287:
templating method. Templating yields an ordered array of mesopores in addition to the disordered network of micropores. It has been shown that the initial crystal structure of the carbide is the primary factor affecting the CDC porosity, especially for low-temperature chlorine treatment. In general, a larger spacing between carbon atoms in the lattice correlates with an increase in the average pore diameter. As the synthesis temperature increases, the average pore diameter increases, while the pore size distribution becomes broader. The overall shape and size of the carbide precursor, however, is largely maintained and CDC formation is usually referred to as a conformal process.
500:/kg at 1 bar and 0 °C. CDCs also store up to 3 wt.% hydrogen at 60 bar and −196 °C, with additional increases possible as a result of chemical or physical activation of the CDC materials. SiOC-CDC with large subnanometer pore volumes are able to store over 5.5 wt.% hydrogen at 60 bar and −196 °C, almost reaching the goal of the US Department of Energy of 6 wt.% storage density for automotive applications. Methane storage densities of over 21.5 wt.% can be achieved for this material at those conditions. In particular, a predominance of pores with subnanometer diameters and large pore volumes are instrumental towards increasing storage densities.
81:. Among carbon materials, microporous CDCs exhibit some of the highest reported specific surface areas (up to more than 3000 m/g). By varying the type of the precursor and the CDC synthesis conditions, microporous and mesoporous structures with controllable average pore size and pore size distributions can be produced. Depending on the precursor and the synthesis conditions, the average pore size control can be applied at sub-Angstrom accuracy. This ability to precisely tune the size and shapes of pores makes CDCs attractive for selective sorption and storage of liquids and gases (e.g., hydrogen, methane, CO
567:
fashion with similarities to a supercapacitor. As an ion-containing water (electrolyte) is flown between two porous electrodes with an applied potential across the system, the corresponding ions assemble into a double layer in the pores of the two terminals, decreasing the ion content in the liquid exiting the purification device. Due to the ability of carbide-derived carbons to closely match the size of ions in the electrolyte, side-by-side comparisons of desalinization devices based on CDCs and activated carbon showed a significant efficiency increase in the 1.2–1.4 V range compared to activated carbon.
479:
small pores, especially when combined with an overall large particle diameter, impose an additional diffusion limitation on the ion mobility during charge/discharge cycling. The prevalence of mesopores in the CDC structure allows for more ions to move past each other during charging and discharging, allowing for faster scan rates and improved rate handling abilities. Conversely, by implementing nanoparticle carbide precursors, shorter pore channels allow for higher electrolyte mobility, resulting in faster charge/discharge rates and higher power densities.
466:
and pore size control that enable to match the porosity metrics of the porous carbon electrode to a certain electrolyte. In particular, when the pore size approaches the size of the (desolvated) ion in the electrolyte, there is a significant increase in the capacitance. The electrically conductive carbon material minimizes resistance losses in supercapacitor devices and enhances charge screening and confinement, maximizing the packing density and subsequent charge storage capacity of microporous CDC electrodes.
544:). The particles diffuse through the material to form Pt particle surfaces, which may serve as catalyst support layers. In particular, in addition to Pt, other noble elements such as gold can be deposited into the pores, with the resulting nanoparticle size controlled by the pore size and overall pore size distribution of the CDC substrate. Such gold or platinum nanoparticles can be smaller than 1 nm even without employing surface coatings. Au nanoparticles in different CDCs (TiC-CDC, Mo
509:
dry conditions. It’s important to mention that graphite cannot operate in dry environments. The porous 3-dimensional network of CDC allows for high ductility and an increased mechanical strength, minimizing fracture of the film under an applied force. Those coatings find applications in dynamic seals. The friction properties can be further tailored with high-temperature hydrogen annealing and subsequent hydrogen termination of
320:
single crystals (wafers) at 1200–1500 °C, metal/metalloid atoms are selectively removed and a layer of 1–3 layer graphene (depending on the treatment time) is formed, undergoing a conformal transformation of 3 layers of silicon carbide into one monolayer of graphene. Also, graphene formation occurs preferentially on the Si-face of the 6H-SiC crystals, while nanotube growth is favored on the c-face of SiC.
576:
Skeleton, which is located in Tartu, Estonia, and Carbon-Ukraine, located in Kiev, Ukraine, have a diverse product line of porous carbons for supercapacitors, gas storage, and filtration applications. In addition, numerous education and research institutions worldwide are engaged in basic research of CDC structure, synthesis, or (indirectly) their application for various high-end applications.
522:
released into the body during a bacterial infection that cause the primary inflammatory response during the attack and increase the potential lethality of sepsis, making their removal a very important concern. The rates and levels of removal of above cytokines (85–100% removed within 30 minutes) are higher than those observed for comparable activated carbons.
191:
153:
57:). CDCs have also been derived from polymer-derived ceramics such as Si-O-C or Ti-C, and carbonitrides, such as Si-N-C. CDCs can occur in various structures, ranging from amorphous to crystalline carbon, from sp- to sp-bonded, and from highly porous to fully dense. Among others, the following carbon structures have been derived from carbide precursors:
470:
291:
106:
carried out in the 1960-1980s mostly by
Russian scientists on the synthesis of CDC via halogen treatment, while hydrothermal treatment was explored as an alternative route to derive CDCs in the 1990s. Most recently, research activities have centered on optimized CDC synthesis and nanoengineered CDC precursors.
566:
As desalinization and purification of water is critical for obtaining deionized water for laboratory research, large-scale chemical synthesis in industry and consumer applications, the use of porous materials for this application has received particular interest. Capacitive deionization operates in a
508:
CDC films obtained by vacuum annealing (ESK) or chlorine treatment of SiC ceramics yield a low friction coefficient. The friction coefficient of SiC, which is widely used in tribological applications for its high mechanical strength and hardness, can therefore decrease from ~0.7 to ~0.2 or less under
198:
The linear growth rate of the solid carbon product phase suggests a reaction-driven kinetic mechanism, but the kinetics become diffusion-limited for thicker films or larger particles. A high mass transport condition (high gas flow rates) facilitates the removal of the chloride and shifts the reaction
148:
as the chlorinated product, metal chloride, is the discarded byproduct and the carbon itself remains largely unreacted. This method is implemented for commercial production of CDC by
Skeleton in Estonia and Carbon-Ukraine. Hydrothermal etching has also been used for synthesis of SiC-CDC which yielded
105:
was first patented in 1918 by Otis
Hutchins, with the process further optimized for higher yields in 1956. The solid porous carbon product was initially regarded as a waste byproduct until its properties and potential applications were investigated in more detail in 1959 by Walter Mohun. Research was
465:
One application of carbide-derived carbons is as active material in electrodes for electric double layer capacitors which have become commonly known as supercapacitors or ultracapacitors. This is motivated by their good electrical conductivity combined with high surface area, large micropore volume,
319:
While carbon nanotube formation occurs when trace oxygen amounts are present, very high vacuum conditions (approaching 10–10 torr) result in the formation of graphene sheets. If the conditions are maintained, graphene transitions into bulk graphite. In particular, by vacuum annealing silicon carbide
521:
Carbide-derived carbons with a mesoporous structure remove large molecules from biofluids. As other carbons, CDCs possess good biocompatibility. CDCs have been demonstrated to remove cytokines such as TNF-alpha, IL-6, and IL-1beta from blood plasma. These are the most common receptor-binding agents
478:
CDC electrodes have been shown to yield a gravimetric capacitance of up to 190 F/g in aqueous electrolytes and 180 F/g in organic electrolytes. The highest capacitance values are observed for matching ion/pore systems, which allow high-density packing of ions in pores in superionic states. However,
430:
Only the last reaction yields solid carbon. The yield of carbon-containing gases increases with pressure (decreasing solid carbon yield) and decreases with temperatures (increasing the carbon yield). The ability to produce a usable porous carbon material is dependent on the solubility of the formed
315:
Like halogen treatment, vacuum decomposition is a conformal process. The resulting carbon structures are, as a result of the higher temperatures, more ordered, and carbon nanotubes and graphene can be obtained. In particular, vertically aligned carbon nanotubes films of high tube density have been
165:
The most common method for producing porous carbide-derived carbons involves high-temperature etching with halogens, most commonly chlorine gas. The following generic equation describes the reaction of a metal carbide with chlorine gas (M: Si, Ti, V; similar equations can be written for other CDC
575:
Having originated as the by-product of industrial metal chloride synthesis, CDC has certainly a potential for large-scale production at a moderate cost. Currently, only small companies engage in production of carbide-derived carbons and their implementation in commercial products. For example,
286:
Most produced CDCs exhibit a prevalence of micropores (< 2 nm) and mesopores (between 2 and 50 nm), with specific distributions affected by carbide precursor and synthesis conditions. Hierarchic porosity can be achieved by using polymer-derived ceramics with or without utilizing a
182:
Halogen treatment at temperatures between 200 and 1000 °C has been shown to yield mostly disordered porous carbons with a porosity between 50 and ~80 vol% depending on the precursor. Temperatures above 1000 °C result in predominantly graphitic carbon and an observed shrinkage of the
311:
Metal or metalloid atoms from carbides can selectively be extracted at high temperatures (usually above 1200 °C) under vacuum. The underlying mechanism is incongruent decomposition of carbides, using the high melting point of carbon compared to corresponding carbide metals that melt and
118:
was adopted that clearly denotes the precursor. For example, CDC derived from silicon carbide has been referred to as SiC-CDC, Si-CDC, or SiCDC. Recently, it was recommended to adhere to a unified precursor-CDC-nomenclature to reflect the chemical composition of the precursor (e.g.,
143:
CDCs have been synthesized using several chemical and physical synthesis methods. Most commonly, dry chlorine treatment is used to selectively etch metal or metalloid atoms from the carbide precursor lattice. The term "chlorine treatment" is to be preferred over
473:
Confinement of solvated ions in pores, such as those present in CDCs. As the pore size approaches the size of the solvation shell, the solvent molecules are removed, resulting in larger ionic packing density and increased charge storage
316:
reported for vacuum decomposition of SiC. The high tube density translates into a high elastic modulus and high buckling resistance which is of particular interest for mechanical and tribological applications.
328:
The removal of metal atoms from carbides has been reported at high temperatures (300–1000 °C) and pressures (2–200 MPa). The following reactions are possible between metal carbides and water:
1914:
Carroll, B.; Gogotsi, Y.; Kovalchenko, A.; Erdemir, A. & McNallan, M. J. (2003). "Effect of
Humidity on the Tribological Properties of Carbide-Derived Carbon (CDC) Films on Silicon Carbide".
85:) and the high electric conductivity and electrochemical stability allows these structures to be effectively implemented in electrical energy storage and capacitive water desalinization.
496:
store up to 21 wt.% of methane at 25 °C at high pressure. CDCs with subnanometer pores in the 0.50–0.88 nm diameter range have shown to store up to 7.1 mol CO
825:
Rose, M.; Kockrick, E.; Senkovska, I. & Kaskel, S. (2010). "High surface area carbide-derived carbon fibers produced by electrospinning of polycarbosilane precursors".
1860:
Vakifahmetoglu, C.; Presser, V.; Yeon, S.-H.; Colombo, P. & Gogotsi, Y. (2011). "Enhanced hydrogen and methane gas storage of silicon oxycarbide derived carbon".
1381:
Hoffman, E. N.; Yushin, G.; El-Raghy, T.; Gogotsi, Y. & Barsoum, M. W. (2008). "Micro and mesoporosity of carbon derived from ternary and binary metal carbides".
199:
equilibrium towards the CDC product. Chlorine treatment has successfully been employed for CDC synthesis from a variety of carbide precursors, including SiC, TiC, B
1230:
2172:
2068:
Niu, J. J.; Presser, V.; Karwacki, C. & Gogotsi, Y. (2011). "Ultrasmall Gold
Nanoparticles with the Size Controlled by the Pores of Carbide-Derived Carbon".
1887:
Erdemir, A.; et al. (2004). "Effects of High-Temperature
Hydrogenation Treatment on Sliding Friction and Wear Behavior of Carbide-Derived Carbon Films".
1044:
Kitaoka, S.; Tsuji, T.; Katoh, T. & Yamaguchi, Y. (1994). "Tribological
Characteristics of SiC Ceramics in High-Temperature and High-Pressure Water".
1644:
Permann, L.; Latt, M.; Leis, J. & Arulepp, M. (2006). "Electrical double layer characteristics of nanoporous carbon derived from titanium carbide".
1671:
Leis, J.; Arulepp, M.; Kuura, A.; Latt, M. & Lust, E. (2006). "Electrical double-layer characteristics of novel carbide-derived carbon materials".
728:
Rose, M.; et al. (2011). "Hierarchical Micro- and
Mesoporous Carbide-Derived Carbon as a High-Performance Electrode Material in Supercapacitors".
435:) in supercritical water. Hydrothermal carbon formation has been reported for SiC, TiC, WC, TaC, and NbC. Insolubility of metal oxides, for example TiO
114:
Historically, various terms have been used for CDC, such as "mineral carbon" or "nanoporous carbon". Later, a more adequate nomenclature introduced by
1194:
Kusunoki, M.; Rokkaku, M. & Suzuki, T. (1997). "Epitaxial carbon nanotube film self-organized by sublimation decomposition of silicon carbide".
1833:
Presser, V.; McDonough, J.; Yeon, S.-H. & Gogotsi, Y. (2011). "Effect of pore size on carbon dioxide sorption by carbide derived carbon".
1009:
Roy, R.; Ravichandran, D.; Badzian, A. & Breval, E. (1996). "Attempted hydrothermal synthesis of diamond by hydrolysis of b-SiC powder".
2165:
958:
Babkin, O. E.; Ivakhnyuk, G. K.; Lukin, Y. N. & Fedorov, N. F. (1988). "Study of structure of carbide derived carbon by XPS".
1949:
Yushin, G.; et al. (2006). "Mesoporous carbide-derived carbon with porosity tuned for efficient adsorption of cytokines".
456:
702:
1328:
Zhou, H.; et al. (2012). "Understanding controls on interfacial wetting at epitaxial graphene: Experiment and theory".
790:
Presser, V.; et al. (2011). "Flexible Nano-Felts of
Carbide-Derived Carbon with Ultra-High Power Handling Capability".
636:
Presser, V.; Heon, M. & Gogotsi, Y. (2011). "Carbide-Derived
Carbons – From Porous Networks to Nanotubes and Graphene".
2559:
2158:
1798:
Portet, C.; Yushin, G. & Gogotsi, Y. (2008). "Effect of Carbon Particle Size on Electrochemical Performance of EDLC".
1078:
Presser, V.; Heon, M. & Gogotsi, Y. (2011). "Carbide-Derived Carbons-From Porous Networks to Nanotubes and Graphene".
1263:
Lee, D. S.; et al. (2008). "Raman Spectra of Epitaxial Graphene on SiC and of Epitaxial Graphene Transferred to SiO
2098:
Porada, S.; et al. (2012). "Water Desalination Using Capacitive Deionization with Microporous Carbon Electrodes".
2538:
1987:
Yachamaneni, S.; et al. (2010). "Mesoporous carbide-derived carbon for cytokine removal from blood plasma".
763:
Yeon, S.-H.; et al. (2010). "Carbide-derived-carbons with hierarchical porosity from a preceramic polymer".
678:
2304:
1113:
Kockrick, E.; et al. (2010). "Ordered mesoporous carbide derived carbons for high pressure gas storage".
1698:
Kondrat, S.; Kornyshev, A. (2011). "Superionic state in double-layer capacitors with nanoporous electrodes".
561:
2569:
2191:
145:
977:
Gordeev, S. K.; Vartanova, A. V. (1994). "New approach for production of block microporous materials".
2024:"Platinum Reactions with Carbon Coatings Produced by High Temperature Chlorination of Silicon Carbide"
536:
Pt nanoparticles can be introduced to the SiC/C interface during chlorine treatment (in the form of Pt
2495:
2490:
2242:
2181:
2035:
1807:
1717:
1610:
1575:
1520:
1459:
1444:
1417:
1347:
1286:
1203:
1152:
1018:
870:
610:
58:
2564:
62:
1408:
Pandolfo, A. G.; Hollenkamp, A. F. (2006). "Carbon properties and their role in supercapacitors".
2279:
2261:
1931:
1780:
1741:
1707:
1626:
1544:
1483:
1363:
1337:
1310:
1276:
1095:
894:
807:
690:
653:
995:
Yoshimura, M. et al. Dense Carbon Coating on Silicon Carbide Finers by Hydrothermal Treatment.
2115:
2004:
1966:
1733:
1536:
1475:
1302:
886:
745:
698:
1601:
Huczko, A.; et al. (2007). "Characterization of 1-D nanoSiC-derived nanoporous carbon".
1143:
Arulepp, M.; et al. (2006). "The advanced carbide-derived carbon based supercapacitor".
2518:
2448:
2354:
2107:
2077:
2043:
1996:
1958:
1923:
1896:
1869:
1842:
1815:
1772:
1725:
1680:
1653:
1618:
1583:
1528:
1467:
1425:
1390:
1355:
1294:
1245:
1211:
1160:
1122:
1087:
1053:
1026:
878:
834:
799:
772:
737:
645:
590:
585:
531:
2403:
2359:
2325:
2267:
605:
102:
66:
1761:""Brick-and-Mortar" Self-Assembly Approach to Graphitic Mesoporous Carbon Nanocomposites"
17:
2039:
1811:
1729:
1721:
1614:
1579:
1564:"Curvature effects in carbon nanomaterials: Exohedral versus endohedral supercapacitors"
1524:
1505:
1463:
1421:
1351:
1290:
1207:
1156:
1022:
874:
2500:
2309:
2273:
2000:
1962:
1229:
Pathak, S.; Cambaz, Z. G.; Kalidindi, S. R.; Swadener, J. G. & Gogotsi, Y. (2009).
1057:
595:
184:
2553:
2533:
2387:
2286:
2252:
1935:
1487:
1367:
1030:
600:
510:
1873:
1784:
1657:
1630:
1548:
1394:
1314:
1099:
898:
811:
657:
2523:
1900:
1745:
1429:
1164:
460:
115:
1684:
1249:
1126:
838:
776:
2485:
2464:
2207:
70:
1359:
41:
precursors, such as binary (e.g. SiC, TiC), or ternary carbides, also known as
1927:
1506:"Anomalous Increase in Carbon Capacitance at Pore Sizes Less Than 1 Nanometer"
928:
913:
42:
858:
2419:
2237:
1532:
997:
International Symposium on Carbon, Tokyo, Japan; The Carbon Society of Japan
2119:
2008:
1970:
1776:
1737:
1622:
1540:
1479:
1306:
1231:"Viscoelasticity and high buckling stress of dense carbon nanotube brushes"
1091:
890:
803:
749:
741:
649:
2081:
1587:
194:
Different bulk porosity of CDCs derived from different carbide precursors.
2528:
2369:
2232:
2226:
304:
98:
78:
74:
190:
152:
2480:
2201:
2150:
1846:
469:
290:
38:
2111:
2048:
2023:
1819:
1298:
156:
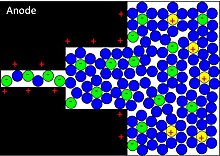
Schematic of chlorine etching of to produce a porous carbon structure.
1471:
1215:
1760:
1563:
882:
718:
Vol. 7, H.S. Nalwa (ed.) pp. 553–574, American Scientific Publishers
439:, is a significant complication for certain metal carbides (e.g., Ti
2135:
1712:
1342:
1281:
468:
289:
189:
151:
911:
Hutchins, O. Method for the Production of Silicon Tetrachlorid.
2154:
2140:
675:
Carbon Materials for Electrochemical Energy Storage Systems
149:
a route for porous carbon films and nanodiamond synthesis.
859:"Nanoporous carbide-derived carbon with tunable pore size"
697:, Y. Gogotsi (ed.) pp. 211–254, CRC Taylor & Francis
294:
Pore size distributions for different carbide precursors.
2145:
2022:
Ersoy, D. A.; McNallan, M. J. & Gogotsi, Y. (2001).
37:, is the common term for carbon materials derived from
312:
eventually evaporate away, leaving the carbon behind.
681:) Ch. 3, 77–113 (CRC Press/Taylor and Francis, 2009).
2509:
2439:
2378:
2345:
2295:
2216:
2188:
926:Andersen, J. N. Silicon Tetrachloride Manufacture.
1179:Carbides. Properties, Production, and Applications
552:C-CDC) catalyze the oxidation of carbon monoxide.
716:Encyclopedia of Nanoscience and Nanotechnology
673:Kyotani, T., Chmiola, J. & Gogotsi, Y. in
2166:
8:
2173:
2159:
2151:
1445:"Materials for electrochemical capacitors"
693:, Nikitin, A. & Gogotsi, Y. (2006) in
2047:
1711:
1341:
1280:
714:Nikitin, A. & Gogotsi, Y. (2004) in
488:Gas storage and carbon dioxide capturing
1046:Journal of the American Ceramic Society
946:Proceedings of the Conference on Carbon
621:
2100:ACS Applied Materials & Interfaces
2093:
2091:
2063:
2061:
2059:
2028:Journal of the Electrochemical Society
1982:
1980:
1800:Journal of the Electrochemical Society
1499:
1497:
1189:
1187:
1138:
1136:
1073:
1071:
1069:
1067:
940:
938:
571:Commercial production and applications
7:
1862:Microporous and Mesoporous Materials
1700:Journal of Physics: Condensed Matter
1383:Microporous and Mesoporous Materials
852:
850:
848:
669:
667:
631:
629:
627:
625:
1759:Fulvio, P. F.; et al. (2011).
251:C, ZrC, ternary carbides such as Ti
2001:10.1016/j.biomaterials.2010.02.054
1963:10.1016/j.biomaterials.2006.07.019
1835:Energy & Environmental Science
1058:10.1111/j.1151-2916.1994.tb07061.x
25:
1504:Chmiola, J.; et al. (2006).
857:Gogotsi, Y.; et al. (2003).
2136:http://nano.materials.drexel.edu
492:TiC-CDC activated with KOH or CO
97:by high temperature reaction of
2208:Lonsdaleite (hexagonal diamond)
1889:Surface and Coatings Technology
1874:10.1016/j.micromeso.2011.03.042
1658:10.1016/j.electacta.2005.06.024
1562:Huang, J.; et al. (2010).
1443:Simon, P.; Gogotsi, Y. (2008).
1395:10.1016/j.micromeso.2007.10.033
457:Electric double-layer capacitor
1901:10.1016/j.surfcoat.2004.07.052
1430:10.1016/j.jpowsour.2006.02.065
1165:10.1016/j.jpowsour.2006.08.014
271:, and carbonitrides such as Ti
1:
1765:Advanced Functional Materials
1730:10.1088/0953-8984/23/2/022201
1568:Journal of Materials Research
1080:Advanced Functional Materials
1011:Diamond and Related Materials
638:Advanced Functional Materials
556:Capacitive deionization (CDI)
1685:10.1016/j.carbon.2006.04.022
1250:10.1016/j.carbon.2009.03.042
1127:10.1016/j.carbon.2010.01.004
1031:10.1016/0925-9635(95)00443-2
839:10.1016/j.carbon.2009.09.043
777:10.1016/j.carbon.2009.09.004
2586:
2539:Aggregated diamond nanorod
1360:10.1103/PhysRevB.85.035406
559:
529:
454:
324:Hydrothermal decomposition
302:
65:carbon, amorphous carbon,
2337:(cyclo[18]carbon)
979:Zhurnal Prikladnoi Khimii
960:Zhurnal Prikladnoi Khimii
948:Vol. 4 pp. 443–453 (1959)
792:Advanced Energy Materials
35:tunable nanoporous carbon
18:Tunable nanoporous carbon
2321:(cyclo[6]carbon)
2305:Linear acetylenic carbon
2141:http://skeletontech.com/
1410:Journal of Power Sources
1177:Kosolapova, T. Y (1971)
1145:Journal of Power Sources
431:metal oxide (such as SiO
27:Type of carbon materials
1928:10.1023/A:1023508006745
1603:Physica Status Solidi B
1533:10.1126/science.1132195
1196:Applied Physics Letters
562:Capacitive deionization
71:nanocrystalline diamond
2365:Carbide-derived carbon
2247:(buckminsterfullerene)
1777:10.1002/adfm.201002641
1623:10.1002/pssb.200776162
1092:10.1002/adfm.201002094
804:10.1002/aenm.201100047
742:10.1002/smll.201001898
650:10.1002/adfm.201002094
475:
295:
195:
157:
93:The production of SiCl
31:Carbide-derived carbon
2146:http://carbon.org.ua/
2082:10.1166/mex.2011.1040
1588:10.1557/JMR.2010.0195
929:U.S. patent 2,739,041
914:U.S. patent 1,271,713
677:(eds F. Beguin &
504:Tribological coatings
483:Proposed applications
472:
341: MC + x H
293:
193:
155:
69:, onion-like carbon,
33:(CDC), also known as
2560:Allotropes of carbon
2182:Allotropes of carbon
695:Carbon Nanomaterials
611:Allotropes of carbon
387:+ CO + (x+1) H
299:Vacuum decomposition
2040:2001JElS..148C.774E
1812:2008JElS..155A.531P
1722:2011JPCM...23b2201K
1646:Electrochimica Acta
1615:2007PSSBR.244.3969H
1580:2010JMatR..25.1525H
1525:2006Sci...313.1760C
1519:(5794): 1760–1763.
1464:2008NatMa...7..845S
1422:2006JPS...157...11P
1352:2012PhRvB..85c5406Z
1291:2008NanoL...8.4320L
1208:1997ApPhL..71.2620K
1157:2006JPS...162.1460A
1023:1996DRM.....5..973R
875:2003NatMa...2..591G
2460:(cyclopropatriene)
2441:hypothetical forms
2262:Fullerene whiskers
1847:10.1039/c1ee01176f
517:Protein adsorption
476:
395:MC + (x+2) H
379:MC + (x+1) H
306:Epitaxial graphene
296:
196:
161:Chlorine treatment
158:
2547:
2546:
2415:(diatomic carbon)
2347:mixed sp/sp forms
2112:10.1021/am201683j
2070:Materials Express
2049:10.1149/1.1415033
2034:(12): C774–C779.
1995:(18): 4789–4795.
1916:Tribology Letters
1820:10.1149/1.2918304
1771:(12): 2208–2215.
1679:(11): 2122–2129.
1609:(11): 3969–3972.
1330:Physical Review B
1299:10.1021/nl802156w
1275:(12): 4320–4325.
1202:(18): 2620–2622.
999:, 552–553 (1998).
178:(gas) + C (solid)
170:MC (solid) + 2 Cl
16:(Redirected from
2577:
2519:Activated carbon
2475:
2474:
2473:
2459:
2458:
2457:
2430:
2429:
2428:
2414:
2413:
2412:
2398:
2397:
2396:
2355:Amorphous carbon
2336:
2335:
2334:
2320:
2319:
2318:
2175:
2168:
2161:
2152:
2124:
2123:
2106:(3): 1194–1199.
2095:
2086:
2085:
2065:
2054:
2053:
2051:
2019:
2013:
2012:
1984:
1975:
1974:
1946:
1940:
1939:
1911:
1905:
1904:
1884:
1878:
1877:
1868:(1–3): 105–112.
1857:
1851:
1850:
1841:(8): 3059–3066.
1830:
1824:
1823:
1806:(7): A531–A536.
1795:
1789:
1788:
1756:
1750:
1749:
1715:
1695:
1689:
1688:
1668:
1662:
1661:
1652:(7): 1274–1281.
1641:
1635:
1634:
1598:
1592:
1591:
1574:(8): 1525–1531.
1559:
1553:
1552:
1510:
1501:
1492:
1491:
1472:10.1038/nmat2297
1452:Nature Materials
1449:
1440:
1434:
1433:
1405:
1399:
1398:
1389:(1–3): 526–532.
1378:
1372:
1371:
1345:
1325:
1319:
1318:
1284:
1260:
1254:
1253:
1244:(8): 1969–1976.
1235:
1226:
1220:
1219:
1216:10.1063/1.120158
1191:
1182:
1175:
1169:
1168:
1151:(2): 1460–1466.
1140:
1131:
1130:
1121:(6): 1707–1717.
1110:
1104:
1103:
1075:
1062:
1061:
1052:(7): 1851–1856.
1041:
1035:
1034:
1006:
1000:
993:
987:
986:
974:
968:
967:
955:
949:
944:Mohun, W. A. in
942:
933:
931:
924:
918:
916:
909:
903:
902:
863:Nature Materials
854:
843:
842:
822:
816:
815:
787:
781:
780:
760:
754:
753:
736:(8): 1108–1117.
725:
719:
712:
706:
688:
682:
671:
662:
661:
633:
591:Hydrogen economy
586:Hydrogen storage
532:Catalyst support
526:Catalyst support
370:
369:
365:
355:
354:
350:
340:
339:
335:
183:material due to
67:carbon nanotubes
21:
2585:
2584:
2580:
2579:
2578:
2576:
2575:
2574:
2550:
2549:
2548:
2543:
2505:
2496:Metallic carbon
2472:
2469:
2468:
2467:
2465:
2456:
2453:
2452:
2451:
2449:
2435:
2427:
2424:
2423:
2422:
2420:
2411:
2408:
2407:
2406:
2404:
2399:(atomic carbon)
2395:
2392:
2391:
2390:
2388:
2374:
2360:Carbon nanofoam
2341:
2333:
2330:
2329:
2328:
2326:
2317:
2314:
2313:
2312:
2310:
2291:
2256:
2246:
2212:
2202:Diamond (cubic)
2184:
2179:
2132:
2127:
2097:
2096:
2089:
2067:
2066:
2057:
2021:
2020:
2016:
1986:
1985:
1978:
1957:(34): 5755–62.
1948:
1947:
1943:
1913:
1912:
1908:
1886:
1885:
1881:
1859:
1858:
1854:
1832:
1831:
1827:
1797:
1796:
1792:
1758:
1757:
1753:
1697:
1696:
1692:
1670:
1669:
1665:
1643:
1642:
1638:
1600:
1599:
1595:
1561:
1560:
1556:
1508:
1503:
1502:
1495:
1458:(11): 845–854.
1447:
1442:
1441:
1437:
1407:
1406:
1402:
1380:
1379:
1375:
1327:
1326:
1322:
1266:
1262:
1261:
1257:
1233:
1228:
1227:
1223:
1193:
1192:
1185:
1176:
1172:
1142:
1141:
1134:
1112:
1111:
1107:
1077:
1076:
1065:
1043:
1042:
1038:
1008:
1007:
1003:
994:
990:
976:
975:
971:
957:
956:
952:
943:
936:
927:
925:
921:
912:
910:
906:
883:10.1038/nmat957
856:
855:
846:
824:
823:
819:
789:
788:
784:
762:
761:
757:
727:
726:
722:
713:
709:
689:
685:
672:
665:
635:
634:
623:
619:
606:Nanoengineering
582:
573:
564:
558:
551:
547:
543:
539:
534:
528:
519:
506:
499:
495:
490:
485:
463:
453:
446:
442:
438:
434:
426:
423:+ C + x H
422:
418:
410:
407:+ (x+2) H
406:
402:
398:
390:
386:
382:
374:
367:
363:
362:
360:
356:
352:
348:
347:
344:
337:
333:
332:
326:
309:
301:
282:
278:
274:
270:
266:
262:
258:
254:
250:
246:
242:
238:
234:
230:
226:
222:
218:
214:
210:
206:
202:
177:
173:
163:
141:
134:
130:
126:
122:
112:
103:silicon carbide
96:
91:
84:
56:
52:
48:
28:
23:
22:
15:
12:
11:
5:
2583:
2581:
2573:
2572:
2567:
2562:
2552:
2551:
2545:
2544:
2542:
2541:
2536:
2531:
2526:
2521:
2515:
2513:
2507:
2506:
2504:
2503:
2501:Penta-graphene
2498:
2493:
2488:
2483:
2478:
2470:
2462:
2454:
2445:
2443:
2437:
2436:
2434:
2433:
2425:
2417:
2409:
2401:
2393:
2384:
2382:
2376:
2375:
2373:
2372:
2367:
2362:
2357:
2351:
2349:
2343:
2342:
2340:
2339:
2331:
2323:
2315:
2307:
2301:
2299:
2293:
2292:
2290:
2289:
2284:
2254:
2244:
2235:
2230:
2222:
2220:
2214:
2213:
2211:
2210:
2205:
2197:
2195:
2186:
2185:
2180:
2178:
2177:
2170:
2163:
2155:
2149:
2148:
2143:
2138:
2131:
2130:External links
2128:
2126:
2125:
2087:
2076:(4): 259–266.
2055:
2014:
1976:
1941:
1906:
1879:
1852:
1825:
1790:
1751:
1690:
1663:
1636:
1593:
1554:
1493:
1435:
1400:
1373:
1320:
1264:
1255:
1221:
1183:
1181:, Plenum Press
1170:
1132:
1105:
1086:(5): 810–833.
1063:
1036:
1017:(9): 973–976.
1001:
988:
969:
950:
934:
919:
904:
869:(9): 591–594.
844:
833:(2): 403–407.
817:
798:(3): 423–430.
782:
755:
720:
707:
683:
663:
644:(5): 810–833.
620:
618:
615:
614:
613:
608:
603:
598:
596:Nanotechnology
593:
588:
581:
578:
572:
569:
557:
554:
549:
545:
541:
537:
527:
524:
518:
515:
511:dangling bonds
505:
502:
497:
493:
489:
486:
484:
481:
452:
449:
444:
440:
436:
432:
428:
427:
424:
420:
416:
415:MC + x H
412:
411:
408:
404:
400:
396:
392:
391:
388:
384:
380:
376:
375:
372:
358:
346:
342:
325:
322:
303:Main article:
300:
297:
280:
276:
272:
268:
264:
260:
256:
252:
248:
244:
240:
236:
232:
228:
224:
220:
216:
212:
208:
204:
200:
185:graphitization
180:
179:
175:
171:
162:
159:
140:
137:
132:
128:
124:
120:
111:
108:
94:
90:
87:
82:
54:
50:
46:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2582:
2571:
2570:Nanomaterials
2568:
2566:
2563:
2561:
2558:
2557:
2555:
2540:
2537:
2535:
2532:
2530:
2527:
2525:
2522:
2520:
2517:
2516:
2514:
2512:
2508:
2502:
2499:
2497:
2494:
2492:
2489:
2487:
2484:
2482:
2479:
2477:
2476:(prismane C8)
2463:
2461:
2447:
2446:
2444:
2442:
2438:
2432:
2418:
2416:
2402:
2400:
2386:
2385:
2383:
2381:
2377:
2371:
2368:
2366:
2363:
2361:
2358:
2356:
2353:
2352:
2350:
2348:
2344:
2338:
2324:
2322:
2308:
2306:
2303:
2302:
2300:
2298:
2294:
2288:
2287:Glassy carbon
2285:
2282:
2281:
2276:
2275:
2270:
2269:
2264:
2263:
2258:
2257:
2249:
2248:
2239:
2236:
2234:
2231:
2229:
2228:
2224:
2223:
2221:
2219:
2215:
2209:
2206:
2204:
2203:
2199:
2198:
2196:
2194:
2193:
2187:
2183:
2176:
2171:
2169:
2164:
2162:
2157:
2156:
2153:
2147:
2144:
2142:
2139:
2137:
2134:
2133:
2129:
2121:
2117:
2113:
2109:
2105:
2101:
2094:
2092:
2088:
2083:
2079:
2075:
2071:
2064:
2062:
2060:
2056:
2050:
2045:
2041:
2037:
2033:
2029:
2025:
2018:
2015:
2010:
2006:
2002:
1998:
1994:
1990:
1983:
1981:
1977:
1972:
1968:
1964:
1960:
1956:
1952:
1945:
1942:
1937:
1933:
1929:
1925:
1921:
1917:
1910:
1907:
1902:
1898:
1894:
1890:
1883:
1880:
1875:
1871:
1867:
1863:
1856:
1853:
1848:
1844:
1840:
1836:
1829:
1826:
1821:
1817:
1813:
1809:
1805:
1801:
1794:
1791:
1786:
1782:
1778:
1774:
1770:
1766:
1762:
1755:
1752:
1747:
1743:
1739:
1735:
1731:
1727:
1723:
1719:
1714:
1709:
1706:(2): 022201.
1705:
1701:
1694:
1691:
1686:
1682:
1678:
1674:
1667:
1664:
1659:
1655:
1651:
1647:
1640:
1637:
1632:
1628:
1624:
1620:
1616:
1612:
1608:
1604:
1597:
1594:
1589:
1585:
1581:
1577:
1573:
1569:
1565:
1558:
1555:
1550:
1546:
1542:
1538:
1534:
1530:
1526:
1522:
1518:
1514:
1507:
1500:
1498:
1494:
1489:
1485:
1481:
1477:
1473:
1469:
1465:
1461:
1457:
1453:
1446:
1439:
1436:
1431:
1427:
1423:
1419:
1415:
1411:
1404:
1401:
1396:
1392:
1388:
1384:
1377:
1374:
1369:
1365:
1361:
1357:
1353:
1349:
1344:
1339:
1336:(3): 035406.
1335:
1331:
1324:
1321:
1316:
1312:
1308:
1304:
1300:
1296:
1292:
1288:
1283:
1278:
1274:
1270:
1259:
1256:
1251:
1247:
1243:
1239:
1232:
1225:
1222:
1217:
1213:
1209:
1205:
1201:
1197:
1190:
1188:
1184:
1180:
1174:
1171:
1166:
1162:
1158:
1154:
1150:
1146:
1139:
1137:
1133:
1128:
1124:
1120:
1116:
1109:
1106:
1101:
1097:
1093:
1089:
1085:
1081:
1074:
1072:
1070:
1068:
1064:
1059:
1055:
1051:
1047:
1040:
1037:
1032:
1028:
1024:
1020:
1016:
1012:
1005:
1002:
998:
992:
989:
984:
980:
973:
970:
965:
961:
954:
951:
947:
941:
939:
935:
930:
923:
920:
915:
908:
905:
900:
896:
892:
888:
884:
880:
876:
872:
868:
864:
860:
853:
851:
849:
845:
840:
836:
832:
828:
821:
818:
813:
809:
805:
801:
797:
793:
786:
783:
778:
774:
770:
766:
759:
756:
751:
747:
743:
739:
735:
731:
724:
721:
717:
711:
708:
704:
700:
696:
692:
687:
684:
680:
679:E. Frackowiak
676:
670:
668:
664:
659:
655:
651:
647:
643:
639:
632:
630:
628:
626:
622:
616:
612:
609:
607:
604:
602:
601:Nanomaterials
599:
597:
594:
592:
589:
587:
584:
583:
579:
577:
570:
568:
563:
555:
553:
533:
525:
523:
516:
514:
512:
503:
501:
487:
482:
480:
471:
467:
462:
458:
450:
448:
414:
413:
394:
393:
378:
377:
331:
330:
329:
323:
321:
317:
313:
308:
307:
298:
292:
288:
284:
192:
188:
186:
169:
168:
167:
166:precursors):
160:
154:
150:
147:
138:
136:
117:
109:
107:
104:
100:
88:
86:
80:
76:
72:
68:
64:
60:
44:
40:
36:
32:
19:
2534:Carbon fiber
2524:Carbon black
2510:
2491:Cubic carbon
2440:
2379:
2364:
2346:
2296:
2278:
2272:
2266:
2260:
2251:
2241:
2240:, including
2225:
2217:
2200:
2189:
2103:
2099:
2073:
2069:
2031:
2027:
2017:
1992:
1989:Biomaterials
1988:
1954:
1951:Biomaterials
1950:
1944:
1919:
1915:
1909:
1892:
1888:
1882:
1865:
1861:
1855:
1838:
1834:
1828:
1803:
1799:
1793:
1768:
1764:
1754:
1703:
1699:
1693:
1676:
1672:
1666:
1649:
1645:
1639:
1606:
1602:
1596:
1571:
1567:
1557:
1516:
1512:
1455:
1451:
1438:
1416:(1): 11–27.
1413:
1409:
1403:
1386:
1382:
1376:
1333:
1329:
1323:
1272:
1269:Nano Letters
1268:
1258:
1241:
1237:
1224:
1199:
1195:
1178:
1173:
1148:
1144:
1118:
1114:
1108:
1083:
1079:
1049:
1045:
1039:
1014:
1010:
1004:
996:
991:
985:: 1375–1377.
982:
978:
972:
966:: 1719–1721.
963:
959:
953:
945:
922:
907:
866:
862:
830:
826:
820:
795:
791:
785:
768:
764:
758:
733:
729:
723:
715:
710:
694:
686:
674:
641:
637:
574:
565:
535:
520:
507:
491:
477:
464:
461:Capa vehicle
451:Applications
429:
327:
318:
314:
310:
305:
285:
247:C, VC, WC, W
197:
181:
164:
146:chlorination
142:
116:Yury Gogotsi
113:
110:Nomenclature
92:
34:
30:
29:
2486:Haeckelites
2431:(tricarbon)
2380:other forms
2280:Nanoscrolls
1895:: 588–593.
771:: 201–210.
474:capability.
174:(gas) → MCl
2565:Capacitors
2554:Categories
2238:Fullerenes
703:0849393868
691:Yushin, G.
617:References
560:See also:
530:See also:
455:See also:
63:mesoporous
43:MAX phases
2268:Nanotubes
1936:137442598
1922:: 51–55.
1713:1010.0921
1488:205401964
1368:118510423
1343:1112.2242
1282:0807.4049
371: CH
139:Synthesis
123:C-CDC, Ti
101:gas with
45:(e.g., Ti
2529:Charcoal
2370:Q-carbon
2297:sp forms
2274:Nanobuds
2233:Graphene
2227:Graphite
2218:sp forms
2120:22329838
2009:20303167
1971:16914195
1785:93644784
1738:21406834
1631:95577444
1549:40027564
1541:16917025
1480:18956000
1315:35392475
1307:19368003
1100:96797238
899:14257229
891:12907942
812:97714605
750:21449047
658:96797238
580:See also
548:C-CDC, B
263:, and Ti
135:C-CDC).
99:chlorine
79:graphite
75:graphene
2511:related
2481:Chaoite
2036:Bibcode
1808:Bibcode
1746:4494305
1718:Bibcode
1611:Bibcode
1576:Bibcode
1521:Bibcode
1513:Science
1460:Bibcode
1418:Bibcode
1348:Bibcode
1287:Bibcode
1204:Bibcode
1153:Bibcode
1019:Bibcode
871:Bibcode
366:⁄
351:⁄
336:⁄
255:AlC, Ti
131:-CDC, W
89:History
49:AlC, Ti
39:carbide
2118:
2007:
1969:
1934:
1783:
1744:
1736:
1673:Carbon
1629:
1547:
1539:
1486:
1478:
1366:
1313:
1305:
1238:Carbon
1115:Carbon
1098:
932:(1956)
917:(1918)
897:
889:
827:Carbon
810:
765:Carbon
748:
701:
656:
419:O → MO
399:O → MO
383:O → MO
239:C, SrC
203:C, BaC
77:, and
59:micro-
2192:forms
1932:S2CID
1781:S2CID
1742:S2CID
1708:arXiv
1627:S2CID
1545:S2CID
1509:(PDF)
1484:S2CID
1448:(PDF)
1364:S2CID
1338:arXiv
1311:S2CID
1277:arXiv
1234:(PDF)
1096:S2CID
895:S2CID
808:S2CID
730:Small
654:S2CID
345:O → M
227:C, Al
223:C, Mo
207:, CaC
2116:PMID
2005:PMID
1967:PMID
1734:PMID
1537:PMID
1476:PMID
1303:PMID
887:PMID
746:PMID
699:ISBN
459:and
403:+ CO
243:, Ta
235:, Nb
219:, Fe
211:, Cr
61:and
2190:sp
2108:doi
2078:doi
2044:doi
2032:148
1997:doi
1959:doi
1924:doi
1897:doi
1893:188
1870:doi
1866:144
1843:doi
1816:doi
1804:155
1773:doi
1726:doi
1681:doi
1654:doi
1619:doi
1607:244
1584:doi
1529:doi
1517:313
1468:doi
1426:doi
1414:157
1391:doi
1387:112
1356:doi
1295:doi
1267:".
1246:doi
1212:doi
1161:doi
1149:162
1123:doi
1088:doi
1054:doi
1027:doi
879:doi
835:doi
800:doi
773:doi
738:doi
646:doi
447:).
443:SiC
281:0.5
277:0.5
275:AlC
267:SiC
259:AlC
127:SiC
53:SiC
2556::
2332:18
2277:,
2271:,
2265:,
2259:,
2255:70
2250:,
2245:60
2114:.
2102:.
2090:^
2072:.
2058:^
2042:.
2030:.
2026:.
2003:.
1993:31
1991:.
1979:^
1965:.
1955:27
1953:.
1930:.
1920:15
1918:.
1891:.
1864:.
1837:.
1814:.
1802:.
1779:.
1769:21
1767:.
1763:.
1740:.
1732:.
1724:.
1716:.
1704:23
1702:.
1677:44
1675:.
1650:51
1648:.
1625:.
1617:.
1605:.
1582:.
1572:25
1570:.
1566:.
1543:.
1535:.
1527:.
1515:.
1511:.
1496:^
1482:.
1474:.
1466:.
1454:.
1450:.
1424:.
1412:.
1385:.
1362:.
1354:.
1346:.
1334:85
1332:.
1309:.
1301:.
1293:.
1285:.
1271:.
1242:47
1240:.
1236:.
1210:.
1200:71
1198:.
1186:^
1159:.
1147:.
1135:^
1119:48
1117:.
1094:.
1084:21
1082:.
1066:^
1050:77
1048:.
1025:.
1013:.
983:67
981:.
964:57
962:.
937:^
893:.
885:.
877:.
865:.
861:.
847:^
831:48
829:.
806:.
794:.
769:48
767:.
744:.
732:.
666:^
652:.
642:21
640:.
624:^
540:Cl
513:.
361:+
283:.
187:.
73:,
2471:6
2466:C
2455:3
2450:C
2426:3
2421:C
2410:2
2405:C
2394:1
2389:C
2327:C
2316:6
2311:C
2283:)
2253:C
2243:C
2174:e
2167:t
2160:v
2122:.
2110::
2104:4
2084:.
2080::
2074:1
2052:.
2046::
2038::
2011:.
1999::
1973:.
1961::
1938:.
1926::
1903:.
1899::
1876:.
1872::
1849:.
1845::
1839:4
1822:.
1818::
1810::
1787:.
1775::
1748:.
1728::
1720::
1710::
1687:.
1683::
1660:.
1656::
1633:.
1621::
1613::
1590:.
1586::
1578::
1551:.
1531::
1523::
1490:.
1470::
1462::
1456:7
1432:.
1428::
1420::
1397:.
1393::
1370:.
1358::
1350::
1340::
1317:.
1297::
1289::
1279::
1273:8
1265:2
1252:.
1248::
1218:.
1214::
1206::
1167:.
1163::
1155::
1129:.
1125::
1102:.
1090::
1060:.
1056::
1033:.
1029::
1021::
1015:5
901:.
881::
873::
867:2
841:.
837::
814:.
802::
796:1
779:.
775::
752:.
740::
734:7
705:.
660:.
648::
550:4
546:2
542:3
538:3
498:2
494:2
445:2
441:3
437:2
433:2
425:2
421:x
417:2
409:2
405:2
401:x
397:2
389:2
385:x
381:2
373:4
368:2
364:x
359:x
357:O
353:2
349:x
343:2
338:2
334:x
279:N
273:2
269:2
265:3
261:2
257:3
253:2
249:2
245:2
241:2
237:2
233:3
231:C
229:4
225:2
221:3
217:2
215:C
213:3
209:2
205:2
201:4
176:4
172:2
133:2
129:2
125:3
121:4
119:B
95:4
83:2
55:2
51:3
47:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.