent-free hybrid ceramic-polymer electrolyte based on Li 7 La 3 Zr 2 O 12 in P(EO) 15 LiTFSI".
622:
up to 1 MPa or higher. Meanwhile, these materials can provide ionic conductivities on the order of 1 mS cm without using flammable solvents. However, gel electrolytes (i.e. "soggy sand" electrolytes) can achieve liquid-like ionic conductivities (~ 10 mS cm) while being in the solid state. Matrix
1015:
Lee, Yong-Gun; Fujiki, Satoshi; Jung, Changhoon; Suzuki, Naoki; Yashiro, Nobuyoshi; Omoda, Ryo; Ko, Dong-Su; Shiratsuchi, Tomoyuki; Sugimoto, Toshinori; Ryu, Saebom; Ku, Jun Hwan; Watanabe, Taku; Park, Youngsin; Aihara, Yuichi; Im, Dongmin; Han, In Taek (9 March 2020). "High-energy long-cycling
216:
During device or car operation the SSBs may undergo large volume variations and face mechanical stress. Also, electrochemical stability at high operating electrode potentials which are of advantage when it comes to high energy density. Hence, it is important that their mechanical, thermal, and
1520:
Bachman, John
Christopher; Muy, Sokseiha; Grimaud, Alexis; Chang, Hao-Hsun; Pour, Nir; Lux, Simon F.; Paschos, Odysseas; Maglia, Filippo; Lupart, Saskia; Lamp, Peter; Giordano, Livia; Shao-Horn, Yang (29 December 2015). "Inorganic Solid-State Electrolytes for Lithium Batteries: Mechanisms and
490:, MgO, zeolite, montmorillonite, ...), with the sole purpose of reducing the crystallinity, or active (LLTO, LLZO, LATP...) if ISE's particles are dispersed and depending on the polymer/inorganic ratio the nomenclature ceramic-in-polymer and polymer-in-ceramic is often used.
179:
Along with high ionic conductivity the candidate must have the ability to be stacked within a single package, so it supplies high energy density to the
Electric Vehicles. A high volumetric energy density is required so that the driving range of EVs can be increased between
1912:
Strangmüller, Stefan; Eickhoff, Henrik; Müller, David; Klein, Wilhelm; Raudaschl-Sieber, Gabriele; Kirchhain, Holger; Sedlmeier, Christian; Baran, Volodymyr; Senyshyn, Anatoliy; Deringer, Volker L.; van Wüllen, Leo; Gasteiger, Hubert A.; Fässler, Thomas F. (2019-09-11).
617:
as a solvent that has improved safety including non-flammability and stability at high temperatures. Matrix materials in ionogels can vary from polymer materials to inorganic nano-materials. These matrix materials (as with all QSSEs) provide mechanical stability with a
120:
However, unresolved fundamental issues remain in order to fully understand the behavior of all-solid batteries, especially in the area of electrochemical interfaces. In recent years the needs of safety and performance improvements with respect to the state-of-the-art
3003:
Liu, Xiaochen; Ding, Guoliang; Zhou, Xinhong; Li, Shizhen; He, Weisheng; Chai, Jingchao; Pang, Chunguang; Liu, Zhihong; Cui, Guanglei (2017). "An interpenetrating network poly(diethylene glycol carbonate)-based polymer electrolyte for solid state lithium batteries".
605:
are useful ways to polymerize in-situ the GPE directly in contact with the electrodes for a perfectly adherent interface. Values of ionic conductivity on the order of 1 mS cm can be easily achieved with GPEs, as demonstrate the numerous research articles published.
140:
published research on an all-solid-state battery (ASSB) using an argyrodite-based solid-state electrolyte with a demonstrated energy density of 900 Wh L and a stable cyclability of more than 1000 cycles, reaching for the first time a value close to the 1000 Wh L.
250:
If SEs contain expensive materials like Ge it will make the production cost go up significantly. The production of an exemplar SSB will require the convergence of uncomplicated fabrication technologies like particle dispersion, mechanical mixing, film formation
2510:
Rohan, Rupesh; Pareek, Kapil; Chen, Zhongxin; Cai, Weiwei; Zhang, Yunfeng; Xu, Guodong; Gao, Zhiqiang; Cheng, Hansong (2015). "A high performance polysiloxane-based single ion conducting polymeric electrolyte membrane for application in lithium ion batteries".
1993:
Asano, Tetsuya; Sakai, Akihiro; Ouchi, Satoru; Sakaida, Masashi; Miyazaki, Akinobu; Hasegawa, Shinya (November 2018). "Solid Halide
Electrolytes with High Lithium-Ion Conductivity for Application in 4 V Class Bulk-Type All-Solid-State Batteries".
1609:
Han, Xiaogang; Gong, Yunhui; Fu, Kun (Kelvin); He, Xingfeng; Hitz, Gregory T.; Dai, Jiaqi; Pearse, Alex; Liu, Boyang; Wang, Howard; Rubloff, Gary; Mo, Yifei; Thangadurai, Venkataraman; Wachsman, Eric D.; Hu, Liangbing (19 December 2016).
2565:
Liu, Bo; Huang, Yun; Cao, Haijun; Song, Amin; Lin, Yuanhua; Wang, Mingshan; Li, Xing (28 October 2017). "A high-performance and environment-friendly gel polymer electrolyte for lithium ion battery based on composited lignin membrane".
740:
Chen, Zhen; Kim, Guk-Tae; Wang, Zeli; Bresser, Dominic; Qin, Bingsheng; Geiger, Dorin; Kaiser, Ute; Wang, Xuesen; Shen, Ze Xiang; Passerini, Stefano (October 2019). "4-V flexible all-solid-state lithium polymer batteries".
255:
It is hard for one material to fulfill all the above criteria, hence a number of other approaches can be used for example a hybrid electrolyte system which combines the advantages of inorganic and polymer electrolytes.
651:
The versatility and properties of the solid-state electrolyte widen the possible applications towards high energy density and cheaper battery chemistries that are otherwise prevented by the current state-of-the-art of
1870:
Restle, Tassilo M. F.; Strangmüller, Stefan; Baran, Volodymyr; Senyshyn, Anatoliy; Kirchhain, Holger; Klein, Wilhelm; Merk, Samuel; Müller, David; Kutsch, Tobias; van Wüllen, Leo; Fässler, Thomas F. (November 2022).
3793:
Long, Canghai; Li, Libo; Zhai, Mo; Shan, Yuhang (November 2019). "Facile preparation and electrochemistry performance of quasi solid-state polymer lithium–sulfur battery with high-safety and weak shuttle effect".
2692:; Lee, Hye Ryoung; Hsu, Po-Chun; Liu, Kai; Cui, Yi (December 2015). "High Ionic Conductivity of Composite Solid Polymer Electrolyte via In Situ Synthesis of Monodispersed SiO Nanospheres in Poly(ethylene oxide)".
2438:
Zhang, Lei; Wang, Shi; Li, Jingyu; Liu, Xu; Chen, Pingping; Zhao, Tong; Zhang, Liaoyun (2019). "A nitrogen-containing all-solid-state hyperbranched polymer electrolyte for superior performance lithium batteries".
294:
state, that conducts ions by diffusion through the lattice. The main advantages of this class of solid-state electrolyte are the high ionic conductivity (of the order of a few mS cm at room-temperature), high
3417:
Bi, Haitao; Sui, Gang; Yang, Xiaoping (December 2014). "Studies on polymer nanofibre membranes with optimized core–shell structure as outstanding performance skeleton materials in gel polymer electrolytes".
2474:
Wang, Qinglei; Zhang, Huanrui; Cui, Zili; Zhou, Qian; Shangguan, Xuehui; Tian, Songwei; Zhou, Xinhong; Cui, Guanglei (December 2019). "Siloxane-based polymer electrolytes for solid-state lithium batteries".
1707:
Liu, Qi; Geng, Zhen; Han, Cuiping; Fu, Yongzhu; Li, Song; He, Yan-bing; Kang, Feiyu; Li, Baohua (June 2018). "Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries".
498:, interpenetration, and blending may also be used as polymer/polymer coordination to tune the properties of the SPEs and achieve better performances, introducing in the polymeric chains polar groups like
2113:
Senevirathne, Keerthi; Day, Cynthia S.; Gross, Michael D.; Lachgar, Abdessadek; Holzwarth, N.A.W. (February 2013). "A new crystalline LiPON electrolyte: Synthesis, properties, and electronic structure".
164:
Historically, SSBs have suffered from low ionic conductivities due to poor interfacial kinetics and mobility of ions in general. Hence an SE with a high ionic conductivity is of primary importance. High
2661:
Berthier, C.; Gorecki, W.; Minier, M.; Armand, M.B.; Chabagno, J.M.; Rigaud, P. (September 1983). "Microscopic investigation of ionic conductivity in alkali metal salts-poly(ethylene oxide) adducts".
2078:
Akin, Mert; Wang, Yuchen; Qiao, Xiaoyao; Yan, Zhiwei; Zhou, Xiangyang (September 2020). "Effect of relative humidity on the reaction kinetics in rubidium silver iodide based all-solid-state battery".
3211:
Liang, Shishuo; Yan, Wenqi; Wu, Xu; Zhang, Yi; Zhu, Yusong; Wang, Hongwei; Wu, Yuping (May 2018). "Gel polymer electrolytes for lithium ion batteries: Fabrication, characterization and performance".
3390:
Gerbaldi, C.; Nair, J.R.; Meligrana, G.; Bongiovanni, R.; Bodoardo, S.; Penazzi, N. (January 2010). "UV-curable siloxane-acrylate gel-copolymer electrolytes for lithium-based battery applications".
41:
and it is the characteristic component of the solid-state battery. It is useful for applications in electrical energy storage (EES) in substitution of the liquid electrolytes found in particular in
1657:
Kraft, Marvin A.; Ohno, Saneyuki; Zinkevich, Tatiana; Koerver, Raimund; Culver, Sean P.; Fuchs, Till; Senyshyn, Anatoliy; Indris, Sylvio; Morgan, Benjamin J.; Zeier, Wolfgang G. (November 2018).
2754:
Kumar, Binod; Scanlon, Lawrence; Marsh, Richard; Mason, Rachel; Higgins, Robert; Baldwin, Richard (March 2001). "Structural evolution and conductivity of PEO:LiBF4–MgO composite electrolytes".
414:(SPE) are defined as a solvent-free salt solution in a polymer host material that conducts ions through the polymer chains. Compared to ISEs, SPEs are much easier to process, generally by
3840:
885:
117:). The first polymeric material able to conduct ions at the solid-state was PEO, discovered in the 1970s by V. Wright. The importance of the discovery was recognized in the early 1980s.
1237:
Sundaramahalingam, K.; Muthuvinayagam, M.; Nallamuthu, N.; Vanitha, D.; Vahini, M. (1 January 2019). "Investigations on lithium acetate-doped PVA/PVP solid polymer blend electrolytes".
3262:
Watanabe, Masayoshi; Kanba, Motoi; Nagaoka, Katsuro; Shinohara, Isao (November 1982). "Ionic conductivity of hybrid films based on polyacrylonitrile and their battery application".
2403:
Mindemark, Jonas; Sun, Bing; Törmä, Erik; Brandell, Daniel (December 2015). "High-performance solid polymer electrolytes for lithium batteries operational at ambient temperature".
2327:
Webb, Michael A.; Jung, Yukyung; Pesko, Danielle M.; Savoie, Brett M.; Yamamoto, Umi; Coates, Geoffrey W.; Balsara, Nitash P.; Wang, Zhen-Gang; Miller, Thomas F. (10 July 2015).
2376:
Hu, Pu; Chai, Jingchao; Duan, Yulong; Liu, Zhihong; Cui, Guanglei; Chen, Liquan (2016). "Progress in nitrile-based polymer electrolytes for high performance lithium batteries".
474:
With the introduction of particles as fillers inside the polymer solution, a composite polymer electrolyte (CPE) is obtained, the particles can be inert to the Li conduction (Al
278:
component aimed to a greatly enhanced safety of the overall device. The main limitation is the ionic conductivity that tends to be much lower compared to a liquid counterpart.
660:
solving the key issue of the polysulfide "shuttle" effect by blocking the dissolution of polysulfide species in the electrolyte that rapidly causes a reduction of capacity.
269:
mechanism: SPEs conducts ions through the interaction with the substitutional groups of the polymer chains, while GPEs conducts ions mainly in the solvent or plasticizer.
1435:
Mindemark, Jonas; Lacey, Matthew J.; Bowden, Tim; Brandell, Daniel (June 2018). "Beyond PEO—Alternative host materials for Li + -conducting solid polymer electrolytes".
430:, but they suffer from the low room-temperature ionic conductivity (10 S cm) due to the high degree of crystallinity. The main alternatives to polyether-based SPEs are
244:
can be used in portable electronic devices. High compatibility with the electrode material can be measured through EIS analysis repeated over more consecutive days.
3453:
Lewandowski, Andrzej; Świderska-Mocek, Agnieszka (December 2009). "Ionic liquids as electrolytes for Li-ion batteries—An overview of electrochemical studies".
1794:
Beister, Heinz Jürgen; Haag, Sabine; Kniep, Rüdiger; Strössner, Klaus; Syassen, Karl (August 1988). "Phase
Transformations of Lithium Nitride under Pressure".
3031:
Rajendran, S; Sivakumar, M; Subadevi, R (February 2004). "Investigations on the effect of various plasticizers in PVA–PMMA solid polymer blend electrolytes".
850:
Kim, Taehoon; Song, Wentao; Son, Dae-Yong; Ono, Luis K.; Qi, Yabing (2019). "Lithium-ion batteries: outlook on present, future, and hybridized technologies".
89:(Toyota, BMW, Honda, Hyundai) expect to integrate these systems into viable devices and to commercialize solid-state battery-based electric vehicles by 2025.
3642:"Amine‐Functionalized Boron Nitride Nanosheets: A New Functional Additive for Robust, Flexible Ion Gel Electrolyte with High Lithium‐Ion Transference Number"
3289:
Appetecchi, G.B.; Croce, F.; Scrosati, B. (June 1995). "Kinetics and stability of the lithium electrode in poly(methylmethacrylate)-based gel electrolytes".
186:
Sufficient power density (W/L) is needed to make energy available when needed which is also a measure of how quickly charging and discharging can take place.
3357:
Verdier, Nina; Lepage, David; Zidani, Ramzi; Prébé, Arnaud; Aymé-Perrot, David; Pellerin, Christian; Dollé, Mickaël; Rochefort, Dominic (27 December 2019).
635:
due to functionalized matrix materials. These new classes of QSSEs are an active area of research to develop the optimal combination of matrix and solvent.
2300:
Sun, Bing; Mindemark, Jonas; Edström, Kristina; Brandell, Daniel (September 2014). "Polycarbonate-based solid polymer electrolytes for Li-ion batteries".
1353:
Zheng, Feng; Kotobuki, Masashi; Song, Shufeng; Lai, Man On; Lu, Li (June 2018). "Review on solid electrolytes for all-solid-state lithium-ion batteries".
1310:
Zheng, Feng; Kotobuki, Masashi; Song, Shufeng; Lai, Man On; Lu, Li (June 2018). "Review on solid electrolytes for all-solid-state lithium-ion batteries".
1566:
Zhao, Qing; Stalin, Sanjuna; Zhao, Chen-Zi; Archer, Lynden A. (5 February 2020). "Designing solid-state electrolytes for safe, energy-dense batteries".
704:
1202:
Agrawal, R C; Pandey, G P (21 November 2008). "Solid polymer electrolytes: materials designing and all-solid-state battery applications: an overview".
2141:
Mizuno, F.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. (4 April 2005). "New, Highly Ion-Conductive
Crystals Precipitated from Li2S-P2S5 Glasses".
81:, in substitution of the traditional low capacity graphite, which exhibits a theoretical capacity of 372 mAh g in its fully lithiated state of LiC
207:
170:
1275:
Appetecchi, G. B. (1996). "A New Class of
Advanced Polymer Electrolytes and Their Relevance in Plastic-like, Rechargeable Lithium Batteries".
157:(SEs) to become a major market challenger it must meet some key performance measurements. The major criteria that an SSB/SE should have are:
3585:
Hyun, Woo Jin; de Moraes, Ana C. M.; Lim, Jin-Myoung; Downing, Julia R.; Park, Kyu-Young; Tan, Mark Tian Zhi; Hersam, Mark C. (2019-08-27).
286:
Inorganic solid electrolyte (ISE) are a particular type of all-solid-state electrolyte that is constituted by an inorganic material in the
722:
557:
mechanical properties of solids and the high transport properties of liquids. A number of polymer hosts have been used in GPEs, including
2178:
1952:
Li, Yutao; Xu, Henghui; Chien, Po-Hsiu; Wu, Nan; Xin, Sen; Xue, Leigang; Park, Kyusung; Hu, Yan-Yan; Goodenough, John B. (9 July 2018).
382:. Some ISEs can be glass ceramics assuming an amorphous state instead of a regular crystalline structure. Popular examples are lithium
2538:
Jacob, M (11 December 1997). "Effect of PEO addition on the electrolytic and thermal properties of PVDF-LiClO4 polymer electrolytes".
2329:"Systematic Computational and Experimental Investigation of Lithium-Ion Transport Mechanisms in Polyester-Based Polymer Electrolytes"
3691:
Yuan, Huadong; Nai, Jianwei; Tian, He; Ju, Zhijin; Zhang, Wenkui; Liu, Yujing; Tao, Xinyong; Lou, Xiong Wen (David) (6 March 2020).
3246:
2953:
Zhang, Yuhang; Lu, Wei; Cong, Lina; Liu, Jia; Sun, Liqun; Mauger, Alain; Julien, Christian M.; Xie, Haiming; Liu, Jun (April 2019).
911:
684:
2273:
Payne, D.R.; Wright, P.V. (May 1982). "Morphology and ionic conductivity of some lithium ion complexes with poly(ethylene oxide)".
3176:
Tripathi, Alok Kumar (2021). "Ionic liquid–based solid electrolytes (ionogels) for application in rechargeable lithium battery".
2601:
Yahya, M.Z.A.; Arof, A.K. (May 2003). "Effect of oleic acid plasticizer on chitosan–lithium acetate solid polymer electrolytes".
535:
35:
3861:
86:
3318:"Effect of PEG as a plasticizer on the electrical and optical properties of polymer blend electrolyte MC-CH-LiBF4 based films"
2783:"Preparation and performance study of a PVDF–LATP ceramic composite polymer electrolyte membrane for solid-state batteries"
2954:
3758:
Li, Linlin; Li, Siyuan; Lu, Yingying (2018). "Suppression of dendritic lithium growth in lithium metal-based batteries".
2878:"PEO/garnet composite electrolytes for solid-state lithium batteries: From "ceramic-in-polymer" to "polymer-in-ceramic""
2630:"High-strength and flexible cellulose/PEG based gel polymer electrolyte with high performance for lithium ion batteries"
3640:
Kim, Donggun; Liu, Xin; Yu, Baozhi; Mateti, Srikanth; O'Dell, Luke A.; Rong, Qiangzhou; Chen, Ying (Ian) (April 2020).
3587:"High-Modulus Hexagonal Boron Nitride Nanoplatelet Gel Electrolytes for Solid-State Rechargeable Lithium-Ion Batteries"
1873:"Super‐Ionic Conductivity in ω‐ Li 9 Tr P 4 ( Tr = Al, Ga, In) and Lithium Diffusion Pathways in Li 9 AlP 4 Polymorphs"
1751:
DeWees, Rachel; Wang, Hui (24 July 2019). "Synthesis and
Properties of NaSICON‐type LATP and LAGP Solid Electrolytes".
954:
GRAY, F; MACCALLUM, J; VINCENT, C (January 1986). "Poly(ethylene oxide) - LiCF3SO3 - polystyrene electrolyte systems".
2246:
Fenton, D.E.; Parker, J.M.; Wright, P.V. (November 1973). "Complexes of alkali metal ions with poly(ethylene oxide)".
1471:
423:
266:
166:
78:
2955:"Cross-linking network based on Poly(ethylene oxide): Solid polymer electrolyte for room temperature lithium battery"
832:
1659:"Inducing High Ionic Conductivity in the Lithium Superionic Argyrodites Li P Ge S I for All-Solid-State Batteries"
2727:
Kumar, B (2 September 1999). "Polymer ceramic composite electrolytes: conductivity and thermal history effects".
2039:"Greatly enhanced energy density of all‐solid‐state rechargeable battery operating in high humidity environments"
566:
426:, thanks to the segmental motion of the polymeric chains because of the great ion complexation capability of the
203:
130:
657:
415:
230:
2628:
Zhao, Lingzhu; Fu, Jingchuan; Du, Zhi; Jia, Xiaobo; Qu, Yanyu; Yu, Feng; Du, Jie; Chen, Yong (January 2020).
570:
65:
suppression in the presence of a solid-state electrolyte membrane. The use of a high capacity anode and low
3316:
Ahmed, Hawzhin T.; Jalal, Viyan J.; Tahir, Dana A.; Mohamad, Azhin H.; Abdullah, Omed Gh. (December 2019).
3866:
679:
372:
226:
1658:
927:
Wright, Peter V. (September 1975). "Electrical conductivity in ionic complexes of poly(ethylene oxide)".
590:
218:
126:
518:
3803:
3704:
3543:
3462:
3427:
3329:
2969:
2919:
2850:
2794:
2412:
2187:
1834:
1717:
1575:
1486:
1362:
1319:
1284:
1168:
1121:
1072:
1025:
989:
632:
530:
compounds consisting of a liquid electrolyte and a solid matrix. This liquid electrolyte serves as a
411:
199:
3531:
2876:
Chen, Long; Li, Yutao; Li, Shuai-Peng; Fan, Li-Zhen; Nan, Ce-Wen; Goodenough, John B. (April 2018).
669:
578:
443:
150:
129:
very appealing and are now considered an encouraging technology to satisfy the need for long range
74:
66:
53:, higher achievable power density and cyclability. This makes possible, for example, the use of a
42:
23:
3359:"Cross-Linked Polyacrylonitrile-Based Elastomer Used as Gel Polymer Electrolyte in Li-Ion Battery"
3856:
3819:
3740:
3622:
3193:
3128:
2985:
2583:
2492:
2456:
2158:
2095:
2060:
2019:
1852:
1776:
1733:
1689:
1591:
1502:
1452:
1378:
1335:
1254:
1219:
1041:
867:
768:
628:
586:
574:
558:
527:
234:
154:
62:
980:
Janek, Jürgen; Zeier, Wolfgang G. (8 September 2016). "A solid future for battery development".
192:
Long cycle and shelf life are needed as conventional Li-ion batteries degrade after a few years.
3146:
Manuel Stephan, A. (January 2006). "Review on gel polymer electrolytes for lithium batteries".
786:
Polymer-Derived SiOC Integrated with a Graphene Aerogel As a Highly Stable Li-Ion Battery Anode
49:, low flammability, non-volatility, mechanical and thermal stability, easy processability, low
3775:
3732:
3673:
3614:
3606:
3567:
3559:
3509:
3242:
3086:
2935:
2822:
2709:
2358:
2011:
1975:
1934:
1894:
1768:
1681:
1639:
1631:
1548:
1414:
1184:
1137:
1090:
907:
594:
582:
562:
110:
1872:
573:, etc. The polymers are synthesized with increased porosity to incorporate solvents such as
3811:
3767:
3722:
3712:
3663:
3653:
3598:
3551:
3501:
3470:
3435:
3399:
3370:
3337:
3298:
3271:
3220:
3185:
3155:
3120:
3076:
3040:
3013:
2977:
2927:
2889:
2858:
2812:
2802:
2763:
2736:
2701:
2670:
2641:
2610:
2575:
2547:
2520:
2484:
2448:
2420:
2385:
2348:
2340:
2309:
2282:
2255:
2226:
2195:
2150:
2123:
2087:
2050:
2003:
1965:
1926:
1884:
1842:
1803:
1760:
1725:
1673:
1623:
1583:
1538:
1530:
1494:
1444:
1406:
1370:
1327:
1292:
1246:
1211:
1176:
1129:
1080:
1033:
997:
959:
936:
859:
811:
758:
750:
653:
491:
619:
364:
98:
2199:
1215:
3807:
3708:
3547:
3466:
3431:
3333:
2973:
2923:
2854:
2798:
2416:
2191:
2037:
Wang, Yuchen; Akin, Mert; Qiao, Xiaoyao; Yan, Zhiwei; Zhou, Xiangyang (September 2021).
1838:
1721:
1579:
1490:
1366:
1323:
1288:
1172:
1125:
1076:
1029:
993:
19:
3727:
3692:
2817:
2782:
2353:
2328:
1448:
785:
674:
503:
431:
122:
102:
50:
46:
3044:
2767:
2740:
2614:
2551:
3850:
3823:
3744:
3668:
3626:
3302:
3197:
3132:
2989:
2674:
2587:
2496:
2460:
2286:
2259:
2099:
2064:
2023:
1821:
de Jongh, P. E.; Blanchard, D.; Matsuo, M.; Udovic, T. J.; Orimo, S. (3 March 2016).
1780:
1737:
1693:
1595:
1456:
1382:
1339:
1258:
1045:
963:
871:
772:
455:
387:
296:
3403:
3159:
2162:
2091:
1856:
1823:"Complex hydrides as room-temperature solid electrolytes for rechargeable batteries"
1506:
1223:
1016:
all-solid-state lithium metal batteries enabled by silver–carbon composite anodes".
3586:
3474:
3439:
2981:
2862:
2424:
1729:
1498:
1472:"Challenges and issues facing lithium metal for solid-state rechargeable batteries"
1374:
1331:
614:
451:
222:
3488:
Osada, Irene; de Vries, Henrik; Scrosati, Bruno; Passerini, Stefano (2016-01-11).
2231:
2214:
3693:"An ultrastable lithium metal anode enabled by designed metal fluoride spansules"
3189:
2894:
2877:
2705:
2646:
2629:
1109:
816:
799:
754:
705:"Japanese Government Partners With Manufacturers On Solid State Battery Research"
57:
metal anode in a practical device, without the intrinsic limitations of a liquid
1954:"A Perovskite Electrolyte That Is Stable in Moist Air for Lithium-Ion Batteries"
1915:"Fast Ionic Conductivity in the Most Lithium-Rich Phosphidosilicate Li 14 SiP 6"
1914:
1534:
1156:
643:
598:
549:
531:
58:
38:
3815:
3342:
3317:
3275:
2488:
2344:
1612:"Negating interfacial impedance in garnet-based solid-state Li metal batteries"
1250:
593:
or other ethers or aprotic organic solvents with high dielectric constant like
3489:
3224:
3108:
2689:
2579:
2313:
2127:
1847:
1822:
1587:
1037:
602:
495:
447:
435:
383:
360:
316:
45:. The main advantages are the absolute safety, no issues of leakages of toxic
3677:
3610:
3563:
3090:
2176:
Hallinan, Daniel T.; Balsara, Nitash P. (July 2013). "Polymer Electrolytes".
1938:
1898:
1188:
1141:
1133:
1094:
1001:
3602:
1180:
467:
308:
3779:
3736:
3717:
3658:
3641:
3618:
3571:
3513:
3505:
3375:
3358:
3124:
3107:
Chen, Nan; Zhang, Haiqin; Li, Li; Chen, Renjie; Guo, Shaojun (April 2018).
3081:
3065:"Nanocomposite Ionogel Electrolytes for Solid-State Rechargeable Batteries"
3064:
2939:
2826:
2713:
2362:
2154:
2015:
2007:
1979:
1970:
1953:
1889:
1807:
1772:
1764:
1685:
1643:
1552:
1418:
1410:
940:
833:"Samsung Reveals Breakthrough: Solid-State EV Battery with 500-Mile Range"
1930:
1677:
1543:
1085:
1060:
463:
2781:
Liang, Xinghua; Han, Di; Wang, Yunting; Lan, Lingxiao; Mao, Jie (2018).
798:
Wang, Renheng; Cui, Weisheng; Chu, Fulu; Wu, Feixiang (September 2020).
763:
3771:
3555:
3109:"Ionogel Electrolytes for High-Performance Lithium Batteries: A Review"
3017:
2807:
2524:
2452:
2389:
863:
610:
553:
545:
540:
507:
439:
352:
344:
336:
312:
304:
287:
137:
70:
54:
3239:
Lithium batteries : new materials, developments, and perspectives
1635:
1611:
1430:
1428:
1296:
627:
nanoparticles are typically paired with low viscosity solvents (e.g.,
2931:
1627:
499:
459:
368:
328:
202:(the closest possible to 1) can be measured through a combination of
2055:
2038:
723:"German Federal Government Invests In Solid State Battery Research"
522:
Comparison of different polymer based quasi-solid-state electrolyes
3530:
Pfaffenhuber, C.; Göbel, M.; Popovic, J.; Maier, J. (2013-10-09).
3490:"Ionic-Liquid-Based Polymer Electrolytes for Battery Applications"
642:
517:
427:
300:
291:
18:
2215:"Review on composite polymer electrolytes for lithium batteries"
609:
Emerging subclasses of QSSEs use matrix materials and solvents.
340:
332:
97:
The first inorganic solid-state electrolytes were discovered by
1470:
Mauger, A.; Armand, M.; Julien, C.M.; Zaghib, K. (June 2017).
221:(at least tens of MPa) can be measured through a traditional
526:
Quasi solid-state electrolytes (QSSEs) are a wide class of
510:
drastically improve the dissolution of the lithium salts.
315:(lithium superionic conductor) (e.g. LGPS, LiSiPS, LiPS),
3063:
Hyun, Woo Jin; Thomas, Cory M.; Hersam, Mark C. (2020).
3171:
3169:
359:), lithium phosphidotrielates and phoshidotetrelates,
169:(at least higher than 10 S cm) can be measured through
1059:
Robinson, Arthur L.; Janek, Jürgen (December 2014).
3532:"Soggy-sand electrolytes: status and perspectives"
1796:Angewandte Chemie International Edition in English
214:Thermal, mechanical and electrochemical Stability:
1108:Janek, Jürgen; Zeier, Wolfgang G. (2016-09-08).
217:electrochemical stability are considered. High
647:The uncontrolled formation of lithium dendrites
339:(sodium superionic conductor) (e.g. LTP, LATP,
138:Samsung Advanced Institute of Technology (SAIT)
311:-based and the crystalline structures include
73:with a specific capacity of 3860 mAh g and a
8:
2213:Manuel Stephan, A.; Nahm, K.S. (July 2006).
171:electrochemical impedance spectroscopy (EIS)
3796:Journal of Physics and Chemistry of Solids
800:"Lithium metal anodes: Present and future"
3726:
3716:
3667:
3657:
3374:
3341:
3080:
2893:
2816:
2806:
2645:
2352:
2230:
2054:
1969:
1888:
1846:
1542:
1084:
815:
762:
458:(e.g. PVDF, PVDF-HFP). Bio-polymers like
229:(at least 4-5 V) can be measured through
16:Type of solid ionic conductor electrolyte
2043:International Journal of Energy Research
1919:Journal of the American Chemical Society
1666:Journal of the American Chemical Society
1110:"A solid future for battery development"
471:and the amorphous-to-crystalline ratio.
3494:Angewandte Chemie International Edition
2568:Journal of Solid State Electrochemistry
1958:Angewandte Chemie International Edition
696:
227:electrochemical stability windows (ESW)
101:in the nineteenth century, these being
36:ionic conductor and electron-insulating
1521:Properties Governing Ion Conduction".
1277:Journal of the Electrochemical Society
3525:
3523:
3102:
3100:
3058:
3056:
3054:
1270:
1268:
1204:Journal of Physics D: Applied Physics
1061:"Solid-state batteries enter EV fray"
975:
973:
788:Applied Materials and Interfaces 2020
7:
2200:10.1146/annurev-matsci-071312-121705
3536:Physical Chemistry Chemical Physics
2179:Annual Review of Materials Research
363:(e.g. lithium lanthanum titanate, "
3264:Journal of Applied Polymer Science
1449:10.1016/j.progpolymsci.2017.12.004
831:Baldwin, Roberto (12 March 2020).
597:can also be mixed the SPE matrix.
248:Economic fabrication technologies:
61:thanks to the property of lithium
14:
685:Research in lithium-ion batteries
282:Inorganic solid electrolyte (ISE)
26:with the solid-state electrolyte.
3006:Journal of Materials Chemistry A
2513:Journal of Materials Chemistry A
2441:Journal of Materials Chemistry A
2378:Journal of Materials Chemistry A
852:Journal of Materials Chemistry A
3404:10.1016/j.electacta.2009.05.055
3160:10.1016/j.eurpolymj.2005.09.017
2092:10.1016/j.electacta.2020.136779
406:Solid polymer electrolyte (SPE)
3475:10.1016/j.jpowsour.2009.06.089
3440:10.1016/j.jpowsour.2014.05.030
2982:10.1016/j.jpowsour.2019.02.090
2863:10.1016/j.jpowsour.2017.04.014
2425:10.1016/j.jpowsour.2015.08.035
1730:10.1016/j.jpowsour.2018.04.019
1499:10.1016/j.jpowsour.2017.04.018
1375:10.1016/j.jpowsour.2018.04.022
1332:10.1016/j.jpowsour.2018.04.022
1216:10.1088/0022-3727/41/22/223001
906:. Cambridge University Press.
231:linear sweep voltammetry (LSV)
1:
3646:Advanced Functional Materials
3045:10.1016/S0167-577X(03)00585-8
2768:10.1016/S0013-4686(00)00747-7
2741:10.1016/S0167-2738(99)00148-4
2615:10.1016/S0014-3057(02)00355-5
2552:10.1016/S0167-2738(97)00422-0
2232:10.1016/j.polymer.2006.05.069
1877:Advanced Functional Materials
1155:Hu, Yong-Sheng (2016-04-07).
514:Quasi-solid-state electrolyte
3363:ACS Applied Energy Materials
3303:10.1016/0013-4686(94)00345-2
3190:10.1016/j.mtener.2021.100643
2895:10.1016/j.nanoen.2017.12.037
2706:10.1021/acs.nanolett.5b04117
2675:10.1016/0167-2738(83)90068-1
2647:10.1016/j.memsci.2019.117428
2287:10.1016/0032-3861(82)90052-0
2260:10.1016/0032-3861(73)90146-8
964:10.1016/0167-2738(86)90127-X
904:Solid State Electrochemistry
817:10.1016/j.jechem.2019.12.024
755:10.1016/j.nanoen.2019.103986
589:(DMC). Low molecular weight
2634:Journal of Membrane Science
1535:10.1021/acs.chemrev.5b00563
1437:Progress in Polymer Science
804:Journal of Energy Chemistry
591:poly(ethylene glycol) (PEG)
273:All-solid-state electrolyte
3883:
3816:10.1016/j.jpcs.2019.06.017
3343:10.1016/j.rinp.2019.102735
3276:10.1002/app.1982.070271110
2910:lithium-metal batteries".
2688:Lin, Dingchang; Liu, Wei;
2489:10.1016/j.ensm.2019.04.016
2345:10.1021/acscentsci.5b00195
1251:10.1007/s00289-018-02670-2
1157:"Batteries: Getting solid"
196:Ionic transference number:
177:Volumetric Energy Density:
3225:10.1016/j.ssi.2017.12.023
3113:Advanced Energy Materials
3069:Advanced Energy Materials
2580:10.1007/s10008-017-3814-x
2314:10.1016/j.ssi.2013.08.014
2128:10.1016/j.ssi.2012.12.013
1848:10.1007/s00339-016-9807-2
1588:10.1038/s41578-019-0165-5
1038:10.1038/s41560-020-0575-z
200:ionic transference number
131:battery electric vehicles
3455:Journal of Power Sources
3420:Journal of Power Sources
3148:European Polymer Journal
2962:Journal of Power Sources
2843:Journal of Power Sources
2603:European Polymer Journal
2477:Energy Storage Materials
2405:Journal of Power Sources
1710:Journal of Power Sources
1568:Nature Reviews Materials
1479:Journal of Power Sources
1355:Journal of Power Sources
1312:Journal of Power Sources
1134:10.1038/nenergy.2016.141
1002:10.1038/nenergy.2016.141
658:lithium-sulfur batteries
595:dimethylsulfoxide (DMSO)
386:(LIPON) and the lithium
3843:. Retrieved 2020-06-26.
3760:Chemical Communications
3603:10.1021/acsnano.9b04989
1181:10.1038/nenergy.2016.42
929:British Polymer Journal
886:"Solid-State Batteries"
235:cyclic voltammetry (CV)
32:solid-state electrolyte
24:All Solid-State Battery
3862:Rechargeable batteries
3718:10.1126/sciadv.aaz3112
3659:10.1002/adfm.201910813
3506:10.1002/anie.201504971
3376:10.1021/acsaem.9b02129
3178:Materials Today Energy
3125:10.1002/aenm.201702675
3082:10.1002/aenm.202002135
2155:10.1002/adma.200401286
2008:10.1002/adma.201803075
1971:10.1002/anie.201804114
1890:10.1002/adfm.202112377
1808:10.1002/anie.198811011
1765:10.1002/cssc.201900725
1411:10.1002/cssc.201700977
680:Lithium-sulfur battery
648:
523:
204:chronoamperometry (CA)
27:
3669:10536/DRO/DU:30135199
941:10.1002/pi.4980070505
646:
623:materials such as SiO
521:
384:phosphorus oxynitride
151:Solid State Batteries
127:solid-state batteries
22:
2762:(10–11): 1515–1521.
1931:10.1021/jacs.9b05301
1678:10.1021/jacs.8b10282
1086:10.1557/mrs.2014.285
133:of the near future.
3841:Solid-state battery
3808:2019JPCS..134..255L
3709:2020SciA....6.3112Y
3548:2013PCCP...1518318P
3542:(42): 18318–18335.
3467:2009JPS...194..601L
3432:2014JPS...267..309B
3392:Electrochimica Acta
3334:2019ResPh..1502735A
3291:Electrochimica Acta
3012:(22): 11124–11130.
2974:2019JPS...420...63Z
2924:2013NatMa..12..452B
2855:2017JPS...353..287K
2799:2018RSCAd...840498L
2793:(71): 40498–40504.
2756:Electrochimica Acta
2519:(40): 20267–20276.
2417:2015JPS...298..166M
2384:(26): 10070–10083.
2333:ACS Central Science
2192:2013AnRMS..43..503H
2080:Electrochimica Acta
2049:(11): 16794–16805.
1925:(36): 14200–14209.
1839:2016ApPhA.122..251D
1722:2018JPS...389..120L
1672:(47): 16330–16339.
1580:2020NatRM...5..229Z
1491:2017JPS...353..333M
1367:2018JPS...389..198Z
1324:2018JPS...389..198Z
1289:1996JElS..143....6A
1173:2016NatEn...116042H
1126:2016NatEn...116141J
1077:2014MRSBu..39.1046R
1030:2020NatEn...5..299L
994:2016NatEn...116141J
670:Solid-state battery
633:transference number
579:propylene carbonate
412:polymer electrolyte
327:X, X = Cl, Br, I),
219:mechanical strength
162:Ionic conductivity:
136:In March 2020, the
75:reduction potential
67:reduction potential
43:lithium-ion battery
3772:10.1039/C8CC02280A
3556:10.1039/C3CP53124D
3322:Results in Physics
3241:. Elsevier. 1994.
3213:Solid State Ionics
3018:10.1039/C7TA02423A
2808:10.1039/C8RA08436J
2729:Solid State Ionics
2663:Solid State Ionics
2540:Solid State Ionics
2525:10.1039/c5ta02628h
2453:10.1039/C9TA00180H
2390:10.1039/C6TA02907H
2302:Solid State Ionics
2143:Advanced Materials
2116:Solid State Ionics
1996:Advanced Materials
958:. 18–19: 282–286.
956:Solid State Ionics
864:10.1039/c8ta10513h
729:. 29 October 2018.
649:
629:ethylene carbonate
613:, for example use
587:dimethyl carbonate
575:ethylene carbonate
524:
167:ionic conductivity
155:Solid Electrolytes
28:
3766:(50): 6648–6661.
3270:(11): 4191–4198.
3033:Materials Letters
2447:(12): 6801–6808.
2225:(16): 5952–5964.
1964:(28): 8587–8591.
1827:Applied Physics A
1759:(16): 3713–3725.
1405:(17): 3490–3496.
1297:10.1149/1.1836379
1245:(11): 5577–5602.
1071:(12): 1046–1047.
583:diethyl carbonate
111:lead(II) fluoride
34:(SSE) is a solid
3874:
3828:
3827:
3790:
3784:
3783:
3755:
3749:
3748:
3730:
3720:
3703:(10): eaaz3112.
3697:Science Advances
3688:
3682:
3681:
3671:
3661:
3637:
3631:
3630:
3597:(8): 9664–9672.
3582:
3576:
3575:
3527:
3518:
3517:
3485:
3479:
3478:
3450:
3444:
3443:
3414:
3408:
3407:
3398:(4): 1460–1467.
3387:
3381:
3380:
3378:
3369:(1): 1099–1110.
3354:
3348:
3347:
3345:
3313:
3307:
3306:
3286:
3280:
3279:
3259:
3253:
3252:
3235:
3229:
3228:
3208:
3202:
3201:
3173:
3164:
3163:
3143:
3137:
3136:
3104:
3095:
3094:
3084:
3060:
3049:
3048:
3028:
3022:
3021:
3000:
2994:
2993:
2959:
2950:
2944:
2943:
2932:10.1038/nmat3602
2912:Nature Materials
2906:
2900:
2899:
2897:
2873:
2867:
2866:
2837:
2831:
2830:
2820:
2810:
2778:
2772:
2771:
2751:
2745:
2744:
2735:(3–4): 239–254.
2724:
2718:
2717:
2685:
2679:
2678:
2658:
2652:
2651:
2649:
2625:
2619:
2618:
2598:
2592:
2591:
2562:
2556:
2555:
2546:(3–4): 267–276.
2535:
2529:
2528:
2507:
2501:
2500:
2471:
2465:
2464:
2435:
2429:
2428:
2400:
2394:
2393:
2373:
2367:
2366:
2356:
2324:
2318:
2317:
2297:
2291:
2290:
2270:
2264:
2263:
2243:
2237:
2236:
2234:
2210:
2204:
2203:
2173:
2167:
2166:
2138:
2132:
2131:
2110:
2104:
2103:
2075:
2069:
2068:
2058:
2034:
2028:
2027:
1990:
1984:
1983:
1973:
1949:
1943:
1942:
1909:
1903:
1902:
1892:
1867:
1861:
1860:
1850:
1818:
1812:
1811:
1802:(8): 1101–1103.
1791:
1785:
1784:
1748:
1742:
1741:
1704:
1698:
1697:
1663:
1654:
1648:
1647:
1628:10.1038/nmat4821
1616:Nature Materials
1606:
1600:
1599:
1563:
1557:
1556:
1546:
1523:Chemical Reviews
1517:
1511:
1510:
1476:
1467:
1461:
1460:
1432:
1423:
1422:
1393:
1387:
1386:
1350:
1344:
1343:
1307:
1301:
1300:
1272:
1263:
1262:
1239:Polymer Bulletin
1234:
1228:
1227:
1199:
1193:
1192:
1152:
1146:
1145:
1105:
1099:
1098:
1088:
1056:
1050:
1049:
1012:
1006:
1005:
977:
968:
967:
951:
945:
944:
924:
918:
917:
900:
894:
893:
882:
876:
875:
858:(7): 2942–2964.
847:
841:
840:
828:
822:
821:
819:
795:
789:
783:
777:
776:
766:
737:
731:
730:
719:
713:
712:
701:
654:Li-ion batteries
548:, which acts as
492:Copolymerization
454:(e.g. PDMS) and
416:solution casting
267:ionic conduction
123:Li-ion chemistry
47:organic solvents
3882:
3881:
3877:
3876:
3875:
3873:
3872:
3871:
3847:
3846:
3837:
3832:
3831:
3792:
3791:
3787:
3757:
3756:
3752:
3690:
3689:
3685:
3652:(15): 1910813.
3639:
3638:
3634:
3584:
3583:
3579:
3529:
3528:
3521:
3487:
3486:
3482:
3452:
3451:
3447:
3416:
3415:
3411:
3389:
3388:
3384:
3356:
3355:
3351:
3315:
3314:
3310:
3288:
3287:
3283:
3261:
3260:
3256:
3249:
3237:
3236:
3232:
3210:
3209:
3205:
3175:
3174:
3167:
3145:
3144:
3140:
3119:(12): 1702675.
3106:
3105:
3098:
3075:(36): 2002135.
3062:
3061:
3052:
3030:
3029:
3025:
3002:
3001:
2997:
2957:
2952:
2951:
2947:
2908:
2907:
2903:
2875:
2874:
2870:
2839:
2838:
2834:
2780:
2779:
2775:
2753:
2752:
2748:
2726:
2725:
2721:
2687:
2686:
2682:
2660:
2659:
2655:
2627:
2626:
2622:
2600:
2599:
2595:
2564:
2563:
2559:
2537:
2536:
2532:
2509:
2508:
2504:
2473:
2472:
2468:
2437:
2436:
2432:
2402:
2401:
2397:
2375:
2374:
2370:
2326:
2325:
2321:
2299:
2298:
2294:
2272:
2271:
2267:
2245:
2244:
2240:
2212:
2211:
2207:
2175:
2174:
2170:
2140:
2139:
2135:
2112:
2111:
2107:
2077:
2076:
2072:
2056:10.1002/er.6928
2036:
2035:
2031:
2002:(44): 1803075.
1992:
1991:
1987:
1951:
1950:
1946:
1911:
1910:
1906:
1883:(46): 2112377.
1869:
1868:
1864:
1820:
1819:
1815:
1793:
1792:
1788:
1750:
1749:
1745:
1706:
1705:
1701:
1661:
1656:
1655:
1651:
1608:
1607:
1603:
1565:
1564:
1560:
1519:
1518:
1514:
1474:
1469:
1468:
1464:
1434:
1433:
1426:
1395:
1394:
1390:
1352:
1351:
1347:
1309:
1308:
1304:
1274:
1273:
1266:
1236:
1235:
1231:
1201:
1200:
1196:
1154:
1153:
1149:
1107:
1106:
1102:
1058:
1057:
1053:
1014:
1013:
1009:
979:
978:
971:
953:
952:
948:
926:
925:
921:
914:
902:
901:
897:
884:
883:
879:
849:
848:
844:
830:
829:
825:
797:
796:
792:
784:
780:
739:
738:
734:
721:
720:
716:
703:
702:
698:
693:
666:
641:
626:
516:
489:
485:
481:
477:
421:
408:
401:
397:
393:
380:
376:
358:
350:
326:
322:
317:argyrodite-like
284:
275:
262:
147:
116:
108:
99:Michael Faraday
95:
84:
17:
12:
11:
5:
3880:
3878:
3870:
3869:
3864:
3859:
3849:
3848:
3845:
3844:
3836:
3835:External links
3833:
3830:
3829:
3785:
3750:
3683:
3632:
3577:
3519:
3500:(2): 500–513.
3480:
3461:(2): 601–609.
3445:
3409:
3382:
3349:
3308:
3297:(8): 991–997.
3281:
3254:
3247:
3230:
3203:
3165:
3138:
3096:
3050:
3039:(5): 641–649.
3023:
2995:
2945:
2918:(5): 452–457.
2901:
2868:
2832:
2773:
2746:
2719:
2700:(1): 459–465.
2680:
2653:
2620:
2609:(5): 897–902.
2593:
2574:(3): 807–816.
2557:
2530:
2502:
2466:
2430:
2395:
2368:
2339:(4): 198–205.
2319:
2292:
2281:(5): 690–693.
2265:
2238:
2205:
2186:(1): 503–525.
2168:
2149:(7): 918–921.
2133:
2105:
2070:
2029:
1985:
1944:
1904:
1862:
1813:
1786:
1743:
1699:
1649:
1622:(5): 572–579.
1601:
1574:(3): 229–252.
1558:
1529:(1): 140–162.
1512:
1462:
1424:
1388:
1345:
1302:
1264:
1229:
1210:(22): 223001.
1194:
1147:
1100:
1051:
1024:(4): 299–308.
1007:
969:
946:
935:(5): 319–327.
919:
912:
895:
892:. 6 July 2019.
877:
842:
837:Car and Driver
823:
790:
778:
732:
714:
695:
694:
692:
689:
688:
687:
682:
677:
675:Li-ion battery
672:
665:
662:
640:
637:
624:
620:storage moduli
544:mainly in the
536:ion conduction
515:
512:
487:
483:
479:
475:
456:fluoropolymers
432:polycarbonates
419:
407:
404:
399:
395:
391:
388:thiophosphates
378:
374:
356:
348:
324:
320:
283:
280:
274:
271:
261:
258:
253:
252:
245:
241:Compatibility:
238:
211:
193:
187:
184:Power density:
181:
174:
146:
143:
114:
106:
103:silver sulfide
94:
91:
82:
77:of -3.04 V vs
51:self-discharge
15:
13:
10:
9:
6:
4:
3:
2:
3879:
3868:
3867:Battery types
3865:
3863:
3860:
3858:
3855:
3854:
3852:
3842:
3839:
3838:
3834:
3825:
3821:
3817:
3813:
3809:
3805:
3801:
3797:
3789:
3786:
3781:
3777:
3773:
3769:
3765:
3761:
3754:
3751:
3746:
3742:
3738:
3734:
3729:
3724:
3719:
3714:
3710:
3706:
3702:
3698:
3694:
3687:
3684:
3679:
3675:
3670:
3665:
3660:
3655:
3651:
3647:
3643:
3636:
3633:
3628:
3624:
3620:
3616:
3612:
3608:
3604:
3600:
3596:
3592:
3588:
3581:
3578:
3573:
3569:
3565:
3561:
3557:
3553:
3549:
3545:
3541:
3537:
3533:
3526:
3524:
3520:
3515:
3511:
3507:
3503:
3499:
3495:
3491:
3484:
3481:
3476:
3472:
3468:
3464:
3460:
3456:
3449:
3446:
3441:
3437:
3433:
3429:
3425:
3421:
3413:
3410:
3405:
3401:
3397:
3393:
3386:
3383:
3377:
3372:
3368:
3364:
3360:
3353:
3350:
3344:
3339:
3335:
3331:
3327:
3323:
3319:
3312:
3309:
3304:
3300:
3296:
3292:
3285:
3282:
3277:
3273:
3269:
3265:
3258:
3255:
3250:
3248:9780444899576
3244:
3240:
3234:
3231:
3226:
3222:
3218:
3214:
3207:
3204:
3199:
3195:
3191:
3187:
3183:
3179:
3172:
3170:
3166:
3161:
3157:
3153:
3149:
3142:
3139:
3134:
3130:
3126:
3122:
3118:
3114:
3110:
3103:
3101:
3097:
3092:
3088:
3083:
3078:
3074:
3070:
3066:
3059:
3057:
3055:
3051:
3046:
3042:
3038:
3034:
3027:
3024:
3019:
3015:
3011:
3007:
2999:
2996:
2991:
2987:
2983:
2979:
2975:
2971:
2967:
2963:
2956:
2949:
2946:
2941:
2937:
2933:
2929:
2925:
2921:
2917:
2913:
2905:
2902:
2896:
2891:
2887:
2883:
2879:
2872:
2869:
2864:
2860:
2856:
2852:
2848:
2844:
2836:
2833:
2828:
2824:
2819:
2814:
2809:
2804:
2800:
2796:
2792:
2788:
2784:
2777:
2774:
2769:
2765:
2761:
2757:
2750:
2747:
2742:
2738:
2734:
2730:
2723:
2720:
2715:
2711:
2707:
2703:
2699:
2695:
2691:
2684:
2681:
2676:
2672:
2668:
2664:
2657:
2654:
2648:
2643:
2639:
2635:
2631:
2624:
2621:
2616:
2612:
2608:
2604:
2597:
2594:
2589:
2585:
2581:
2577:
2573:
2569:
2561:
2558:
2553:
2549:
2545:
2541:
2534:
2531:
2526:
2522:
2518:
2514:
2506:
2503:
2498:
2494:
2490:
2486:
2482:
2478:
2470:
2467:
2462:
2458:
2454:
2450:
2446:
2442:
2434:
2431:
2426:
2422:
2418:
2414:
2410:
2406:
2399:
2396:
2391:
2387:
2383:
2379:
2372:
2369:
2364:
2360:
2355:
2350:
2346:
2342:
2338:
2334:
2330:
2323:
2320:
2315:
2311:
2307:
2303:
2296:
2293:
2288:
2284:
2280:
2276:
2269:
2266:
2261:
2257:
2253:
2249:
2242:
2239:
2233:
2228:
2224:
2220:
2216:
2209:
2206:
2201:
2197:
2193:
2189:
2185:
2181:
2180:
2172:
2169:
2164:
2160:
2156:
2152:
2148:
2144:
2137:
2134:
2129:
2125:
2121:
2117:
2109:
2106:
2101:
2097:
2093:
2089:
2085:
2081:
2074:
2071:
2066:
2062:
2057:
2052:
2048:
2044:
2040:
2033:
2030:
2025:
2021:
2017:
2013:
2009:
2005:
2001:
1997:
1989:
1986:
1981:
1977:
1972:
1967:
1963:
1959:
1955:
1948:
1945:
1940:
1936:
1932:
1928:
1924:
1920:
1916:
1908:
1905:
1900:
1896:
1891:
1886:
1882:
1878:
1874:
1866:
1863:
1858:
1854:
1849:
1844:
1840:
1836:
1832:
1828:
1824:
1817:
1814:
1809:
1805:
1801:
1797:
1790:
1787:
1782:
1778:
1774:
1770:
1766:
1762:
1758:
1754:
1747:
1744:
1739:
1735:
1731:
1727:
1723:
1719:
1715:
1711:
1703:
1700:
1695:
1691:
1687:
1683:
1679:
1675:
1671:
1667:
1660:
1653:
1650:
1645:
1641:
1637:
1633:
1629:
1625:
1621:
1617:
1613:
1605:
1602:
1597:
1593:
1589:
1585:
1581:
1577:
1573:
1569:
1562:
1559:
1554:
1550:
1545:
1544:1721.1/109539
1540:
1536:
1532:
1528:
1524:
1516:
1513:
1508:
1504:
1500:
1496:
1492:
1488:
1484:
1480:
1473:
1466:
1463:
1458:
1454:
1450:
1446:
1442:
1438:
1431:
1429:
1425:
1420:
1416:
1412:
1408:
1404:
1400:
1392:
1389:
1384:
1380:
1376:
1372:
1368:
1364:
1360:
1356:
1349:
1346:
1341:
1337:
1333:
1329:
1325:
1321:
1317:
1313:
1306:
1303:
1298:
1294:
1290:
1286:
1282:
1278:
1271:
1269:
1265:
1260:
1256:
1252:
1248:
1244:
1240:
1233:
1230:
1225:
1221:
1217:
1213:
1209:
1205:
1198:
1195:
1190:
1186:
1182:
1178:
1174:
1170:
1166:
1162:
1161:Nature Energy
1158:
1151:
1148:
1143:
1139:
1135:
1131:
1127:
1123:
1119:
1115:
1114:Nature Energy
1111:
1104:
1101:
1096:
1092:
1087:
1082:
1078:
1074:
1070:
1066:
1062:
1055:
1052:
1047:
1043:
1039:
1035:
1031:
1027:
1023:
1019:
1018:Nature Energy
1011:
1008:
1003:
999:
995:
991:
987:
983:
982:Nature Energy
976:
974:
970:
965:
961:
957:
950:
947:
942:
938:
934:
930:
923:
920:
915:
913:9780511524790
909:
905:
899:
896:
891:
887:
881:
878:
873:
869:
865:
861:
857:
853:
846:
843:
838:
834:
827:
824:
818:
813:
809:
805:
801:
794:
791:
787:
782:
779:
774:
770:
765:
760:
756:
752:
748:
744:
736:
733:
728:
727:CleanTechnica
724:
718:
715:
711:. 7 May 2018.
710:
709:CleanTechnica
706:
700:
697:
690:
686:
683:
681:
678:
676:
673:
671:
668:
667:
663:
661:
659:
655:
645:
639:Opportunities
638:
636:
634:
630:
621:
616:
615:ionic liquids
612:
607:
604:
603:cross-linking
600:
596:
592:
588:
584:
580:
576:
572:
568:
564:
560:
555:
551:
547:
542:
537:
533:
529:
520:
513:
511:
509:
505:
501:
497:
493:
472:
469:
465:
461:
457:
453:
449:
445:
441:
437:
433:
429:
425:
417:
413:
405:
403:
389:
385:
381:
370:
366:
362:
354:
346:
342:
338:
334:
330:
318:
314:
310:
306:
302:
298:
293:
289:
281:
279:
272:
270:
268:
259:
257:
249:
246:
242:
239:
236:
232:
228:
224:
220:
215:
212:
209:
205:
201:
197:
194:
191:
188:
185:
182:
178:
175:
172:
168:
163:
160:
159:
158:
156:
152:
144:
142:
139:
134:
132:
128:
124:
118:
112:
104:
100:
92:
90:
88:
80:
76:
72:
68:
64:
60:
56:
52:
48:
44:
40:
37:
33:
25:
21:
3799:
3795:
3788:
3763:
3759:
3753:
3700:
3696:
3686:
3649:
3645:
3635:
3594:
3590:
3580:
3539:
3535:
3497:
3493:
3483:
3458:
3454:
3448:
3423:
3419:
3412:
3395:
3391:
3385:
3366:
3362:
3352:
3325:
3321:
3311:
3294:
3290:
3284:
3267:
3263:
3257:
3238:
3233:
3216:
3212:
3206:
3181:
3177:
3154:(1): 21–42.
3151:
3147:
3141:
3116:
3112:
3072:
3068:
3036:
3032:
3026:
3009:
3005:
2998:
2965:
2961:
2948:
2915:
2911:
2904:
2885:
2881:
2871:
2846:
2842:
2835:
2790:
2787:RSC Advances
2786:
2776:
2759:
2755:
2749:
2732:
2728:
2722:
2697:
2694:Nano Letters
2693:
2683:
2669:(1): 91–95.
2666:
2662:
2656:
2637:
2633:
2623:
2606:
2602:
2596:
2571:
2567:
2560:
2543:
2539:
2533:
2516:
2512:
2505:
2480:
2476:
2469:
2444:
2440:
2433:
2408:
2404:
2398:
2381:
2377:
2371:
2336:
2332:
2322:
2305:
2301:
2295:
2278:
2274:
2268:
2251:
2247:
2241:
2222:
2218:
2208:
2183:
2177:
2171:
2146:
2142:
2136:
2119:
2115:
2108:
2083:
2079:
2073:
2046:
2042:
2032:
1999:
1995:
1988:
1961:
1957:
1947:
1922:
1918:
1907:
1880:
1876:
1865:
1830:
1826:
1816:
1799:
1795:
1789:
1756:
1752:
1746:
1713:
1709:
1702:
1669:
1665:
1652:
1619:
1615:
1604:
1571:
1567:
1561:
1526:
1522:
1515:
1482:
1478:
1465:
1440:
1436:
1402:
1398:
1391:
1358:
1354:
1348:
1315:
1311:
1305:
1280:
1276:
1242:
1238:
1232:
1207:
1203:
1197:
1167:(4): 16042.
1164:
1160:
1150:
1120:(9): 16141.
1117:
1113:
1103:
1068:
1065:MRS Bulletin
1064:
1054:
1021:
1017:
1010:
988:(9): 16141.
985:
981:
955:
949:
932:
928:
922:
903:
898:
890:FutureBridge
889:
880:
855:
851:
845:
836:
826:
807:
803:
793:
781:
764:10356/149966
746:
742:
735:
726:
717:
708:
699:
650:
608:
601:and thermal
525:
496:crosslinking
473:
452:polysiloxane
450:(e.g. PEI),
446:(e.g. PVA),
444:polyalcohols
442:(e.g. PAN),
440:polynitriles
428:ether groups
409:
371:(LYC, LYB).,
367:"), lithium
351:N), lithium
285:
276:
263:
254:
247:
240:
223:tensile test
213:
195:
189:
183:
176:
161:
148:
135:
119:
96:
31:
29:
3802:: 255–261.
3426:: 309–315.
2888:: 176–184.
2882:Nano Energy
2849:: 287–297.
2690:Liu, Yayuan
2483:: 466–490.
2411:: 166–170.
2308:: 738–742.
2254:(11): 589.
1753:ChemSusChem
1716:: 120–134.
1485:: 333–342.
1443:: 114–143.
1399:ChemSusChem
1361:: 198–213.
1318:: 198–213.
1283:(1): 6–12.
810:: 145–159.
743:Nano Energy
585:(DEC), and
550:plasticizer
534:pathway of
532:percolating
424:ion hopping
361:perovskites
343:), lithium
288:crystalline
190:Cycle life:
125:are making
59:electrolyte
3851:Categories
3328:: 102735.
3184:: 100643.
2640:: 117428.
2122:: 95–101.
2086:: 136779.
1833:(3): 251.
749:: 103986.
691:References
448:polyamines
436:polyesters
309:phosphates
260:Categories
145:Properties
3857:Chemistry
3824:197395956
3745:212739571
3678:1616-301X
3627:197665200
3611:1936-0851
3564:1463-9084
3198:233581904
3133:102749351
3091:1614-6840
2990:107653475
2968:: 63–72.
2588:103666062
2497:149575379
2461:104471195
2100:225553692
2065:236256757
2024:205288274
1939:0002-7863
1899:1616-301X
1781:167209150
1738:104174556
1694:207195755
1596:211028485
1457:102876830
1383:104174202
1340:104174202
1259:104442538
1189:2058-7546
1142:2058-7546
1095:0883-7694
1046:216386265
872:104366580
773:201287650
528:composite
504:carbonyls
468:cellulose
210:analysis.
173:analysis.
153:(SSBs) /
3780:29796542
3737:32181364
3619:31318524
3591:ACS Nano
3572:24080900
3514:26783056
3219:: 2–18.
2940:23542871
2827:35557886
2714:26595277
2363:27162971
2163:95505293
2016:30216562
1980:29734500
1857:53402745
1773:31132230
1686:30380843
1644:27992420
1553:26713396
1507:99108693
1419:28731629
1224:94704160
664:See also
611:Ionogels
571:PVDF-HFP
508:nitriles
464:chitosan
353:hydrides
347:(e.g. Li
345:nitrides
319:(e.g. Li
305:sulfides
180:charges.
63:dendrite
39:material
3804:Bibcode
3728:7060059
3705:Bibcode
3544:Bibcode
3463:Bibcode
3428:Bibcode
3330:Bibcode
2970:Bibcode
2920:Bibcode
2851:Bibcode
2818:9091465
2795:Bibcode
2413:Bibcode
2354:4827473
2275:Polymer
2248:Polymer
2219:Polymer
2188:Bibcode
1835:Bibcode
1718:Bibcode
1636:1433807
1576:Bibcode
1487:Bibcode
1363:Bibcode
1320:Bibcode
1285:Bibcode
1169:Bibcode
1122:Bibcode
1073:Bibcode
1026:Bibcode
990:Bibcode
554:solvent
546:solvent
541:Ionogel
369:halides
337:NASICON
329:garnets
313:LISICON
297:modulus
225:. Wide
109:S) and
93:History
71:lithium
69:, like
55:lithium
3822:
3778:
3743:
3735:
3725:
3676:
3625:
3617:
3609:
3570:
3562:
3512:
3245:
3196:
3131:
3089:
2988:
2938:
2825:
2815:
2712:
2586:
2495:
2459:
2361:
2351:
2161:
2098:
2063:
2022:
2014:
1978:
1937:
1897:
1855:
1779:
1771:
1736:
1692:
1684:
1642:
1634:
1594:
1551:
1505:
1455:
1417:
1381:
1338:
1257:
1222:
1187:
1140:
1093:
1044:
910:
870:
771:
581:(PC),
577:(EC),
552:. The
500:ethers
460:lignin
410:Solid
301:oxides
292:glassy
3820:S2CID
3741:S2CID
3623:S2CID
3194:S2CID
3129:S2CID
2986:S2CID
2958:(PDF)
2584:S2CID
2493:S2CID
2457:S2CID
2159:S2CID
2096:S2CID
2061:S2CID
2020:S2CID
1853:S2CID
1777:S2CID
1734:S2CID
1690:S2CID
1662:(PDF)
1592:S2CID
1503:S2CID
1475:(PDF)
1453:S2CID
1379:S2CID
1336:S2CID
1255:S2CID
1220:S2CID
1042:S2CID
868:S2CID
769:S2CID
486:, SiO
482:, TiO
355:(LiBH
198:High
3776:PMID
3733:PMID
3674:ISSN
3615:PMID
3607:ISSN
3568:PMID
3560:ISSN
3510:PMID
3243:ISBN
3087:ISSN
2936:PMID
2823:PMID
2710:PMID
2359:PMID
2012:PMID
1976:PMID
1935:ISSN
1895:ISSN
1769:PMID
1682:PMID
1640:PMID
1632:OSTI
1549:PMID
1415:PMID
1185:ISSN
1138:ISSN
1091:ISSN
908:ISBN
567:PMMA
466:and
373:RbAg
365:LLTO
341:LAGP
333:LLZO
251:etc.
206:and
149:For
113:(PbF
87:OEMs
3812:doi
3800:134
3768:doi
3723:PMC
3713:doi
3664:hdl
3654:doi
3599:doi
3552:doi
3502:doi
3471:doi
3459:194
3436:doi
3424:267
3400:doi
3371:doi
3338:doi
3299:doi
3272:doi
3221:doi
3217:318
3186:doi
3156:doi
3121:doi
3077:doi
3041:doi
3014:doi
2978:doi
2966:420
2928:doi
2890:doi
2859:doi
2847:353
2813:PMC
2803:doi
2764:doi
2737:doi
2733:124
2702:doi
2671:doi
2642:doi
2638:593
2611:doi
2576:doi
2548:doi
2544:104
2521:doi
2485:doi
2449:doi
2421:doi
2409:298
2386:doi
2349:PMC
2341:doi
2310:doi
2306:262
2283:doi
2256:doi
2227:doi
2196:doi
2151:doi
2124:doi
2120:233
2088:doi
2084:355
2051:doi
2004:doi
1966:doi
1927:doi
1923:141
1885:doi
1843:doi
1831:122
1804:doi
1761:doi
1726:doi
1714:389
1674:doi
1670:140
1624:doi
1584:doi
1539:hdl
1531:doi
1527:116
1495:doi
1483:353
1445:doi
1407:doi
1371:doi
1359:389
1328:doi
1316:389
1293:doi
1281:143
1247:doi
1212:doi
1177:doi
1130:doi
1081:doi
1034:doi
998:doi
960:doi
937:doi
860:doi
812:doi
759:hdl
751:doi
563:PAN
559:PEO
506:or
402:).
394:S–P
390:(Li
335:),
307:or
290:or
233:or
208:EIS
105:(Ag
79:SHE
3853::
3818:.
3810:.
3798:.
3774:.
3764:54
3762:.
3739:.
3731:.
3721:.
3711:.
3699:.
3695:.
3672:.
3662:.
3650:30
3648:.
3644:.
3621:.
3613:.
3605:.
3595:13
3593:.
3589:.
3566:.
3558:.
3550:.
3540:15
3538:.
3534:.
3522:^
3508:.
3498:55
3496:.
3492:.
3469:.
3457:.
3434:.
3422:.
3396:55
3394:.
3365:.
3361:.
3336:.
3326:15
3324:.
3320:.
3295:40
3293:.
3268:27
3266:.
3215:.
3192:.
3182:20
3180:.
3168:^
3152:42
3150:.
3127:.
3115:.
3111:.
3099:^
3085:.
3073:10
3071:.
3067:.
3053:^
3037:58
3035:.
3008:.
2984:.
2976:.
2964:.
2960:.
2934:.
2926:.
2916:12
2914:.
2886:46
2884:.
2880:.
2857:.
2845:.
2821:.
2811:.
2801:.
2789:.
2785:.
2760:46
2758:.
2731:.
2708:.
2698:16
2696:.
2667:11
2665:.
2636:.
2632:.
2607:39
2605:.
2582:.
2572:22
2570:.
2542:.
2515:.
2491:.
2481:23
2479:.
2455:.
2443:.
2419:.
2407:.
2380:.
2357:.
2347:.
2335:.
2331:.
2304:.
2279:23
2277:.
2252:14
2250:.
2223:47
2221:.
2217:.
2194:.
2184:43
2182:.
2157:.
2147:17
2145:.
2118:.
2094:.
2082:.
2059:.
2047:45
2045:.
2041:.
2018:.
2010:.
2000:30
1998:.
1974:.
1962:57
1960:.
1956:.
1933:.
1921:.
1917:.
1893:.
1881:32
1879:.
1875:.
1851:.
1841:.
1829:.
1825:.
1800:27
1798:.
1775:.
1767:.
1757:12
1755:.
1732:.
1724:.
1712:.
1688:.
1680:.
1668:.
1664:.
1638:.
1630:.
1620:16
1618:.
1614:.
1590:.
1582:.
1570:.
1547:.
1537:.
1525:.
1501:.
1493:.
1481:.
1477:.
1451:.
1441:81
1439:.
1427:^
1413:.
1403:10
1401:.
1377:.
1369:.
1357:.
1334:.
1326:.
1314:.
1291:.
1279:.
1267:^
1253:.
1243:76
1241:.
1218:.
1208:41
1206:.
1183:.
1175:.
1163:.
1159:.
1136:.
1128:.
1116:.
1112:.
1089:.
1079:.
1069:39
1067:.
1063:.
1040:.
1032:.
1020:.
996:.
984:.
972:^
931:.
888:.
866:.
854:.
835:.
808:48
806:.
802:.
767:.
757:.
747:64
745:.
725:.
707:.
599:UV
569:,
565:,
561:,
502:,
494:,
462:,
438:,
434:,
323:PS
303:,
30:A
3826:.
3814::
3806::
3782:.
3770::
3747:.
3715::
3707::
3701:6
3680:.
3666::
3656::
3629:.
3601::
3574:.
3554::
3546::
3516:.
3504::
3477:.
3473::
3465::
3442:.
3438::
3430::
3406:.
3402::
3379:.
3373::
3367:3
3346:.
3340::
3332::
3305:.
3301::
3278:.
3274::
3251:.
3227:.
3223::
3200:.
3188::
3162:.
3158::
3135:.
3123::
3117:8
3093:.
3079::
3047:.
3043::
3020:.
3016::
3010:5
2992:.
2980::
2972::
2942:.
2930::
2922::
2898:.
2892::
2865:.
2861::
2853::
2829:.
2805::
2797::
2791:8
2770:.
2766::
2743:.
2739::
2716:.
2704::
2677:.
2673::
2650:.
2644::
2617:.
2613::
2590:.
2578::
2554:.
2550::
2527:.
2523::
2517:3
2499:.
2487::
2463:.
2451::
2445:7
2427:.
2423::
2415::
2392:.
2388::
2382:4
2365:.
2343::
2337:1
2316:.
2312::
2289:.
2285::
2262:.
2258::
2235:.
2229::
2202:.
2198::
2190::
2165:.
2153::
2130:.
2126::
2102:.
2090::
2067:.
2053::
2026:.
2006::
1982:.
1968::
1941:.
1929::
1901:.
1887::
1859:.
1845::
1837::
1810:.
1806::
1783:.
1763::
1740:.
1728::
1720::
1696:.
1676::
1646:.
1626::
1598:.
1586::
1578::
1572:5
1555:.
1541::
1533::
1509:.
1497::
1489::
1459:.
1447::
1421:.
1409::
1385:.
1373::
1365::
1342:.
1330::
1322::
1299:.
1295::
1287::
1261:.
1249::
1226:.
1214::
1191:.
1179::
1171::
1165:1
1144:.
1132::
1124::
1118:1
1097:.
1083::
1075::
1048:.
1036::
1028::
1022:5
1004:.
1000::
992::
986:1
966:.
962::
943:.
939::
933:7
916:.
874:.
862::
856:7
839:.
820:.
814::
775:.
761::
753::
625:2
488:2
484:2
480:3
478:O
476:2
420:g
400:5
398:S
396:2
392:2
379:5
377:I
375:4
357:4
349:3
331:(
325:5
321:6
237:.
115:2
107:2
83:6
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.