47:
296:
203:
38:
525:
1049:
56:
638:
1079:
studies of revealed stacks of partially oxidized TTF molecules adjacent to anionic stacks of TCNQ molecules. This "segregated stack" motif was unexpected and is responsible for the distinctive electrical properties, i.e. high and
533:
505:
1448:
Frere, P.; Skabara, P. J. (2005). "Salts of
Extended Tetrathiafulvalene analogues: relationships Between Molecular Structure, Electrochemical Properties and Solid State Organization".
1386:
Rovira, C. (2004). "Bis(ethylenethio)tetrathiafulvalene (BET-TTF) and
Related Dissymmetrical Electron Donors: From the Molecule to Functional Molecular Materials and Devices (OFETs)".
651:
1360:
1479:
Gorgues, Alain; Hudhomme, Pietrick; Salle, Marc. (2004). "Highly
Functionalized Tetrathiafulvalenes: Riding along the Synthetic Trail from Electrophilic Alkynes".
345:
1091:
TTF), tetramethylselenafulvalenes (TMTSFs), and bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF, CAS ). Several tetramethyltetrathiafulvalene salts (called
743:
The high level of interest in TTFs has spawned the development of many syntheses of TTF and its analogues. Most preparations entail the coupling of cyclic
1312:
Ferraris, J.; Cowan, D. O.; Walatka, V. V. Jr.; Perlstein, J. H. (1973). "Electron transfer in a new highly conducting donor-acceptor complex".
1087:. Since these early discoveries, numerous analogues of TTF have been prepared. Well studied analogues include tetramethyltetrathiafulvalene (Me
1207:
1040:
6π-electron configuration, consequently leaving the central double bond essentially a single bond, as all π-electrons occupy ring orbitals.
1153:(2004). "Tetrathiafulvalenes, Oligoacenenes, and Their Buckminsterfullerene Derivatives: The Brick and Mortar of Organic Electronics".
310:
1529:
1052:
Edge-on view of portion of crystal structure of hexamethyleneTTF/TCNQ charge transfer salt, highlighting the segregated stacking.
1284:
Wudl, F.; Wobschall, D.; Hufnagel, E. J. (1972). "Electrical
Conductivity by the Bis(1,3-dithiole)-bis(1,3-dithiolium) System".
1559:
1554:
486:
943:
Bulk TTF itself has unremarkable electrical properties. Distinctive properties are, however, associated with salts of its
571:
658:
561:
253:
579:
274:
1190:
Wudl, F.; Kaplan, M. L. (1979). "2,2′-Bi-L,3-Dithiolylidene (Tetrathiafulvalene, TTF) and its
Radical Cation Salts".
524:
210:
1450:
1128:
1084:
959:
781:
198:
1096:
128:
1354:
1033:
709:
678:
547:
517:
1256:
966:
68:
291:
94:
954:
The high electrical conductivity of TTF salts can be attributed to the following features of TTF:
1564:
1217:
1150:
1015:
617:
567:
140:
1245:"Réexamen de la structure du complexe hexaméthylène-tétrathiafulvalène-tétracyanoquinodiméthane"
1533:
1498:
1467:
1436:
1405:
1346:
1314:
1286:
1203:
1172:
1076:
46:
1525:
1490:
1481:
1459:
1428:
1419:
1397:
1388:
1322:
1294:
1264:
1195:
1164:
1155:
981:
833:
762:
682:
438:
368:
758:-2-thion or the related 1,3-dithiole-2-ones. For TTF itself, the synthesis begins with the
262:
180:
104:
1229:
759:
575:
1516:
Segura, José L.; Martín, Nazario (2001). "New
Concepts in Tetrathiafulvalene Chemistry".
1509:
295:
202:
1260:
735:
with sulfur atoms. Over 10,000 scientific publications discuss TTF and its derivatives.
583:
160:
1417:
Iyoda, M; Hasegawa, M; Miyake, Y (2004). "Bi-TTF, Bis-TTF, and
Related TTF Oligomers".
1108:
977:
629:
1548:
1032:
Each dithiolylidene ring in TTF has 7π electrons: 2 for each sulfur atom, 1 for each
887:
732:
427:
417:
191:
1048:
587:
1067:
was reported to be a semiconductor in 1972. Subsequently, the charge-transfer salt
1019:
857:
755:
705:
553:
242:
37:
1341:
1081:
997:
785:
17:
1269:
1244:
1199:
449:
396:
171:
970:
713:
612:
1537:
1502:
1471:
1440:
1409:
1176:
55:
1530:
10.1002/1521-3773(20010417)40:8<1372::aid-anie1372>3.0.co;2-i
1072:
1037:
944:
1401:
1326:
1298:
229:
211:
1494:
1432:
1168:
1463:
1011:
976:
its ability to undergo oxidation at mild potentials to give a stable
466:
628:
Except where otherwise noted, data are given for materials in their
811:(1,3-dithiole-2-yl methyl thioether), which is treated as follows:
1047:
473:
151:
127:
117:
1068:
607:
984:
measurements show that TTF can be oxidized twice reversibly:
279:
1342:"2,2'-Bi-5,6-Dihydro-1,3-Dithiolodithiinylidene (BEDT-TTF)"
422:
116 to 119 °C (241 to 246 °F; 389 to 392 K)
1243:
D. Chasseau; G. Comberton; J. Gaultier; C. Hauw (1978).
646:
1036:
carbon atom. Thus, oxidation converts each ring to an
241:
319:InChI=1S/C6H4S4/c1-2-8-5(7-1)6-9-3-4-10-6/h1-4H
103:
329:InChI=1/C6H4S4/c1-2-8-5(7-1)6-9-3-4-10-6/h1-4H
8:
1359:: CS1 maint: multiple names: authors list (
708:compound contributed to the development of
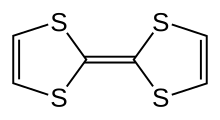
27:Organosulfuric compound with formula C6H4S4
1144:
1142:
294:
201:
179:
29:
1510:Physical properties of Tetrathiafulvalene
1268:
261:
1022:
947:derivatives, such as salts derived from
930:
926:
922:
918:
914:
910:
906:
902:
897:
893:
889:
883:
879:
875:
871:
859:
853:
849:
845:
841:
835:
829:
825:
821:
817:
806:
802:
798:
794:
775:
771:
767:
750:
746:
727:
723:
719:
700:
696:
692:
688:
1518:Angewandte Chemie International Edition
1120:
350:
315:
290:
1352:
1225:
1215:
192:
322:Key: FHCPAXDKURNIOZ-UHFFFAOYSA-N
159:
7:
712:. TTF is related to the hydrocarbon
332:Key: FHCPAXDKURNIOZ-UHFFFAOYAZ
232:
965:its high symmetry, which promotes
25:
1249:Acta Crystallographica Section B
1194:. Vol. 19. pp. 27–30.
636:
523:
380:
54:
45:
36:
1340:Larsen, J.; Lenoir, C. (1998).
632:(at 25 °C , 100 kPa).
487:Occupational safety and health
386:
374:
1:
962:of its oxidized derivatives,
958:its planarity, which allows
1095:) are of some relevance as
73:2,2′-Bi(1,3-dithiolylidene)
1581:
1370:, vol. 9, p. 72
1270:10.1107/S0567740878003830
1200:10.1002/9780470132500.ch7
1071:was shown to be a narrow
791:and then reduced to give
731:, by replacement of four
626:
593:
504:
484:
479:
459:
361:
341:
306:
87:
79:
67:
62:
53:
44:
35:
1451:Chemical Society Reviews
1133:pubchem.ncbi.nlm.nih.gov
754:building blocks such as
562:Precautionary statements
1097:organic superconductors
1085:electrical conductivity
1560:Organic semiconductors
1149:Bendikov, M; Wudl, F;
1053:
1555:Molecular electronics
1051:
969:, thereby minimizing
967:charge delocalization
782:1,3-dithiole-2-thione
710:molecular electronics
679:organosulfur compound
1512:from the literature.
1129:"Tetrathiafulvalene"
452:in organic solvents
82:Δ2,2-Bi-1,3-dithiole
69:Preferred IUPAC name
1327:10.1021/ja00784a066
1299:10.1021/ja00757a079
1261:1978AcCrB..34..689C
1192:Inorganic Syntheses
439:Solubility in water
404: g·mol
141:Beilstein Reference
32:
31:Tetrathiafulvalene
1054:
704:. Studies on this
671:Tetrathiafulvalene
659:Infobox references
618:Tetracyanoethylene
594:Related compounds
30:
1495:10.1021/cr0306485
1489:(11): 5151–5184.
1433:10.1021/cr030651o
1427:(11): 5085–5113.
1402:10.1021/cr030663+
1396:(11): 5289–5317.
1368:Collected Volumes
1347:Organic Syntheses
1315:J. Am. Chem. Soc.
1287:J. Am. Chem. Soc.
1209:978-0-470-13250-0
1169:10.1021/cr030666m
1163:(11): 4891–4945.
1077:X-ray diffraction
667:Chemical compound
665:
664:
600:Related compounds
548:Hazard statements
353:S1C=CSC1=C2SC=CS2
275:CompTox Dashboard
129:Interactive image
16:(Redirected from
1572:
1541:
1524:(8): 1372–1409.
1506:
1482:Chemical Reviews
1475:
1464:10.1039/b316392j
1444:
1420:Chemical Reviews
1413:
1389:Chemical Reviews
1373:
1371:
1364:
1358:
1350:
1337:
1331:
1330:
1309:
1303:
1302:
1281:
1275:
1274:
1272:
1240:
1234:
1233:
1227:
1223:
1221:
1213:
1187:
1181:
1180:
1156:Chemical Reviews
1146:
1137:
1136:
1125:
1066:
1065:
1064:
1026:
1005:
991:
950:
939:Redox properties
934:
864:
810:
779:
763:trithiocarbonate
753:
730:
703:
649:
643:
640:
639:
589:
585:
581:
577:
573:
569:
555:
527:
403:
388:
382:
376:
369:Chemical formula
299:
298:
283:
281:
265:
245:
234:
213:
205:
194:
183:
163:
131:
107:
58:
49:
40:
33:
21:
18:Tetrathiafulvene
1580:
1579:
1575:
1574:
1573:
1571:
1570:
1569:
1545:
1544:
1515:
1478:
1447:
1416:
1385:
1382:
1380:Further reading
1377:
1376:
1366:
1351:
1339:
1338:
1334:
1311:
1310:
1306:
1283:
1282:
1278:
1242:
1241:
1237:
1224:
1214:
1210:
1189:
1188:
1184:
1151:Perepichka, D F
1148:
1147:
1140:
1127:
1126:
1122:
1117:
1105:
1090:
1075:semiconductor.
1063:
1061:
1060:
1059:
1057:
1046:
1024:
1020:
1003:
989:
982:Electrochemical
973:repulsions, and
948:
941:
932:
928:
924:
920:
916:
912:
908:
904:
899:
895:
891:
885:
881:
877:
873:
869:
861:
855:
851:
847:
843:
837:
831:
827:
823:
819:
815:
808:
804:
800:
796:
792:
777:
773:
769:
765:
752:
748:
744:
741:
729:
725:
721:
717:
702:
698:
694:
690:
686:
668:
661:
656:
655:
654: ?)
645:
641:
637:
633:
622:
601:
564:
550:
536:
520:
497:
469:
441:
401:
391:
385:
379:
371:
357:
354:
349:
348:
337:
334:
333:
330:
324:
323:
320:
314:
313:
302:
284:
277:
268:
248:
235:
223:
186:
166:
143:
134:
121:
110:
97:
83:
75:
74:
28:
23:
22:
15:
12:
11:
5:
1578:
1576:
1568:
1567:
1562:
1557:
1547:
1546:
1543:
1542:
1513:
1507:
1476:
1445:
1414:
1381:
1378:
1375:
1374:
1332:
1321:(3): 948–949.
1304:
1293:(2): 670–672.
1276:
1235:
1226:|journal=
1208:
1182:
1138:
1119:
1118:
1116:
1113:
1112:
1111:
1109:Bechgaard salt
1104:
1101:
1088:
1062:
1045:
1042:
1030:
1029:
1010:= 0.78 V, vs.
1001:
986:
985:
978:radical cation
974:
963:
940:
937:
936:
935:
917:+ 2 [NH(CH
866:
865:
740:
737:
666:
663:
662:
657:
635:
634:
630:standard state
627:
624:
623:
621:
620:
615:
610:
604:
602:
599:
596:
595:
591:
590:
565:
560:
557:
556:
551:
546:
543:
542:
537:
532:
529:
528:
521:
516:
513:
512:
502:
501:
498:
495:
492:
491:
482:
481:
477:
476:
470:
465:
462:
461:
457:
456:
453:
446:
445:
442:
437:
434:
433:
430:
424:
423:
420:
414:
413:
410:
406:
405:
399:
393:
392:
389:
383:
377:
372:
367:
364:
363:
359:
358:
356:
355:
352:
344:
343:
342:
339:
338:
336:
335:
331:
328:
327:
325:
321:
318:
317:
309:
308:
307:
304:
303:
301:
300:
287:
285:
273:
270:
269:
267:
266:
258:
256:
250:
249:
247:
246:
238:
236:
228:
225:
224:
222:
221:
217:
215:
207:
206:
196:
188:
187:
185:
184:
176:
174:
168:
167:
165:
164:
156:
154:
148:
147:
144:
139:
136:
135:
133:
132:
124:
122:
115:
112:
111:
109:
108:
100:
98:
93:
90:
89:
85:
84:
81:
77:
76:
72:
71:
65:
64:
60:
59:
51:
50:
42:
41:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1577:
1566:
1563:
1561:
1558:
1556:
1553:
1552:
1550:
1539:
1535:
1531:
1527:
1523:
1519:
1514:
1511:
1508:
1504:
1500:
1496:
1492:
1488:
1484:
1483:
1477:
1473:
1469:
1465:
1461:
1457:
1453:
1452:
1446:
1442:
1438:
1434:
1430:
1426:
1422:
1421:
1415:
1411:
1407:
1403:
1399:
1395:
1391:
1390:
1384:
1383:
1379:
1369:
1362:
1356:
1349:
1348:
1343:
1336:
1333:
1328:
1324:
1320:
1317:
1316:
1308:
1305:
1300:
1296:
1292:
1289:
1288:
1280:
1277:
1271:
1266:
1262:
1258:
1254:
1250:
1246:
1239:
1236:
1231:
1219:
1211:
1205:
1201:
1197:
1193:
1186:
1183:
1178:
1174:
1170:
1166:
1162:
1158:
1157:
1152:
1145:
1143:
1139:
1134:
1130:
1124:
1121:
1114:
1110:
1107:
1106:
1102:
1100:
1098:
1094:
1086:
1083:
1078:
1074:
1070:
1050:
1043:
1041:
1039:
1035:
1027:
1017:
1013:
1009:
1004:TTF → TTF + e
1002:
999:
995:
990:TTF → TTF + e
988:
987:
983:
979:
975:
972:
968:
964:
961:
957:
956:
955:
952:
946:
938:
900:
868:
867:
863:
839:
814:
813:
812:
790:
788:
783:
764:
761:
757:
738:
736:
734:
715:
711:
707:
684:
680:
676:
672:
660:
653:
648:
631:
625:
619:
616:
614:
611:
609:
606:
605:
603:
598:
597:
592:
566:
563:
559:
558:
552:
549:
545:
544:
541:
538:
535:
531:
530:
526:
522:
519:
515:
514:
510:
508:
503:
499:
494:
493:
489:
488:
483:
478:
475:
471:
468:
467:Dipole moment
464:
463:
458:
454:
451:
448:
447:
443:
440:
436:
435:
431:
429:
428:Boiling point
426:
425:
421:
419:
418:Melting point
416:
415:
412:Yellow solid
411:
408:
407:
400:
398:
395:
394:
373:
370:
366:
365:
360:
351:
347:
340:
326:
316:
312:
305:
297:
293:
292:DTXSID6067620
289:
288:
286:
276:
272:
271:
264:
260:
259:
257:
255:
252:
251:
244:
240:
239:
237:
231:
227:
226:
219:
218:
216:
214:
209:
208:
204:
200:
197:
195:
193:ECHA InfoCard
190:
189:
182:
178:
177:
175:
173:
170:
169:
162:
158:
157:
155:
153:
150:
149:
145:
142:
138:
137:
130:
126:
125:
123:
119:
114:
113:
106:
102:
101:
99:
96:
92:
91:
86:
78:
70:
66:
61:
57:
52:
48:
43:
39:
34:
19:
1521:
1517:
1486:
1480:
1458:(1): 69–98.
1455:
1449:
1424:
1418:
1393:
1387:
1367:
1355:cite journal
1345:
1335:
1318:
1313:
1307:
1290:
1285:
1279:
1252:
1248:
1238:
1191:
1185:
1160:
1154:
1132:
1123:
1092:
1055:
1031:
1007:
993:
960:π-π stacking
953:
942:
786:
784:), which is
756:1,3-dithiole
742:
706:heterocyclic
674:
670:
669:
539:
506:
500:combustible
496:Main hazards
485:
88:Identifiers
80:Other names
1093:Fabre salts
1082:anisotropic
789:-methylated
739:Preparation
534:Signal word
490:(OHS/OSH):
432:Decomposes
409:Appearance
362:Properties
199:100.045.979
161:CHEBI:52444
1549:Categories
1255:(2): 689.
1115:References
882:CH][BF
852:CH][BF
518:Pictograms
460:Structure
450:Solubility
444:Insoluble
397:Molar mass
263:HY1EN16W9T
172:ChemSpider
116:3D model (
105:31366-25-3
95:CAS Number
1565:Dithioles
1228:ignored (
1218:cite book
1056:The salt
1028:solution)
971:coulombic
733:CH groups
714:fulvalene
681:with the
613:Thiophene
580:P333+P313
576:P302+P352
509:labelling
220:250-593-7
212:EC Number
1538:11317287
1503:15535646
1472:15643491
1441:15535643
1410:15535651
1177:15535637
1103:See also
1073:band gap
1038:aromatic
945:oxidized
929:][BF
870:2 [H
840:→ [H
834:H[BF
677:) is an
480:Hazards
455:Soluble
146:1282106
1257:Bibcode
1044:History
996:= 0.34
683:formula
652:what is
650: (
540:Warning
230:PubChem
1536:
1501:
1470:
1439:
1408:
1206:
1175:
886:] + 2
828:CH(SCH
805:CH(SCH
760:cyclic
647:verify
644:
402:204.34
346:SMILES
63:Names
311:InChI
243:99451
181:89848
152:ChEBI
118:JSmol
1534:PMID
1499:PMID
1468:PMID
1437:PMID
1406:PMID
1361:link
1230:help
1204:ISBN
1173:PMID
1069:TCNQ
1016:AgCl
901:→ (H
888:N(CH
856:] +
832:) +
608:TCNQ
588:P501
584:P363
572:P280
568:P261
554:H317
254:UNII
1526:doi
1491:doi
1487:104
1460:doi
1429:doi
1425:104
1398:doi
1394:104
1323:doi
1295:doi
1265:doi
1196:doi
1165:doi
1161:104
1018:in
949:TTF
778:C=S
675:TTF
507:GHS
280:EPA
233:CID
1551::
1532:.
1522:40
1520:.
1497:.
1485:.
1466:.
1456:34
1454:.
1435:.
1423:.
1404:.
1392:.
1365:;
1357:}}
1353:{{
1344:.
1319:95
1291:94
1263:.
1253:34
1251:.
1247:.
1222::
1220:}}
1216:{{
1202:.
1171:.
1159:.
1141:^
1131:.
1099:.
1058:Cl
1034:sp
1025:CN
1021:CH
1012:Ag
980:.
951:.
921:CH
913:C)
892:CH
862:SH
858:CH
838:]
718:(C
716:,
586:,
582:,
578:,
574:,
570:,
511::
472:0
1540:.
1528::
1505:.
1493::
1474:.
1462::
1443:.
1431::
1412:.
1400::
1372:.
1363:)
1329:.
1325::
1301:.
1297::
1273:.
1267::
1259::
1232:)
1212:.
1198::
1179:.
1167::
1135:.
1089:4
1023:3
1014:/
1008:E
1006:(
1000:)
998:V
994:E
992:(
933:]
931:4
927:3
925:)
923:3
919:2
915:2
911:2
909:S
907:2
905:C
903:2
898:3
896:)
894:3
890:2
884:4
880:2
878:S
876:2
874:C
872:2
860:3
854:4
850:2
848:S
846:2
844:C
842:2
836:4
830:3
826:2
824:S
822:2
820:C
818:2
816:H
809:)
807:3
803:2
801:S
799:2
797:C
795:2
793:H
787:S
780:(
776:2
774:S
772:2
770:C
768:2
766:H
751:2
749:S
747:3
745:C
728:2
726:)
724:4
722:H
720:5
701:2
699:)
697:2
695:S
693:2
691:H
689:3
687:C
685:(
673:(
642:Y
474:D
390:4
387:S
384:4
381:H
378:6
375:C
282:)
278:(
120:)
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.