750:. In general the salts are preferred over the acid form because they are more soluble in water, but the active form is the acid. The optimal pH for the antimicrobial activity is below pH 6.5. Sorbates are generally used at concentrations of 0.025% to 0.10%. Adding sorbate salts to food will, however, raise the pH of the food slightly so the pH may need to be adjusted to assure safety. It is found in foods such as various kinds of cheese, bread, muffins, donuts, pies, cookies, protein bars, syrups, lemonades, fruit juices, dried meats, sausages, nuggets, burgers, sandwiches, tacos, pizzas, smoked fish, margarine, sauces, soups, and more.
288:
198:
43:
480:
34:
483:
485:
531:
484:
694:
of sorbic acid, which he converted to sorbic acid by hydrolysis. Its antimicrobial activities were discovered in the late 1930s and 1940s, and it became commercially available in the late 1940s and 1950s. Beginning in the 1980s, sorbic acid and its salts were used as inhibitors of
544:
1136:"Benzoate and Sorbate Salts: A Systematic Review of the Potential Hazards of These Invaluable Preservatives and the Expanding Spectrum of Clinical Uses for Sodium Benzoate"
1213:
941:
486:
494:
337:
1024:(For the crystalline acid, I suggest the name "sorbic acid", whereby an old name of the malic acid that's found in rowan berries gains new meaning.)
508:
501:
1045:
925:
302:
42:
619:
551:
245:
1203:
1035:
266:
1208:
935:
683:
443:
115:
646:. It can also be prepared from isomeric hexadienoic acids, which are available via a nickel-catalyzed reaction of
995:
193:
1198:
155:
1118:
697:
1193:
55:
738:, are antimicrobial agents often used as preservatives in food and drinks to prevent the growth of
283:
81:
33:
1061:
1022:
vor, wodurch ein alter Name der in den
Vogelbeeren gefundenen Aepfelsäure neue Bedeutung gewinnt."
853:
598:
1157:
1099:
1041:
921:
771:
731:
1178:
1147:
1091:
1007:
964:
913:
829:
795:
627:
579:
571:
432:
360:
175:
254:
883:
852:
Sorbic acid and sorbate salts have a very low mammalian toxicity and carcinogenicity. Its
803:
777:
735:
687:
655:
91:
287:
197:
135:
765:
659:
647:
643:
522:
1187:
878:
739:
421:
411:
186:
20:
837:
706:
675:
639:
575:
234:
873:
833:
791:
785:
1095:
841:
807:
388:
166:
1011:
968:
917:
1152:
1135:
818:
651:
1161:
1103:
868:
814:
754:
702:
610:
507:
500:
493:
466:
213:
205:
1082:
Kinderlerer JL, Hatton PV (1990). "Fungal metabolites of sorbic acid".
822:
691:
615:
401:
221:
663:
602:
146:
521:
Except where otherwise noted, data are given for materials in their
799:
747:
743:
679:
126:
114:
104:
1062:"Sorbic Acid (E200) – Overview, Uses, Side Effects & More"
718:
638:
The traditional route to sorbic acid involves condensation of
622:
readily. It was first isolated from the unripe berries of the
908:
Erich Lück, Martin Jager, Nico Raczek (2000). "Sorbic Acid".
311:
InChI=1S/C6H8O2/c1-2-3-4-5-6(7)8/h2-5H,1H3,(H,7,8)/b3-2+,5-4+
321:
InChI=1/C6H8O2/c1-2-3-4-5-6(7)8/h2-5H,1H3,(H,7,8)/b3-2+,5-4+
271:
478:
821:. Other detoxification reactions include reduction to 4-
727:
of 4.76, sorbic acid is about as acidic as acetic acid.
539:
1140:
Comprehensive
Reviews in Food Science and Food Safety
1018:"Ich schlage für die krystallinische Säure den Namen
206:
836:, and as an intermediate in the manufacture of some
955:C. F. H. Allen; J. VanAllan (1944). "Sorbic Acid".
666:. An estimated 30,000 tons are produced annually.
813:. The pentadiene manifests as a typical odor of
658:. The route used commercially, however, is from
233:
482:
90:
998:[New volatile acid of rowan berries].
910:Ullmann's Encyclopedia of Industrial Chemistry
8:
982:Ashford's Dictionary of Industrial Chemicals
940:: CS1 maint: multiple names: authors list (
859:is estimated to be between 7.4 and 10 g/kg.
614:. It is a colourless solid that is slightly
286:
196:
174:
25:
1214:Substances discovered in the 19th century
1151:
903:
901:
899:
253:
604:
592:
588:
584:
895:
701:in meat products to replace the use of
342:
307:
282:
1016:Hofmann named sorbic acid on p. 133:
996:"Neue flüchtige Säure der Vogelbeeren"
933:
730:Sorbic acid and its salts, especially
426:228 °C (442 °F; 501 K)
416:135 °C (275 °F; 408 K)
187:
1123:. John Wiley & Sons. p. 547.
314:Key: WSWCOQWTEOXDQX-MQQKCMAXSA-N
154:
134:
7:
674:Sorbic acid was isolated in 1859 by
828:Sorbic acid can also be used as an
324:Key: WSWCOQWTEOXDQX-MQQKCMAXBN
224:
1037:Natural food antimicrobial systems
14:
802:are able to detoxify sorbates by
705:, which can produce carcinogenic
1000:Annalen der Chemie und Pharmacie
984:, Third edition, 2011, page 8482
529:
372:
41:
32:
525:(at 25 °C , 100 kPa).
16:Organic compound (CH3(CH)4COOH)
1117:Bingham E, Cohrssen B (2012).
378:
366:
1:
1134:Piper JD, Piper PW (2017).
1064:. HealthKnight. 21 May 2022
1230:
1040:. CRC Press. p. 637.
18:
1096:10.1080/02652039009373931
1034:A. S. Naidu, ed. (2000).
783:Some molds (notably some
519:
460:
353:
333:
298:
74:
54:
49:
40:
31:
1012:10.1002/jlac.18591100202
969:10.15227/orgsyn.024.0092
918:10.1002/14356007.a24_507
19:Not to be confused with
1153:10.1111/1541-4337.12284
912:. Weinheim: Wiley-VCH.
630:tree), hence its name.
68:)-Hexa-2,4-dienoic acid
994:Hofmann, A.W. (1859).
489:
438:1.6 g/L at 20 °C
825:and 4-hexenoic acid.
698:Clostridium botulinum
488:
568:2,4-hexadienoic acid
471:(fire diamond)
56:Preferred IUPAC name
713:Properties and uses
456:4.76 at 25 °C
433:Solubility in water
396: g·mol
28:
1204:E-number additives
1120:Patty's Toxicology
552:Infobox references
490:
26:
1209:Conjugated dienes
1179:Sorbic inchem.org
1084:Food Addit Contam
936:cite encyclopedia
772:Potassium sorbate
732:potassium sorbate
684:A. W. von Hofmann
560:Chemical compound
558:
557:
267:CompTox Dashboard
116:Interactive image
1221:
1166:
1165:
1155:
1131:
1125:
1124:
1114:
1108:
1107:
1079:
1073:
1072:
1070:
1069:
1058:
1052:
1051:
1031:
1025:
1015:
991:
985:
979:
973:
972:
952:
946:
945:
939:
931:
905:
761:E200 Sorbic acid
624:Sorbus aucuparia
613:
596:
580:chemical formula
572:organic compound
542:
536:
533:
532:
510:
503:
496:
481:
395:
380:
374:
368:
361:Chemical formula
345:O=C(O)\C=C\C=C\C
291:
290:
275:
273:
257:
237:
226:
208:
200:
189:
178:
158:
138:
118:
94:
45:
36:
29:
1229:
1228:
1224:
1223:
1222:
1220:
1219:
1218:
1184:
1183:
1175:
1170:
1169:
1133:
1132:
1128:
1116:
1115:
1111:
1081:
1080:
1076:
1067:
1065:
1060:
1059:
1055:
1048:
1033:
1032:
1028:
993:
992:
988:
980:
976:
954:
953:
949:
932:
928:
907:
906:
897:
892:
884:Parasorbic acid
865:
857:
850:
811:-1,3-pentadiene
804:decarboxylation
778:Calcium sorbate
736:calcium sorbate
725:
715:
688:parasorbic acid
686:. This affords
672:
656:carbon monoxide
636:
606:
601:
594:
590:
586:
582:
574:used as a food
570:, is a natural
561:
554:
549:
548:
547: ?)
538:
534:
530:
526:
515:
514:
513:
512:
505:
498:
491:
487:
479:
452:
435:
393:
383:
377:
371:
363:
349:
346:
341:
340:
329:
326:
325:
322:
316:
315:
312:
306:
305:
294:
276:
269:
260:
240:
227:
214:(preservatives)
181:
161:
141:
121:
108:
97:
84:
70:
69:
24:
17:
12:
11:
5:
1227:
1225:
1217:
1216:
1211:
1206:
1201:
1196:
1186:
1185:
1182:
1181:
1174:
1173:External links
1171:
1168:
1167:
1146:(5): 868–880.
1126:
1109:
1074:
1053:
1046:
1026:
1006:(2): 129–140.
986:
974:
947:
926:
894:
893:
891:
888:
887:
886:
881:
876:
871:
864:
861:
855:
849:
846:
781:
780:
774:
768:
766:Sodium sorbate
762:
723:
714:
711:
671:
668:
660:crotonaldehyde
648:allyl chloride
644:crotonaldehyde
635:
632:
559:
556:
555:
550:
528:
527:
523:standard state
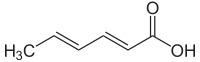
520:
517:
516:
506:
499:
492:
477:
476:
475:
474:
472:
463:
462:
458:
457:
454:
450:
440:
439:
436:
431:
428:
427:
424:
418:
417:
414:
408:
407:
404:
398:
397:
391:
385:
384:
381:
375:
369:
364:
359:
356:
355:
351:
350:
348:
347:
344:
336:
335:
334:
331:
330:
328:
327:
323:
320:
319:
317:
313:
310:
309:
301:
300:
299:
296:
295:
293:
292:
279:
277:
265:
262:
261:
259:
258:
250:
248:
242:
241:
239:
238:
230:
228:
220:
217:
216:
210:
202:
201:
191:
183:
182:
180:
179:
171:
169:
163:
162:
160:
159:
151:
149:
143:
142:
140:
139:
131:
129:
123:
122:
120:
119:
111:
109:
102:
99:
98:
96:
95:
87:
85:
80:
77:
76:
72:
71:
59:
58:
52:
51:
47:
46:
38:
37:
15:
13:
10:
9:
6:
4:
3:
2:
1226:
1215:
1212:
1210:
1207:
1205:
1202:
1200:
1199:Preservatives
1197:
1195:
1192:
1191:
1189:
1180:
1177:
1176:
1172:
1163:
1159:
1154:
1149:
1145:
1141:
1137:
1130:
1127:
1122:
1121:
1113:
1110:
1105:
1101:
1097:
1093:
1090:(5): 657–69.
1089:
1085:
1078:
1075:
1063:
1057:
1054:
1049:
1047:0-8493-2047-X
1043:
1039:
1038:
1030:
1027:
1023:
1019:
1013:
1009:
1005:
1002:(in German).
1001:
997:
990:
987:
983:
978:
975:
970:
966:
962:
958:
951:
948:
943:
937:
929:
927:3-527-30673-0
923:
919:
915:
911:
904:
902:
900:
896:
889:
885:
882:
880:
879:Acids in wine
877:
875:
872:
870:
867:
866:
862:
860:
858:
847:
845:
843:
839:
835:
831:
826:
824:
820:
816:
812:
810:
805:
801:
797:
794:
793:
788:
787:
779:
775:
773:
769:
767:
763:
760:
759:
758:
756:
751:
749:
745:
741:
737:
733:
728:
726:
722:
712:
710:
708:
704:
700:
699:
693:
689:
685:
681:
677:
669:
667:
665:
661:
657:
653:
649:
645:
641:
633:
631:
629:
625:
621:
618:in water and
617:
612:
609:−CH=CH−CH=CH−
608:
600:
581:
578:. It has the
577:
573:
569:
565:
553:
546:
541:
524:
518:
511:
504:
497:
473:
470:
469:
465:
464:
459:
455:
449:
445:
442:
441:
437:
434:
430:
429:
425:
423:
422:Boiling point
420:
419:
415:
413:
412:Melting point
410:
409:
405:
403:
400:
399:
392:
390:
387:
386:
365:
362:
358:
357:
352:
343:
339:
332:
318:
308:
304:
297:
289:
285:
284:DTXSID3021277
281:
280:
278:
268:
264:
263:
256:
252:
251:
249:
247:
244:
243:
236:
232:
231:
229:
223:
219:
218:
215:
211:
209:
204:
203:
199:
195:
192:
190:
188:ECHA InfoCard
185:
184:
177:
173:
172:
170:
168:
165:
164:
157:
153:
152:
150:
148:
145:
144:
137:
133:
132:
130:
128:
125:
124:
117:
113:
112:
110:
106:
101:
100:
93:
89:
88:
86:
83:
79:
78:
73:
67:
63:
57:
53:
48:
44:
39:
35:
30:
22:
21:Ascorbic acid
1143:
1139:
1129:
1119:
1112:
1087:
1083:
1077:
1066:. Retrieved
1056:
1036:
1029:
1021:
1017:
1003:
999:
989:
981:
977:
960:
956:
950:
909:
851:
838:plasticizers
827:
808:
806:, producing
790:
784:
782:
752:
729:
720:
716:
707:nitrosamines
696:
676:distillation
673:
640:malonic acid
637:
623:
576:preservative
567:
563:
562:
467:
447:
156:ChEMBL250212
75:Identifiers
65:
61:
27:Sorbic acid
1194:Enoic acids
1020:Sorbinsäure
874:Polysorbate
834:cold rubber
792:Penicillium
786:Trichoderma
564:Sorbic acid
406:1.204 g/cm
354:Properties
194:100.003.427
136:CHEBI:38358
1188:Categories
1068:2022-08-04
957:Org. Synth
890:References
842:lubricants
680:rowanberry
634:Production
389:Molar mass
255:X045WJ989B
167:ChemSpider
103:3D model (
82:CAS Number
819:petroleum
755:E numbers
652:acetylene
599:structure
1162:33371618
869:Sorbitol
863:See also
830:additive
815:kerosene
703:nitrites
620:sublimes
597:and the
468:NFPA 704
461:Hazards
207:E number
92:110-44-1
1104:2253810
823:hexenol
796:strains
717:With a
692:lactone
682:oil by
670:History
616:soluble
611:C(=O)OH
545:what is
543: (
444:Acidity
402:Density
394:112.128
222:PubChem
1160:
1102:
1044:
963:: 92.
924:
848:Safety
800:yeasts
798:) and
746:, and
690:, the
664:ketene
654:, and
540:verify
537:
338:SMILES
235:643460
176:558605
147:ChEMBL
50:Names
809:trans
776:E203
770:E202
764:E201
757:are:
748:fungi
744:yeast
628:rowan
566:, or
303:InChI
212:E200
127:ChEBI
105:JSmol
1158:PMID
1100:PMID
1042:ISBN
942:link
922:ISBN
840:and
832:for
789:and
753:The
740:mold
734:and
662:and
642:and
587:(CH)
246:UNII
1148:doi
1092:doi
1008:doi
1004:110
965:doi
914:doi
817:or
678:of
272:EPA
225:CID
1190::
1156:.
1144:16
1142:.
1138:.
1098:.
1086:.
961:24
959:.
938:}}
934:{{
920:.
898:^
856:50
854:LD
844:.
742:,
709:.
650:,
591:CO
583:CH
453:)
446:(p
64:,4
60:(2
1164:.
1150::
1106:.
1094::
1088:7
1071:.
1050:.
1014:.
1010::
971:.
967::
944:)
930:.
916::
724:a
721:K
719:p
626:(
607:C
605:3
603:H
595:H
593:2
589:4
585:3
535:N
509:0
502:1
495:2
451:a
448:K
382:2
379:O
376:8
373:H
370:6
367:C
274:)
270:(
107:)
66:E
62:E
23:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.