314:
about 0.5, where the equilibrium constant is defined as K = /. Hemiacetals of ketones (sometimes called hemiketals) are even less stable than those of aldehydes. However, cyclic hemiacetals and hemiacetals bearing electron withdrawing groups are stable. Electron-withdrawing groups attached to the carbonyl atom shift the equilibrium constant toward the hemiacetal. They increase the polarization of the carbonyl group, which already has a positively polarized carbonyl carbon, and make it even more prone to attack by a nucleophile. The chart below shows the extent of hydration of some carbonyl compounds.
345:
consumed for every one produced. In contrast, the formation of cyclic hemiacetals involves a single molecule reacting with itself, making the reaction more favorable. Another way to understand the stability of cyclic hemiacetals is to look at the equilibrium constant as the ratio of the forward and backward reaction rate. For a cyclic hemiacetal the reaction is intramolecular so the nucleophile is always held close to the carbonyl group ready to attack, so the forward rate of reaction is much higher than the backward rate. Many biologically relevant sugars, such as
234:
285:
89:
365:
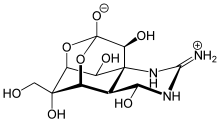
215:-brosylmitomycin A, crystallized in 1967. The tetrahedral carbon C17 forms a 136.54 pm bond with O3, which is shorter than C8-O3 bond (142.31 pm). In contrast, C17-N2 bond (149.06 pm) is longer than N1-C1 bond (148.75 pm) and N1-C11 bond (147.85 pm) due to donation of O3 lone pair into σ* orbital of C17-N2. This model however is forced into tetracyclic sceleton, and tetrahedral O3 is methylated which makes it a poor model overall.
381:
219:
120:
353:
421:
326:
469:
340:(bond angles forced to be 60˚), sp hybridization is more favorable than sp hybridization. For the sp hybridized hydrate the bonds have to be distorted by about 49˚, while for the sp hybridized ketone the bond angle distortion is about 60˚. So the addition to the carbonyl group allows some of the strain inherent in the small ring to be released, which is why cyclopropanone and
165:
439:
binding site of the ligand, to produce a solvated complex. Because this necessarily means that the interaction is entropically disfavored, highly favorable enthalpic contacts between the protein and the ligand must compensate for the entropic loss. The design of new ligands is usually based on the modification of known ligands for the target proteins.
372:
Acetals, as already pointed out, are stable tetrahedral intermediates so they can be used as protective groups in organic synthesis. Acetals are stable under basic conditions, so they can be used to protect ketones from a base. The acetal group is hydrolyzed under acidic conditions. An example with a
153:
with the carbonyl group, which means that addition to the carbonyl group is thermodynamically less favored than addition to corresponding aldehyde or ketone. Stable tetrahedral intermediates of carboxylic acid derivatives do exist and they usually possess at least one of the following four structural
144:
Although the tetrahedral intermediates are usually transient intermediates, many compounds of this general structures are known. The reactions of aldehydes, ketones, and their derivatives frequently have a detectable tetrahedral intermediate, while for the reactions of derivatives of carboxylic acids
438:
A solvated ligand that binds the protein of interest is likely to exist as an equilibrium mixture of several conformers. Likewise the solvated protein also exists as several conformers in equilibrium. Formation of protein-ligand complex includes displacement of the solvent molecules that occupy the
313:
in synthetic chemistry. A very well known reaction occurs when acetaldehyde is dissolved in methanol, producing a hemiacetal. Most hemiacetals are unstable with respect to their parent aldehydes and alcohols. For example, the equilibrium constant for reaction of acetaldehyde with simple alcohols is
203:
The first X-ray crystal structures of tetrahedral intermediates were obtained in 1973 from bovine trypsin crystallized with bovine pancreatic trypsin inhibitor, and in 1974 from porcine trypsin crystallized with soybean trypsin inhibitor. In both cases the tetrahedral intermediate is stabilized in
464:
Stabilization of tetrahedral intermediates inside of the enzyme active site has been investigated using tetrahedral intermediate mimics. The specific binding forces involved in stabilizing the transition state have been describe crystallographycally. In the mammalian serine proteases, trypsin and
110:
in 1951. He labeled carboxylic acid derivatives with oxygen isotope O18 and reacted these derivatives with water to make labeled carboxylic acids. At the end of the reaction he found that the remaining starting material had a decreased proportion of labeled oxygen, which is consistent with the
443:
are enzymes that catalyze hydrolysis of a peptide bond. These proteins have evolved to recognize and bind the transition state of peptide hydrolysis reaction which is a tetrahedral intermediate. Therefore, the main protease inhibitors are tetrahedral intermediate mimics having an alcohol or a
360:
In the presence of acid, hemiacetals can undergo an elimination reaction, losing the oxygen atom that once belonged to the parent aldehyde’s carbonyl group. These oxonium ions are powerful electrophiles, and react rapidly with a second molecule of alcohol to form new, stable compounds, called
344:
are very reactive electrophiles. For larger rings, where the bond angles are not as distorted, the stability of the hemiacetals is due to entropy and the proximity of the nucleophile to the carbonyl group. Formation of an acyclic acetal involves a decrease in entropy because two molecules are
229:
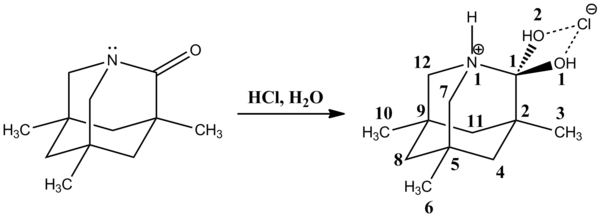
The more recent x-ray crystal structure of 1-aza-3,5,7-trimethyladamantan-2-one is a good model for cationic tetrahedral intermediate. The C1-N1 bond is rather long , and C1-O1(2) bonds are shortened . The protonated nitrogen atom N1 is a great amine leaving group.
412:
five-membered ring intermediate. Quantum mechanical calculations have shown that the tetrahedral adduct is formed easily and it is fairly stable, in agreement with the experimental results. The very facile reaction of
Weinreb amides with organolithium and
465:
chymotrypsin, two peptide NH groups of the polypeptide backbone form the so-called oxyanion hole by donating hydrogen bonds to the negatively charged oxygen atom of the tetrahedral intermediate. A simple diagram describing the interaction is shown below.
734:
Ruhlmann, A.; Kukla D.; Schwager P.; Bartels K.; Huber R. (1973). "Structure of the complex formed by bovine trypsin and bovine pancreatic trypsin inhibitor. Crystal structure determination and stereochemistry of the contact region".
79:
with sodium benzyloxide, he observed a white precipitate which under acidic conditions yields benzyl benzoate, methyl benzoate, methanol, and benzyl alcohol. He named the likely common intermediate “
195:
These compounds were used to study the kinetics of tetrahedral intermediate decomposition into its respective carbonyl species, and to measure the IR, UV, and NMR spectra of the tetrahedral adduct.
131:. The angle between the line of nucleophilic attack and the C-O bond is greater than 90˚ due to a better orbital overlap between the HOMO of the nucleophile and the π* LUMO of the C-O double bond.
39:
attached to the new tetrahedral carbon atom to leave with the negative charge. Tetrahedral intermediates are very significant in organic syntheses and biological systems as a key intermediate in
257:-OH bond which is about 143.2 pm. The elongated C1-N1, and shortened C1-O1 bonds are explained with an anomeric effect resulting from the interaction of the oxygen lone pairs with the σ*
844:
Kirby, A. J.; Komarov I.V.; Feeder N. (1998). "Spontaneous, Millisecond
Formation of a Twisted Amide from the Amino Acid, and the Crystal Structure of a Tetrahedral Intermediate".
770:
Sweet, R.M.; Wright H.T.; Clothia C.H.; Blow D.M. (1974). "Crystal structure of the complex of porcine trypsin with soybean trypsin inhibitor (Kunitz) at 2.6 Å resolution".
417:
results from the chelate stabilization in the tetrahedral adduct and, more importantly, the transition state leading to the adduct. The tetrahedral adducts are shown below.
309:
are essentially tetrahedral intermediates. They form when nucleophiles add to a carbonyl group, but unlike tetrahedral intermediates they can be very stable and used as
27:
in which the bond arrangement around an initially double-bonded carbon atom has been transformed from trigonal to tetrahedral. Tetrahedral intermediates result from
245:-acylpyrroles with organometallic compounds, followed by protonation with ammonium chloride producing a carbinol. The C1-N1 bond is longer than the usual C
364:
871:
Evans, D. A.; G. Borg; K. A. Scheidt (2002). "Remarkably Stable
Tetrahedral Intermediates: Carbinols from Nucleophilic Additions to N–Acylpyrroles".
233:
703:"Mono S-Acylated 1,8-Naphthalenedithiol. Isolation and Characterization of Tetrahedral Intermediate in the Intramolecular Acyl Transfer Reaction"
420:
284:
919:
884:
989:
Adler, M.; Adler S.; Boche G. (2005). "Tetrahedral intermediates in reactions of carboxylic acid derivatives with nucleophiles".
1116:
404:-methylcarboxylic acid amides. Weinreb amides are reacted with organometallic compounds to give, on protonation, ketones (see
88:
106:
The first evidence for tetrahedral intermediates in the substitution reactions of carboxylic derivatives was provided by
380:
1126:
1121:
1016:
Babine, R. E.; Bender S. L. (1997). "Molecular
Recognition of Protein−Ligand Complexes: Applications to Drug Design".
128:
572:(1901). "Mixed organomagnesium combinations and their application in acid, alcohol, and hydrocarbon synthesis".
325:
678:
Cerrini, S.; Fedeli W.; Mazza F. (1971). "X-Ray crystallographic proof of a cyclol structure in a tripeptide".
405:
218:
336:- three-membered ring ketones- are also hydrated to a significant extent. Since three-membered rings are very
651:
Gideon, Fraenkel; Watson Debra (1975). "Alkoxide adduct of an amide. Mean lifetime of an intimate ion pair".
352:
145:
this is not the case. At the oxidation level of carboxylic acid derivatives, the groups such as OR, OAr, NR
211:
Some insight into the structure of tetrahedral intermediate can be obtained from the crystal structure of
621:
535:
508:
99:
assumed the existence of unstable tetrahedral intermediate in 1901, while investigating the reaction of
64:
28:
24:
241:
In 2002 David Evans et al. observed a very stable neutral tetrahedral intermediate in the reaction of
1064:
253:
bond which range from 141.2-145.8 pm. In contrast, the C1-O1 bond is shorter than the average C
191:
compounds with sulfur atoms bonded to the anomeric centre (e.g., S-acylated-1,8-naphthalenedithiol)
44:
468:
408:). It is generally accepted that the high yields of ketones are due to the high stability of the
594:(1951). "Oxygen Exchange as Evidence for the Existence of an Intermediate in Ester Hydrolysis".
322:
reacts with water so readily because its substituents are very small- a purely steric effect.
184:
compounds with donor groups that are poorly conjugated with the potential carbonyl group (e.g.
1092:
1033:
951:
915:
826:
787:
752:
315:
150:
1082:
1072:
1025:
998:
971:
907:
880:
853:
818:
779:
744:
714:
683:
660:
633:
603:
551:
494:
414:
310:
273:
bonds which are 151.3 pm. Also, the C1-C11 bond is slightly shorter than the average C
205:
72:
591:
569:
521:
107:
96:
76:
68:
1068:
119:
333:
1087:
1052:
975:
911:
265:
orbital should be responsible for the lengthened C1-C2 bond compared to the average C
1110:
748:
393:
341:
173:
compounds with a strong electron-withdrawing group attached to the acyl carbon (e.g.
36:
319:
159:
361:
acetals. The whole mechanism of acetal formation from hemiacetal is drawn below.
805:
Tulinsky, A.; Van den Hende J.H. (1967). "The crystal and molecular structure of
337:
35:
group. The stability of tetrahedral intermediate depends on the ability of the
555:
445:
302:
1077:
499:
453:
449:
374:
1053:"Site-directed mutagenesis and the role of the oxyanion hole in subtilisin"
1037:
885:
10.1002/1521-3757(20020902)114:17<3320::aid-ange3320>3.0.co;2-u
1096:
830:
791:
756:
164:
1051:
Bryan, P.; Pantoliano M. W.; Quill S. G.; Hsiao H. Y.; Poulos T. (1986).
719:
702:
687:
440:
52:
32:
822:
783:
664:
637:
607:
490:
409:
346:
63:
One of the earliest accounts of the tetrahedral intermediate came from
1029:
898:
Bell, R. P. (1966). "The reversible hydration of carbonyl compounds".
857:
306:
185:
100:
48:
1002:
539:
163:
118:
40:
368:
Acid catalyzed acetal formation from the corresponding hemiacetal
261:
orbital. Similarly, an interaction of an oxygen lone pair with σ*
204:
the active sites of enzymes, which have evolved to stabilize the
424:
Weinreb ketone synthesis and tetrahedral intermediate stability
127:
The nucleophilic attack on the carbonyl group proceeds via the
624:; Gougoutas, J. Z. (1964). "The Structure of Tetrodotoxin".
467:
419:
379:
363:
351:
324:
283:
232:
217:
87:
935:
Clayden J.; Greeves N.; Warren S. & Wothers P. (2001).
540:"Ueber die Einwirkung von Natriumalkylaten auf Benzaldehyd"
318:
is probably the most hydrated carbonyl compound possible.
55:, hydride reductions, and other chemical reactions.
47:, ester hydrolysis, formation and hydrolysis of
962:-methylamides as effective acylating agents".
8:
701:Tagaki, M.; Ishahara R.; Matsudu T. (1977).
80:
111:existence of the tetrahedral intermediate.
902:. Advances in Physical Organic Chemistry.
1086:
1076:
718:
498:
482:
517:
506:
293:Stability of tetrahedral intermediates
135:Structure of tetrahedral intermediates
199:X-ray crystal structure determination
7:
237:1-aza-3,5,7-trimethyladamantan-2-one
14:
377:protecting group is given below.
349:, are cyclic hemiacetals.
288:Carbinol tetrahedral intermediate
281:bond which is around 153.0 pm.
329:Hydration equilibrium constants
103:with organomagnesium reagents.
444:phosphate group. Examples are
16:Chemical reaction intermediate
1:
976:10.1016/s0040-4039(01)91316-4
912:10.1016/S0065-3160(08)60351-2
749:10.1016/0022-2836(73)90448-8
491:"IUPAC Gold Book definition"
181:-dimethyltrifluoroacetamide)
158:polycyclic structures (e.g.
67:in 1887. In the reaction of
429:Applications in biomedicine
384:Dioxolane ketone protection
1143:
1057:Proc. Natl. Acad. Sci. USA
939:. Oxford University Press.
556:10.1002/cber.188702001148
81:
406:Weinreb ketone synthesis
21:tetrahedral intermediate
1078:10.1073/pnas.83.11.3743
500:10.1351/goldbook.T06289
298:Acetals and hemiacetals
208:of peptide hydrolysis.
129:Bürgi-Dunitz trajectory
123:Burgi-Dunitz trajectory
92:Claisen's 1887 reaction
82:additionelle Verbindung
1117:Reactive intermediates
516:Cite journal requires
473:
425:
385:
369:
357:
330:
289:
238:
226:
169:
124:
93:
809:-brosylmitomycin A".
471:
423:
383:
367:
355:
328:
287:
236:
221:
167:
122:
91:
65:Rainer Ludwig Claisen
29:nucleophilic addition
25:reaction intermediate
900:Adv. Phys. Org. Chem
720:10.1246/bcsj.50.2193
707:Bull. Chem. Soc. Jpn
688:10.1039/C29710001607
1069:1986PNAS...83.3743B
823:10.1021/ja00988a018
784:10.1021/bi00717a024
665:10.1021/ja00834a063
638:10.1021/ja01076a076
608:10.1021/ja01148a063
45:transesterification
1127:Addition reactions
1122:Carbonyl compounds
991:J. Phys. Org. Chem
474:
460:Enzymatic activity
426:
386:
370:
358:
356:Cyclic hemiacetals
331:
290:
239:
227:
225:-brosylmitomycin A
170:
125:
115:Reaction mechanism
94:
1030:10.1021/cr960370z
937:Organic Chemistry
873:Angewandte Chemie
858:10.1021/ja980700s
852:(28): 7101–7102.
817:(12): 2905–2911.
778:(20): 4212–4228.
682:(24): 1607–1608.
415:Grignard reagents
316:Hexafluoroacetone
311:protective groups
1134:
1101:
1100:
1090:
1080:
1048:
1042:
1041:
1024:(5): 1359–1472.
1013:
1007:
1006:
986:
980:
979:
964:Tetrahedron Lett
947:
941:
940:
932:
926:
925:
895:
889:
888:
868:
862:
861:
846:J. Am. Chem. Soc
841:
835:
834:
811:J. Am. Chem. Soc
802:
796:
795:
767:
761:
760:
731:
725:
724:
722:
713:(8): 2193–2194.
698:
692:
691:
675:
669:
668:
653:J. Am. Chem. Soc
648:
642:
641:
626:J. Am. Chem. Soc
618:
612:
611:
602:(4): 1626–1629.
596:J. Am. Chem. Soc
588:
582:
581:
566:
560:
559:
532:
526:
525:
519:
514:
512:
504:
502:
487:
206:transition state
140:General features
84:
83:
73:sodium methoxide
1142:
1141:
1137:
1136:
1135:
1133:
1132:
1131:
1107:
1106:
1105:
1104:
1050:
1049:
1045:
1015:
1014:
1010:
1003:10.1002/poc.807
988:
987:
983:
970:(39): 3815–18.
949:
948:
944:
934:
933:
929:
922:
897:
896:
892:
879:(17): 3320–23.
870:
869:
865:
843:
842:
838:
804:
803:
799:
769:
768:
764:
733:
732:
728:
700:
699:
695:
677:
676:
672:
650:
649:
645:
622:Woodward, R. B.
620:
619:
615:
590:
589:
585:
574:Ann. Chim. Phys
568:
567:
563:
534:
533:
529:
515:
505:
489:
488:
484:
479:
462:
436:
431:
391:
334:Cyclopropanones
300:
295:
280:
276:
272:
268:
264:
260:
256:
252:
248:
201:
148:
142:
137:
117:
108:Myron L. Bender
97:Victor Grignard
77:methyl benzoate
69:benzyl benzoate
61:
17:
12:
11:
5:
1140:
1138:
1130:
1129:
1124:
1119:
1109:
1108:
1103:
1102:
1063:(11): 3743–5.
1043:
1008:
997:(3): 193–209.
981:
952:Weinreb, S. M.
942:
927:
920:
890:
863:
836:
797:
762:
743:(3): 417–436.
726:
693:
670:
659:(1): 231–232.
643:
613:
583:
561:
550:(1): 646–650.
527:
518:|journal=
481:
480:
478:
475:
461:
458:
435:
432:
430:
427:
394:Weinreb amides
390:
389:Weinreb amides
387:
299:
296:
294:
291:
278:
274:
270:
266:
262:
258:
254:
250:
246:
200:
197:
193:
192:
189:
182:
171:
146:
141:
138:
136:
133:
116:
113:
60:
57:
41:esterification
15:
13:
10:
9:
6:
4:
3:
2:
1139:
1128:
1125:
1123:
1120:
1118:
1115:
1114:
1112:
1098:
1094:
1089:
1084:
1079:
1074:
1070:
1066:
1062:
1058:
1054:
1047:
1044:
1039:
1035:
1031:
1027:
1023:
1019:
1012:
1009:
1004:
1000:
996:
992:
985:
982:
977:
973:
969:
965:
961:
957:
953:
946:
943:
938:
931:
928:
923:
921:9780120335046
917:
913:
909:
905:
901:
894:
891:
886:
882:
878:
874:
867:
864:
859:
855:
851:
847:
840:
837:
832:
828:
824:
820:
816:
812:
808:
801:
798:
793:
789:
785:
781:
777:
773:
766:
763:
758:
754:
750:
746:
742:
738:
730:
727:
721:
716:
712:
708:
704:
697:
694:
689:
685:
681:
680:Chem. Commun.
674:
671:
666:
662:
658:
654:
647:
644:
639:
635:
631:
627:
623:
617:
614:
609:
605:
601:
597:
593:
592:Bender, M. L.
587:
584:
579:
575:
571:
565:
562:
557:
553:
549:
545:
541:
537:
531:
528:
523:
510:
501:
496:
492:
486:
483:
476:
472:Oxyanion hole
470:
466:
459:
457:
455:
451:
447:
442:
433:
428:
422:
418:
416:
411:
407:
403:
399:
395:
388:
382:
378:
376:
366:
362:
354:
350:
348:
343:
342:cyclobutanone
339:
335:
327:
323:
321:
317:
312:
308:
304:
297:
292:
286:
282:
244:
235:
231:
224:
220:
216:
214:
209:
207:
198:
196:
190:
187:
183:
180:
176:
172:
166:
161:
157:
156:
155:
152:
139:
134:
132:
130:
121:
114:
112:
109:
104:
102:
98:
90:
86:
78:
74:
70:
66:
58:
56:
54:
50:
46:
42:
38:
34:
30:
26:
22:
1060:
1056:
1046:
1021:
1017:
1011:
994:
990:
984:
967:
963:
959:
955:
945:
936:
930:
903:
899:
893:
876:
872:
866:
849:
845:
839:
814:
810:
806:
800:
775:
772:Biochemistry
771:
765:
740:
737:J. Mol. Biol
736:
729:
710:
706:
696:
679:
673:
656:
652:
646:
632:(22): 5030.
629:
625:
616:
599:
595:
586:
577:
573:
570:Grignard, V.
564:
547:
543:
530:
509:cite journal
485:
463:
437:
401:
397:
392:
371:
359:
332:
320:Formaldehyde
301:
242:
240:
228:
222:
212:
210:
202:
194:
178:
174:
168:Tetrodotoxin
160:tetrodotoxin
149:, or Cl are
143:
126:
105:
95:
62:
20:
18:
906:(1): 1–29.
536:Claisen, L.
434:Drug design
303:Hemiacetals
154:features:
1111:Categories
950:Nahm, S.;
580:: 433–490.
477:References
446:saquinavir
151:conjugated
1018:Chem. Rev
958:-methoxy-
954:(1981). "
544:Chem. Ber
454:pepstatin
450:ritonavir
441:Proteases
400:-methoxy-
375:dioxolane
1038:11851455
538:(1887).
410:chelated
338:strained
53:peptides
33:carbonyl
1097:3520553
1065:Bibcode
831:6043811
792:4472048
757:4737866
456:, etc.
347:glucose
307:acetals
251:pyrrole
59:History
1095:
1088:323599
1085:
1036:
918:
829:
790:
755:
186:cyclol
101:esters
75:, and
49:amides
37:groups
71:with
31:to a
23:is a
1093:PMID
1034:PMID
916:ISBN
827:PMID
788:PMID
753:PMID
522:help
396:are
305:and
85:.”
51:and
1083:PMC
1073:doi
1026:doi
999:doi
972:doi
908:doi
881:doi
877:114
854:doi
850:120
819:doi
780:doi
745:doi
715:doi
684:doi
661:doi
634:doi
604:doi
552:doi
495:doi
279:sp3
275:sp3
271:sp2
267:sp2
263:C-C
259:C-N
255:sp3
247:sp3
1113::
1091:.
1081:.
1071:.
1061:83
1059:.
1055:.
1032:.
1022:97
1020:.
995:18
993:.
968:22
966:.
914:.
875:.
848:.
825:.
815:89
813:.
786:.
776:13
774:.
751:.
741:77
739:.
711:50
709:.
705:.
657:97
655:.
630:86
628:.
600:73
598:.
578:24
576:.
548:20
546:.
542:.
513::
511:}}
507:{{
493:.
452:,
448:,
277:-C
269:-C
249:-N
162:)
43:,
19:A
1099:.
1075::
1067::
1040:.
1028::
1005:.
1001::
978:.
974::
960:N
956:N
924:.
910::
904:4
887:.
883::
860:.
856::
833:.
821::
807:N
794:.
782::
759:.
747::
723:.
717::
690:.
686::
667:.
663::
640:.
636::
610:.
606::
558:.
554::
524:)
520:(
503:.
497::
402:N
398:N
243:N
223:N
213:N
188:)
179:N
177:,
175:N
147:2
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.