312:
384:
909:
865:
749:
652:
442:
825:
789:
204:
688:
604:
506:
559:
140:
38:
61:, is a chemical process that comprises at least two consecutive reactions such that each subsequent reaction occurs only in virtue of the chemical functionality formed in the previous step. In cascade reactions, isolation of intermediates is not required, as each reaction composing the sequence occurs spontaneously. In the strictest definition of the term, the reaction conditions do not change among the consecutive steps of a cascade and no new reagents are added after the initial step. By contrast,
85:. Since then, the use of cascade reactions has proliferated in the area of total synthesis. Similarly, the development of cascade-driven organic methodology has also grown tremendously. This increased interest in cascade sequences is reflected by the numerous relevant review articles published in the past couple of decades. A growing area of focus is the development of asymmetric catalysis of cascade processes by employing chiral organocatalysts or chiral transition-metal complexes.
264:
89:
in which two or more classes of reaction are included in a cascade, the distinction becomes rather arbitrary and the process is labeled according to what can be arguably considered the “major theme”. In order to highlight the remarkable synthetic utility of cascade reactions, the majority of the examples below come from the total syntheses of complex molecules.
980:
88:
Classification of cascade reactions is sometimes difficult due to the diverse nature of the many steps in the transformation. K. C. Nicolaou labels the cascades as nucleophilic/electrophilic, radical, pericyclic or transition-metal-catalyzed, based on the mechanism of the steps involved. In the cases
65:
similarly allow at least two reactions to be carried out consecutively without any isolation of intermediates, but do not preclude the addition of new reagents or the change of conditions after the first reaction. Thus, any cascade reaction is also a one-pot procedure, while the reverse does not hold
527:
Possibly the most widely encountered kind of process in cascade transformations, pericyclic reactions include cycloadditions, electrocyclic reactions and sigmatropic rearrangements. Although some of the abovementioned instances of nucleophilic/electrophilic and radical cascades involved pericyclic
77:
and reduction of waste generated by the several chemical processes, as well as of the time and work required to carry them out. The efficiency and utility of a cascade reaction can be measured in terms of the number of bonds formed in the overall sequence, the degree of increase in the structural
930:
Multistep tandem reactions (or cascade reactions) are a sequence of chemical transformations (usually more than two steps) that happens consecutively to convert a starting material to a complex product. This kind of organic reactions are designed to construct difficult structures encountered in
948:
routiennocin 1 (Fig. 1), the central spiroketal skeleton was constructed by a multistep tandem reaction (Fig. 2). Fragment A and fragment B were coupled in a single step to form the key intermediate G that could be further elaborated to afford the final product routiennocin.
709:
Transition-metal-catalyzed cascade sequences combine the novelty and power of organometallic chemistry with the synthetic utility and economy of cascade reactions, providing an even more ecologically and economically desirable approach to organic synthesis.
575:
A pericyclic sequence involving intramolecular hetero-cycloaddition reactions was employed in the total synthesis of naturally occurring alkaloid (–)-vindorosine (Scheme 9). Rapid access to the target was achieved from a solution of 1,3,4-oxadiazole
45:'s synthesis of dihydroprotodaphniphylline features a highly efficient cascade involving two aldehyde/amine condensations, a Prins-like cyclization, and a 1,5-hydride transfer to afford a pentacyclic structure from an acyclic starting material.
311:
908:
405:
Radical cascades are those in which the key step constitutes a radical reaction. The high reactivity of free radical species renders radical-based synthetic approaches decidedly suitable for cascade reactions.
409:
One of the most widely recognized examples of the synthetic utility of radical cascades is the cyclization sequence employed in the total synthesis of (±)-hirsutene, in 1985 (Scheme 6). Herein, alkyl iodide
864:
383:
748:
100:
An example of such a cascade is seen in the short enantioselective synthesis of the broad-spectrum antibiotic (–)-chloramphenicol, reported by Rao et al. (Scheme 1). Herein, the chiral epoxy-alcohol
620:
The total synthesis of (–)-colombiasin A reported in 2005 by the
Harrowven group included an electrocyclic cascade (Scheme 10). When subjected to heat via microwave irradiation, squarate derivative
228:
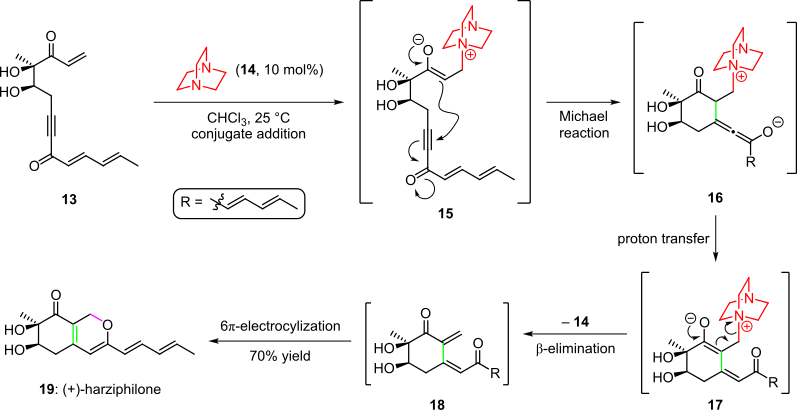
An organocatalytic cascade was employed in the total synthesis of the natural product harziphilone, reported by
Sorensen et al. in 2004 (Scheme 3). Herein, treatment of the enone starting material
668:
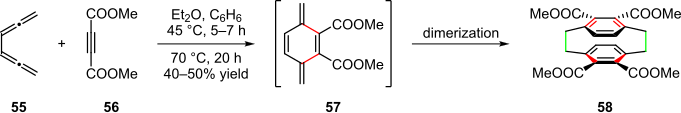
Certain paracyclophanes can also be obtained via pericyclic cascades, as reported by the Hopf group in 1981 (Scheme 11). In this sequence, a Diels-Alder reaction between 1,2,4,5-hexatetraene
976:
formation reaction. This multistep tandem reaction greatly simplified the construction of this complex spiroketal structure and eased the path towards the total synthesis of routiennocin.
809:
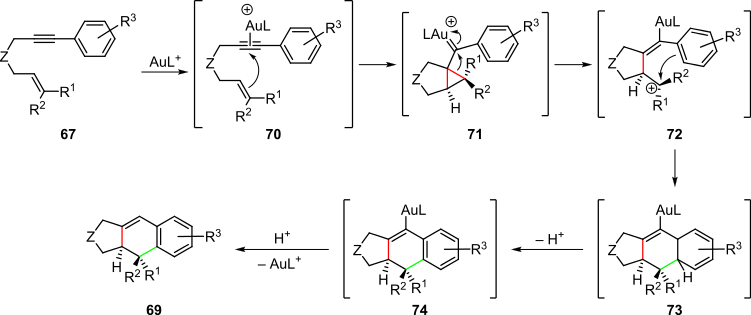
mediated by gold catalysis is another example of a transition-metal-catalyzed cascade (Scheme 14). A variety of 1,6-enynes reacted under mild conditions in the presence of Au(I) complexes
788:
441:
893:. This step is followed by the dissociation of the triflate anion, association of the neighboring olefin and 1,2-insertion of the naphthyl group into the olefin to yield intermediate
824:
651:
773:
Rhodium catalysis was also employed to initiate a cyclization/cycloaddition cascade in the synthesis of a tigliane reported by the Dauben group (Scheme 13). Treatment of diazoimide
558:
964:
formed carbon anion that attacked the alkyl iodide part of fragment B to generate intermediate C (step 1). Then a 3, 4-dihydropyran derivative D was formed through base-mediated
304:) with moderate to excellent diastereoselectivity and complete enantiocontrol (Scheme 4). The transformation is mediated by the readily available proline-derived organocatalyst
203:
603:
505:
687:
66:
true. Although often composed solely of intramolecular transformations, cascade reactions can also occur intermolecularly, in which case they also fall under the category of
336:
The transformation was proposed to proceed via a
Michael addition/Michael addition/aldol condensation sequence (Scheme 5). In the first step, Michael addition of aldehyde
248:
after proton transfer and tautomerization. The cascade was completed by elimination of the organocatalyst and a spontaneous 6π-electrocyclic ring closure of the resultant
881:
An example of palladium-catalyzed cascades is represented by the asymmetric polyene Heck cyclization used in the preparation of (+)-xestoquinone from triflate substrate
139:
580:
in triisopropyl benzene subjected to high temperatures and reduced pressure. First an inverse-electron-demand hetero-Diels-Alder reaction occurred to give intermediate
528:
processes, this section contains only cascade sequences that are solely composed of pericyclic reactions or in which such a reaction arguably constitutes the key step.
531:
A representative example of a pericyclic cascade is the endiandric acid cascade reported by
Nicolaou et al. in 1982 (Scheme 8). Herein the highly unsaturated system
225:
A subcategory of nucleophilic/electrophilic sequences is constituted by organocatalytic cascades, in which the key nucleophilic attack is driven by organocatalysis.
905:
in 82% overall yield and with moderate enantioselectivity. The palladium(0) catalyst is also regenerated in this step, thus allowing the cascade to be reinitiated.
781:
after an intramolecular cyclization with the neighboring carbonyl group. An intramolecular cycloaddition then spontaneously occurred to afford the target tigliane
721:-chromen products in a hydroformylation cascade (Scheme 12). First, selective rhodium-catalyzed hydroformylation of the less sterically hindered olefin bond in
972:
moiety in intermediate D was removed by acid treatment to give the diol product E (step 3). The spiroketal product G was generated via intramolecular
240:
via conjugate addition. Subsequent cyclization by the intramolecular
Michael addition of the enolate into the triple bond of the system gave species
861:. Due to the nature of the interaction of gold complexes with unsaturated systems, this process could also be considered an electrophilic cascade.
97:
Nucleophilic/electrophilic cascades are defined as the cascade sequences in which the key step constitutes a nucleophilic or electrophilic attack.
156:
A nucleophilic cascade was also employed in the total synthesis of the natural product pentalenene (Scheme 2). In this procedure, squarate ester
551:, the geometry and stereochemistry of which favored a subsequent intramolecular Diels-Alder reaction. The methyl ester of endiandric acid B (
624:
underwent an electrocyclic opening of the cyclobutene ring, followed by a 6π-electrocyclic ring closure that yielded bicyclic intermediate
1755:
Diez-Martin, D. Kotecha, N. R.; Ley, S. V.; Mantegani, S.; Menendez, J. C.; Organ, H. M.; White, A. D., Tetrahedron, 1992, 48, 1899-7938.
478:
stereoselectively in virtue of the stereochemistry of the ether linkage. In the next step of the cascade, the geometric constraints of
885:(Scheme 16). Oxidative addition of the aryl–triflate bond into the palladium(0) complex in the presence of chiral diphosphine ligand (
953:
172:, which spontaneously underwent a 4π-conrotatory electrocyclic opening of the cyclobutene ring. The resulting conjugated species
30:
This article is about cascade reactions in synthetic organic chemistry. For series of consecutive biochemical reactions, see
841:
This formal cycloaddition was proposed to proceed via the cascade process shown in Scheme 15. Complexation of the 1,6-enyne
1773:
1795:
41:
Cascade reactions are often key steps in the efficient total synthesis of complex natural products. The key step in
1790:
192:
was obtained selectively. The cascade was completed by an intramolecular aldol condensation that afforded product
849:, in which the activated triple bond is attacked by the olefin functionality to yield substituted cyclopropane
458:
A cascade radical process was also used in one of the total syntheses of (–)-morphine (Scheme 7). Aryl bromide
67:
539:, which upon heating underwent an 8π-conrotatory electrocyclic ring closure, yielding cyclic intermediate
37:
180:, which more readily underwent an 8π-conrotatory electrocyclization to the highly strained intermediate
588:. A spontaneous intramolecular cycloaddition of the 1,3-dipole and the indole system then formed the
276:
An outstanding triple organocatalytic cascade was reported by Raabe et al. in 2006. Linear aldehydes (
965:
31:
857:, which undergoes a Friedel Crafts-type reaction and then rearomatizes to give tricyclic product
960:
Four chemical transformations happened in this tandem reaction. First, treating fragment A with
104:
was first treated with dichloroacetonitrile in the presence of NaH. The resulting intermediate
1746:
Nicolaou, K. C.; Edmonds, David J.; Bulger, Paul G. Angew. Chem. Int. Ed. 2006, 45, 7134-7186.
543:. A second spontaneous electrocyclization, this time a 6π-disrotatory ring closure, converted
62:
42:
116:
O-mediated cascade reaction. Intramolecular opening of the epoxide ring yielded intermediate
584:. Thermodynamically favorable loss of nitrogen generated the 1,3-dipole-containing species
935:
932:
494:-trig cyclization. Subsequent elimination of the phenyl sulfinyl radical afforded product
263:
161:
983:
Fig. 2: Representative examples of synthetic targeting using polyring forming processes
961:
922:
Palladium-catalyzed Heck cascade in the enantioselective synthesis of (+)-xestoquinone
915:
Palladium-catalyzed Heck cascade in the enantioselective synthesis of (+)-xestoquinone
1784:
713:
For instance, rhodium catalysis was used to convert acyclic monoterpenes of the type
1768:
733:
via a carbonyl-ene reaction. A second rhodium-catalyzed hydroformylation to species
368:, which is prone to undergo an intramolecular aldol condensation to iminium species
333:-substituted cyclohexane carbaldehydes via a triple organocatalytic cascade reaction
322:-substituted cyclohexane carbaldehydes via a triple organocatalytic cascade reaction
78:
complexity via the process, and its applicability to broader classes of substrates.
74:
878:
Proposed cascade process in the formal intramolecular cycloaddition of 1,6-enynes
871:
Proposed cascade process in the formal intramolecular cycloaddition of 1,6-enynes
777:
with rhodium(II) acetate dimer generated a carbenoid that yielded reactive ylide
196:
in 76% overall yield. Further elaboration afforded the target (±)-pentalenene (
17:
945:
897:. A second migratory insertion into the remaining olefin group followed by a
596:
in 78% overall yield. Further elaboration yielded the target natural product
942:
82:
853:. Electrophilic opening of the three-membered ring forms cationic species
397:
Proposed catalytic cycle for the asymmetric triple organocatalytic cascade
390:
Proposed catalytic cycle for the asymmetric triple organocatalytic cascade
644:
via a heat-facilitated Diels-Alder reaction followed by cleavage of the
979:
952:
802:
Rhodium(II)-carbenoid-initiated cascade in the synthesis of a tigliane
795:
Rhodium(II)-carbenoid-initiated cascade in the synthesis of a tigliane
486:-trig cyclization pathway; instead secondary benzylic radical species
729:, which under the same conditions was then converted to intermediate
498:
in 30% overall yield, which was further elaborated to (–)-morphine (
805:
The formal intramolecular cycloaddition of 1,6-enynes of the type
766:
Rhodium-catalyzed hydroformylation cascade for the preparation of 4
755:
Rhodium-catalyzed hydroformylation cascade for the preparation of 4
455:
Cascade radical cyclization in the total synthesis of (±)-hirsutene
448:
Cascade radical cyclization in the total synthesis of (±)-hirsutene
360:
with the organocatalyst then facilitates the conjugate addition of
1726:
Maddaford, S. P.; Andersen, N. G.; Cristofoli, W. A.; Keay, B. A.
978:
973:
951:
572:
Pericyclic cascade in the synthesis of endiandric acid derivatives
565:
Pericyclic cascade in the synthesis of endiandric acid derivatives
273:
Organocatalytic cascade in the total synthesis of (+)-harziphilone
1546:
Elliott, G. I.; Velcicky, J.; Ishikawa, H.; Li, Y.; Boger, D. L.
838:
Gold-catalyzed formal intramolecular cycloaddition of 1,6-enynes
831:
Gold-catalyzed formal intramolecular cycloaddition of 1,6-enynes
665:
Electrocyclic cascade in the total synthesis of (–)-colombiasin A
658:
Electrocyclic cascade in the total synthesis of (–)-colombiasin A
969:
1569:
Harrowven, D. C.; Pascoe, D. D.; Demurtas, D.; Bourne, H. O.
1523:
Nicolaou, K. C.; Petasis, N. A.; Zipkin, R. E.; Uenishi, J.
907:
863:
823:
787:
747:
686:
650:
617:
Pericyclic cascade in the total synthesis of (–)-vindorosine
610:
Pericyclic cascade in the total synthesis of (–)-vindorosine
602:
557:
519:
Cascade radical cyclization in the synthesis of (–)-morphine
512:
Cascade radical cyclization in the synthesis of (–)-morphine
504:
440:
382:
310:
296:) could be condensed together organocatalytically to afford
262:
202:
138:
845:
with the cationic form of the catalyst yields intermediate
164:. The two nucleophilic attacks occurred predominantly with
153:
Synthesis of (–)-chloramphenicol via a nucleophilic cascade
146:
Synthesis of (–)-chloramphenicol via a nucleophilic cascade
535:
was first hydrogenated to the conjugated tetraene species
217:
Cascade reaction in the total synthesis of (±)-pentalenene
210:
Cascade reaction in the total synthesis of (±)-pentalenene
184:. The potential to release strain directed protonation of
160:
was treated with (5-methylcyclopent-1-en-1-yl)lithium and
1253:
Wasilke, J. C.; Obrey, S. J.; Baker, R. T.; Bazan, G. C.
968:
on intermediate C (step 2). The protecting group on 1, 3-
701:
Pericyclic sequence for the synthesis of paracyclophanes
694:
Pericyclic sequence for the synthesis of paracyclophanes
680:, which subsequently dimerized to yield paracyclophane
344:
occurs through enamine catalysis, yielding nitroalkane
83:
the synthesis of tropinone reported in 1917 by
Robinson
81:
The earliest example of a cascade reaction is arguably
434:, which upon quenching gave the target (±)-hirsutene (
632:, which upon exposure to air was oxidized to product
474:-trig cyclization then occurred to give intermediate
376:
is regenerated by hydrolysis, along with the product
1414:
Enders, D.; Hüttl, M. R. M.; Grondal, C.; Raabe, G.
1339:
Bhaskar, G.; Satish Kumar, V.; Venkateswara Rao, B.
636:
in 80% overall yield. The target (–)-colombiasin A (
628:. Tautomerization thereof gave the aromatic species
73:
The main benefits of cascade sequences include high
462:was converted to the corresponding radical species
1703:Nieto-Oberhuber, C.; López, S.; Echavarren, A. M.
414:was converted to the primary radical intermediate
1160:Nicolaou, K. C.; Edmonds, D. J.; Bulger, P. G.
676:first formed the highly reactive intermediate
430:-dig radical cyclization lead to intermediate
422:-trig cyclization to afford reactive species
8:
889:)-binap yields chiral palladium(II) complex
901:-elimination then occurs to afford product
490:was obtained via a geometrically-allowed 6-
1385:Stark, L. M.; Pekari, K.; Sorensen, E. J.
555:) was thus obtained in 23% overall yield.
1296:Enders, D.; Grondal, C.; Hüttl, M. R. M.
380:, thus closing the triple cascade cycle.
1645:Dauben, W. G.; Dinges, J.; Smith, T. C.
300:-substituted cyclohexane carbaldehydes (
36:
993:
1722:
1720:
1699:
1697:
1695:
1693:
1672:
1670:
1668:
1666:
1664:
1662:
1641:
1639:
1611:
1609:
1588:
1586:
1565:
1563:
1542:
1540:
1519:
1517:
1489:
1487:
1460:
1458:
1433:
1431:
1410:
1408:
1406:
1404:
1402:
737:was followed by condensation to form 4
1676:Jiménez-Núñez, E.; Echavarren, A. M.
1592:Hopf, H.; Bohm, I.; Kleinschroth, J.
1381:
1379:
1358:
1356:
1335:
1333:
1292:
1290:
1249:
1247:
1245:
1243:
1156:
1154:
1152:
1150:
1148:
1146:
1144:
1142:
1140:
1138:
1136:
1134:
1132:
1130:
1128:
1126:
1124:
1122:
1120:
1118:
1116:
941:In the total synthesis of spiroketal
7:
1316:Grondal, C.; Jeanty, M.; Enders, D.
1222:
1220:
1179:
1177:
1114:
1112:
1110:
1108:
1106:
1104:
1102:
1100:
1098:
1096:
1075:
1073:
1071:
1069:
1067:
1065:
1063:
1042:
1040:
1038:
1036:
1034:
1032:
1011:
1009:
1007:
1005:
1003:
1001:
999:
997:
956:Fig. 1: Structure of Routiennocin 1
705:Transition-metal-catalyzed cascades
124:hydrolysis facilitated by excess BF
93:Nucleophilic/electrophilic cascades
25:
821:in moderate to excellent yields.
482:forbid the kinetically favored 5-
132:O, afforded (–)-chloramphenicol (
817:to yield the tricyclic products
168:addition to afford intermediate
1387:Proc. Natl. Acad. Sci. U. S. A.
1615:Roggenbuck, R.; Eilbracht, P.
741:-chromen products of the type
244:, which afforded intermediate
1:
725:yielded unsaturated aldehyde
364:to give intermediate enamine
1774:The Periodic Table of Videos
1437:Curran, D. P.; Chen, M.-H.
1015:Tietze, L. F.; Beifuss, U.
1812:
1777:(University of Nottingham)
1362:Paquette, L. A.; Geng, F.
926:Multistep tandem reactions
176:equilibrated to conformer
29:
1493:Parker, K. A.; Fokas, D.
1464:Parker, K. A.; Fokas, D.
648:-butyl protecting group.
640:) was then obtained from
547:to the bicyclic species
438:) in 80% overall yield.
329:Asymmetric synthesis of
318:Asymmetric synthesis of
292:-unsaturated aldehydes (
221:Organocatalytic cascades
68:multicomponent reactions
1273:Chapman, C.; Frost, C.
745:in 40% overall yield.
470:-butyltin hydride. A 5-
260:) in 70% overall yield.
136:) in 71% overall yield.
1341:Tetrahedron: Asymmetry
1046:Padwa, A.; Bur, S. K.
984:
957:
916:
872:
832:
796:
760:
695:
659:
611:
566:
513:
466:by treatment with tri-
449:
418:, which underwent a 5-
391:
356:-unsaturated aldehyde
323:
267:
211:
147:
46:
1571:Angew. Chem. Int. Ed.
1548:Angew. Chem. Int. Ed.
1298:Angew. Chem. Int. Ed.
1162:Angew. Chem. Int. Ed.
1017:Angew. Chem. Int. Ed.
982:
955:
911:
867:
827:
791:
751:
690:
654:
606:
561:
508:
444:
386:
314:
266:
256:to (+)-harziphilone (
236:yielded intermediate
206:
142:
40:
1205:J. Chem. Soc. Trans.
966:elimination reaction
232:with organocatalyst
1275:Synthesis (Stuttg).
523:Pericyclic cascades
108:then underwent a BF
32:Biochemical cascade
1796:Chemical synthesis
985:
958:
917:
873:
833:
797:
761:
696:
660:
612:
567:
514:
450:
392:
348:. Condensation of
324:
268:
212:
188:such that species
148:
120:, which, after an
63:one-pot procedures
53:, also known as a
47:
1791:Organic chemistry
1728:J. Am. Chem. Soc.
1705:J. Am. Chem. Soc.
1525:J. Am. Chem. Soc.
426:. A subsequent 5-
372:. Organocatalyst
280:), nitroalkenes (
16:(Redirected from
1803:
1756:
1753:
1747:
1744:
1738:
1724:
1715:
1701:
1688:
1674:
1657:
1643:
1634:
1613:
1604:
1590:
1581:
1567:
1558:
1544:
1535:
1521:
1512:
1491:
1482:
1462:
1453:
1435:
1426:
1412:
1397:
1383:
1374:
1360:
1351:
1337:
1328:
1314:
1308:
1294:
1285:
1271:
1265:
1251:
1238:
1224:
1215:
1201:
1195:
1181:
1172:
1158:
1091:
1077:
1058:
1044:
1027:
1013:
401:Radical cascades
51:cascade reaction
27:Chemical process
21:
1811:
1810:
1806:
1805:
1804:
1802:
1801:
1800:
1781:
1780:
1765:
1760:
1759:
1754:
1750:
1745:
1741:
1725:
1718:
1702:
1691:
1675:
1660:
1644:
1637:
1614:
1607:
1591:
1584:
1568:
1561:
1545:
1538:
1522:
1515:
1492:
1485:
1463:
1456:
1436:
1429:
1413:
1400:
1384:
1377:
1361:
1354:
1338:
1331:
1315:
1311:
1295:
1288:
1272:
1268:
1252:
1241:
1226:Pellissier, H.
1225:
1218:
1202:
1198:
1182:
1175:
1159:
1094:
1079:Pellissier, H.
1078:
1061:
1045:
1030:
1014:
995:
990:
936:total synthesis
933:natural product
928:
923:
879:
839:
803:
771:
707:
702:
672:and dienophile
666:
618:
573:
525:
520:
456:
403:
398:
340:to nitroalkene
334:
274:
223:
218:
162:propynyllithium
154:
131:
127:
115:
111:
95:
59:tandem reaction
55:domino reaction
35:
28:
23:
22:
18:Tandem reaction
15:
12:
11:
5:
1809:
1807:
1799:
1798:
1793:
1783:
1782:
1779:
1778:
1769:Chemical Knots
1764:
1763:External links
1761:
1758:
1757:
1748:
1739:
1737:, 10766–10773.
1716:
1689:
1658:
1635:
1605:
1582:
1559:
1536:
1513:
1483:
1469:Am. Chem. Soc.
1454:
1427:
1398:
1396:, 12064–12066.
1375:
1352:
1329:
1309:
1286:
1266:
1239:
1216:
1196:
1183:Tietze, L. F.
1173:
1092:
1059:
1028:
992:
991:
989:
986:
962:n-butyllithium
927:
924:
918:
874:
834:
798:
762:
706:
703:
697:
661:
613:
568:
524:
521:
515:
451:
402:
399:
393:
325:
269:
222:
219:
213:
149:
129:
125:
113:
109:
94:
91:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1808:
1797:
1794:
1792:
1789:
1788:
1786:
1776:
1775:
1770:
1767:
1766:
1762:
1752:
1749:
1743:
1740:
1736:
1732:
1729:
1723:
1721:
1717:
1713:
1709:
1706:
1700:
1698:
1696:
1694:
1690:
1686:
1682:
1679:
1673:
1671:
1669:
1667:
1665:
1663:
1659:
1655:
1651:
1648:
1647:J. Org. Chem.
1642:
1640:
1636:
1633:
1629:
1625:
1621:
1618:
1612:
1610:
1606:
1602:
1598:
1595:
1589:
1587:
1583:
1579:
1575:
1572:
1566:
1564:
1560:
1556:
1552:
1549:
1543:
1541:
1537:
1533:
1529:
1526:
1520:
1518:
1514:
1511:
1507:
1503:
1499:
1496:
1490:
1488:
1484:
1481:
1477:
1473:
1470:
1467:
1461:
1459:
1455:
1451:
1447:
1443:
1440:
1434:
1432:
1428:
1424:
1420:
1417:
1411:
1409:
1407:
1405:
1403:
1399:
1395:
1391:
1388:
1382:
1380:
1376:
1372:
1368:
1365:
1359:
1357:
1353:
1349:
1345:
1342:
1336:
1334:
1330:
1326:
1322:
1319:
1313:
1310:
1306:
1302:
1299:
1293:
1291:
1287:
1283:
1279:
1276:
1270:
1267:
1263:
1259:
1256:
1250:
1248:
1246:
1244:
1240:
1236:
1232:
1229:
1223:
1221:
1217:
1213:
1209:
1206:
1203:Robinson, R.
1200:
1197:
1193:
1189:
1186:
1180:
1178:
1174:
1170:
1166:
1163:
1157:
1155:
1153:
1151:
1149:
1147:
1145:
1143:
1141:
1139:
1137:
1135:
1133:
1131:
1129:
1127:
1125:
1123:
1121:
1119:
1117:
1115:
1113:
1111:
1109:
1107:
1105:
1103:
1101:
1099:
1097:
1093:
1089:
1085:
1082:
1076:
1074:
1072:
1070:
1068:
1066:
1064:
1060:
1056:
1052:
1049:
1043:
1041:
1039:
1037:
1035:
1033:
1029:
1025:
1021:
1018:
1012:
1010:
1008:
1006:
1004:
1002:
1000:
998:
994:
987:
981:
977:
975:
971:
967:
963:
954:
950:
947:
944:
939:
937:
934:
925:
921:
914:
910:
906:
904:
900:
896:
892:
888:
884:
877:
870:
866:
862:
860:
856:
852:
848:
844:
837:
830:
826:
822:
820:
816:
812:
808:
801:
794:
790:
786:
784:
780:
776:
769:
765:
758:
754:
750:
746:
744:
740:
736:
732:
728:
724:
720:
716:
711:
704:
700:
693:
689:
685:
683:
679:
675:
671:
664:
657:
653:
649:
647:
643:
639:
635:
631:
627:
623:
616:
609:
605:
601:
599:
595:
591:
587:
583:
579:
571:
564:
560:
556:
554:
550:
546:
542:
538:
534:
529:
522:
518:
511:
507:
503:
501:
497:
493:
489:
485:
481:
477:
473:
469:
465:
461:
454:
447:
443:
439:
437:
433:
429:
425:
421:
417:
413:
407:
400:
396:
389:
385:
381:
379:
375:
371:
367:
363:
359:
355:
351:
347:
343:
339:
332:
328:
321:
317:
313:
309:
307:
303:
299:
295:
291:
287:
283:
279:
272:
265:
261:
259:
255:
251:
247:
243:
239:
235:
231:
226:
220:
216:
209:
205:
201:
199:
195:
191:
187:
183:
179:
175:
171:
167:
163:
159:
152:
145:
141:
137:
135:
123:
119:
107:
103:
98:
92:
90:
86:
84:
79:
76:
71:
69:
64:
60:
56:
52:
44:
39:
33:
19:
1772:
1751:
1742:
1734:
1730:
1727:
1714:, 6178–6179.
1711:
1707:
1704:
1684:
1680:
1677:
1656:, 7635–7637.
1653:
1649:
1646:
1631:
1627:
1623:
1619:
1616:
1600:
1596:
1593:
1580:, 1221–1222.
1577:
1573:
1570:
1554:
1550:
1547:
1534:, 5555–5557.
1531:
1527:
1524:
1509:
1505:
1501:
1497:
1494:
1479:
1475:
1471:
1468:
1465:
1452:, 4991–4994.
1449:
1445:
1441:
1438:
1422:
1418:
1415:
1393:
1389:
1386:
1373:, 4547–4549.
1370:
1366:
1363:
1350:, 1279–1283.
1347:
1343:
1340:
1324:
1320:
1317:
1312:
1307:, 1570–1581.
1304:
1300:
1297:
1281:
1277:
1274:
1269:
1264:, 1001–1020.
1261:
1257:
1254:
1237:, 2143–2173.
1234:
1230:
1227:
1211:
1207:
1204:
1199:
1191:
1187:
1184:
1171:, 7134–7186.
1168:
1164:
1161:
1090:, 1619–1665.
1087:
1083:
1080:
1057:, 5341–5378.
1054:
1050:
1047:
1023:
1019:
1016:
959:
940:
929:
919:
912:
902:
898:
894:
890:
886:
882:
880:
875:
868:
858:
854:
850:
846:
842:
840:
835:
828:
818:
814:
810:
806:
804:
799:
792:
782:
778:
774:
772:
767:
763:
756:
752:
742:
738:
734:
730:
726:
722:
718:
714:
712:
708:
698:
691:
681:
677:
673:
669:
667:
662:
655:
645:
641:
637:
633:
629:
625:
621:
619:
614:
607:
597:
593:
589:
585:
581:
577:
574:
569:
562:
552:
548:
544:
540:
536:
532:
530:
526:
516:
509:
499:
495:
491:
487:
483:
479:
475:
471:
467:
463:
459:
457:
452:
445:
435:
431:
427:
423:
419:
415:
411:
408:
404:
394:
387:
377:
373:
369:
365:
361:
357:
353:
349:
345:
341:
337:
335:
330:
326:
319:
315:
305:
301:
297:
293:
289:
285:
281:
277:
275:
270:
257:
253:
249:
245:
241:
237:
233:
229:
227:
224:
214:
207:
197:
193:
189:
185:
181:
177:
173:
169:
165:
157:
155:
150:
143:
133:
121:
117:
105:
101:
99:
96:
87:
80:
75:atom economy
72:
58:
54:
50:
48:
1630:, 7455–7456
1617:Tetrahedron
1594:Org. Synth.
1478:, 9688–9689
1439:Tetrahedron
1228:Tetrahedron
1081:Tetrahedron
1048:Tetrahedron
1785:Categories
1678:Chem. Rev.
1557:, 620–622.
1425:, 861–863.
1364:Org. Lett.
1327:, 167–178.
1318:Nat. Chem.
1255:Chem. Rev.
1194:, 115–136.
1185:Chem. Rev.
1026:, 131–163.
988:References
946:antibiotic
920:Scheme 16.
913:Scheme 16.
876:Scheme 15.
869:Scheme 15.
836:Scheme 14.
829:Scheme 14.
800:Scheme 13.
793:Scheme 13.
764:Scheme 12.
753:Scheme 12.
699:Scheme 11.
692:Scheme 11.
663:Scheme 10.
656:Scheme 10.
1508:, 449–455
1498:Org. Chem
943:ionophore
770:-chromens
759:-chromens
615:Scheme 9.
608:Scheme 9.
592:-product
570:Scheme 8.
563:Scheme 8.
517:Scheme 7.
510:Scheme 7.
453:Scheme 6.
446:Scheme 6.
395:Scheme 5.
388:Scheme 5.
327:Scheme 4.
316:Scheme 4.
271:Scheme 3.
252:-dienone
215:Scheme 2.
208:Scheme 2.
151:Scheme 1.
144:Scheme 1.
43:Heathcock
1687:, 3326.
1284:, 1–21.
122:in situ
1416:Nature
1214:, 762.
284:) and
1603:, 41.
974:ketal
331:tetra
320:tetra
298:tetra
166:trans
1731:1996
1708:2005
1681:2008
1650:1993
1624:1999
1620:Lett
1597:1981
1574:2005
1551:2006
1528:1982
1502:2006
1472:1992
1446:1985
1442:Lett
1419:2006
1390:2004
1367:2002
1344:2004
1321:2010
1301:2007
1282:2007
1278:2007
1258:2005
1231:2006
1208:1917
1188:1996
1165:2006
1084:2006
1051:2007
1020:1993
970:diol
717:to 4
646:tert
590:endo
492:endo
1771:at
1735:118
1712:127
1685:108
1532:104
1476:114
1423:441
1394:101
1262:105
1212:111
811:68a
502:).
484:exo
472:exo
428:exo
420:exo
250:cis
128:·Et
112:·Et
57:or
1787::
1733:,
1719:^
1710:,
1692:^
1683:,
1661:^
1654:58
1652:,
1638:^
1628:40
1626:,
1622:.
1608:^
1601:60
1599:,
1585:^
1578:44
1576:,
1562:^
1555:45
1553:,
1539:^
1530:,
1516:^
1506:71
1504:,
1500:.
1495:J.
1486:^
1474:,
1466:J.
1457:^
1450:26
1448:,
1444:.
1430:^
1421:,
1401:^
1392:,
1378:^
1369:,
1355:^
1348:15
1346:,
1332:^
1323:,
1305:46
1303:,
1289:^
1280:,
1260:,
1242:^
1235:62
1233:,
1219:^
1210:,
1192:96
1190:,
1176:^
1169:45
1167:,
1095:^
1088:62
1086:,
1062:^
1055:63
1053:,
1031:^
1024:32
1022:,
996:^
938:.
903:81
895:79
891:77
883:75
859:69
855:72
851:71
847:70
843:67
819:69
807:67
785:.
783:66
779:65
775:64
743:63
735:62
731:61
727:60
723:59
715:59
684:.
682:58
678:57
674:56
670:55
642:53
638:54
634:53
630:52
626:51
622:49
600:.
598:48
594:47
586:46
582:45
578:44
553:43
549:42
545:41
541:41
537:40
533:39
500:38
496:37
488:36
480:35
476:35
464:34
460:33
436:32
432:31
424:30
416:29
412:28
378:24
374:23
370:27
366:26
362:25
358:22
346:25
342:21
338:20
308:.
306:23
302:24
294:22
282:21
278:20
258:19
254:18
246:17
242:16
238:15
234:14
230:13
200:).
198:12
194:11
190:10
70:.
49:A
1632:.
1510:.
1480:.
1371:4
1325:2
899:β
887:S
815:b
813:–
768:H
757:H
739:H
719:H
468:n
354:β
352:,
350:α
290:β
288:,
286:α
186:9
182:9
178:8
174:7
170:6
158:5
134:4
130:2
126:3
118:3
114:2
110:3
106:2
102:1
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.