640:
669:
607:
573:, and others. In addition, thioesters are formed as key intermediates in several particularly ancient processes that result in the assembly of ATP. In both these instances, the thioester is closer than ATP to the process that uses or yields energy. In other words, thioesters could have actually played the role of ATP in a "thioester world" initially devoid of ATP. Eventually, thioesters could have served to usher in ATP through its ability to support the formation of bonds between
591:
318:
413:
420:
31:
443:
581:
However, due to the high free energy change of thioester's hydrolysis and correspondingly their low equilibrium constants, it is unlikely that these compounds could have accumulated abiotically to any significant extent especially in hydrothermal vent conditions.
335:
In a related reaction, thioesters can be converted into esters. Thioacetate esters can also be cleaved with methanethiol in the presence of stoichiometric base, as illustrated in the preparation of pent-4-yne-1-thiol:
502:. These thioesters arise analogously to those prepared synthetically, the difference being that the dehydration agent is ATP. In addition, thioesters play an important role in the tagging of proteins with
1105:
Dansette, P. M.; Rosi, J.; Debernardi, J.; Bertho, G.; Mansuy, D. (2012). "Metabolic
Activation of Prasugrel: Nature of the Two Competitive Pathways Resulting in the Opening of Its Thiophene Ring".
730:
Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's
Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764.
893:
1422:
796:"Synthesis of Multifunctionalized Ketones Through the Fukuyama Coupling Reaction Catalyzed by Pearlman's Catalyst: Preparation of Ethyl 6-oxotridecanoate"
2341:
975:
Wan Kit Chan; S. Masamune; Gary O. Spessard (1983). "Preparation of O-esters From The
Corresponding Thiol Esters: Tert-butyl Cyclohexanecarboxylate".
2346:
1070:
1019:
764:
910:
1239:
1048:
437:
243:
239:
1255:
Newton, Josiah J.; Britton, Robert; Friesen, Chadron M. (4 October 2018). "Base-Catalyzed
Transesterification of Thionoesters".
1415:
1107:
827:
Jordan, Andrew; Sneddon, Helen F. (2019). "Development of a solvent-reagent selection guide for the formation of thioesters".
701:
620:
are isomeric with thioesters. In a thionoester, sulfur replaces the carbonyl oxygen in an ester. Methyl thionobenzoate is C
317:
2245:
242:. Efforts to improve the sustainability of thioester synthesis have also been reported utilising safer coupling reagent
639:
2369:
1818:
1408:
706:
1173:
Chandru, Kuhan; Gilbert, Alexis; Butch, Christopher; Aono, Masashi; Cleaves, Henderson James II (21 July 2016).
1855:
862:
Volante, R. (1981). "A new, highly efficient method for the conversion of alcohols to thiolesters and thiols".
424:
325:
2374:
2328:
2228:
542:
55:
412:
2335:
2223:
668:
455:
153:
647:
498:, which comprises a large fraction of the Earth's land biomass, proceeds via a thioester derivative of
174:
The analogous alkylation of an acetate salt is rarely practiced. The alkylation can be conducted using
606:
2304:
1749:
1186:
483:
2379:
1610:
864:
691:
662:
145:
780:
1386:
1327:
1280:
1155:
844:
308:
The carbonyl center in thioesters is more reactive toward amine than oxygen nucleophiles, giving
262:
590:
70:) with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the
541:
It is revealing that thioesters are obligatory intermediates in several key processes in which
2294:
2264:
2022:
1644:
1378:
1370:
1319:
1272:
1235:
1212:
1124:
1087:
1044:
1015:
957:
906:
800:
760:
633:
534:
404:
400:
329:
63:
47:
1298:
Monteith, John J.; Scotchburn, Katerina; Mills, L. Reginald; Rousseaux, Sophie A. L. (2022).
553:. They also participate in the synthesis of a number of other cellular components, including
216:
Thioesters can be prepared by condensation of thiols and carboxylic acids in the presence of
1999:
1493:
1431:
1362:
1311:
1264:
1202:
1194:
1116:
1079:
1007:
984:
947:
939:
898:
873:
836:
752:
731:
655:
610:
2218:
1977:
1972:
1955:
1938:
1739:
1488:
574:
471:
266:
79:
1190:
928:"Chemoselectivity in chemical biology: Acyl transfer reactions with sulfur and selenium"
2289:
2284:
2160:
2155:
2150:
1943:
1910:
1694:
1676:
1666:
1207:
1174:
952:
927:
251:
247:
75:
877:
747:
Fujiwara, S.; Kambe, N. (2005). "Thio-, Seleno-, and
Telluro-Carboxylic Acid Esters".
2363:
2309:
2257:
2188:
2074:
2064:
2059:
2049:
2044:
1994:
1989:
1905:
1900:
1890:
1744:
1699:
1661:
1649:
1620:
1498:
1390:
1331:
1037:
848:
696:
479:
475:
273:
123:
1284:
682:
can be transformed to thionoesters under metal-catalyzed cross-coupling conditions.
2240:
2127:
2122:
2099:
1850:
1689:
1615:
1552:
1547:
1525:
1481:
1466:
1456:
1345:
Liu, Yinbo; Mo, Xiaofeng; Majeed, Irfan; Zhang, Mei; Wang, Hui; Zeng, Zhuo (2022).
665:
of an existing methyl thionoester with an alcohol under base-catalyzed conditions.
499:
487:
175:
127:
95:
902:
533:
As posited in a "Thioester World", thioesters are possible precursors to life. As
450:, a thioester that is a key intermediate in the biosynthesis of many biomolecules.
2299:
2252:
2213:
2094:
1982:
1967:
1962:
1950:
1515:
1510:
1476:
1471:
1461:
1439:
1315:
795:
651:
518:
514:
510:
467:
1175:"The Abiotic Chemistry of Thiolated Acetate Derivatives and the Origin of Life"
1011:
735:
545:
is either used or regenerated. Thioesters are involved in the synthesis of all
2208:
2199:
2079:
2034:
1930:
1895:
1885:
1825:
1761:
1684:
1632:
1299:
1083:
558:
491:
463:
459:
447:
149:
103:
99:
17:
1374:
988:
2175:
2054:
2039:
2027:
1870:
1845:
1654:
1268:
679:
570:
522:
503:
217:
30:
1382:
1323:
1276:
1216:
1128:
1091:
1002:
Matteo
Minozzi; Daniele Nanni; Piero Spagnolo (2008). "4-Pentyne-1-thiol".
961:
458:
in many biosynthetic reactions, including the formation and degradation of
442:
419:
258:
also give thioesters upon treatment with thiols in the presence of a base.
2183:
2137:
2104:
1800:
1706:
1580:
1535:
1520:
675:
599:
111:
39:
1346:
1159:
1143:
2145:
2069:
1920:
1915:
1880:
1865:
1860:
1830:
1813:
1637:
1564:
1530:
1366:
840:
595:
566:
554:
255:
107:
35:
1347:"An efficient and straightforward approach for accessing thionoesters
1198:
1120:
943:
2233:
2165:
2009:
1718:
1711:
1605:
1586:
1575:
1559:
1505:
562:
546:
495:
281:
277:
141:
1400:
756:
513:) is postulated in the bioactivation of the antithrombotic prodrugs
1066:"Sulfenic acids as reactive intermediates in xenobiotic metabolism"
1065:
2114:
2084:
2017:
1875:
1840:
1835:
1808:
1756:
1723:
1627:
1451:
605:
589:
550:
441:
418:
309:
87:
71:
29:
751:. Vol. 251. Berlin / Heidelberg: Springer. pp. 87–140.
1542:
1404:
632:. Such compounds are typically prepared by the reaction of the
261:
Thioesters can be conveniently prepared from alcohols by the
667:
638:
1300:"Ni-Catalyzed Synthesis of Thiocarboxylic Acid Derivatives"
148:. For example, thioacetate esters are commonly prepared by
292:
Thioesters hydrolyze to thiols and the carboxylic acid:
594:
General structure of a thionoester, where R and R' are
34:
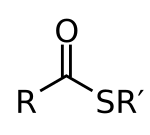
General structure of a thioester, where R and R' are
1035:
Lehninger, A. L.; Nelson, D. L.; Cox, M. M. (2000).
891:
Bertleff, W.; Roeper, M.; Sava, X. "Carbonylation".
122:
One route to thioesters involves the reaction of an
2277:
2197:
2174:
2136:
2113:
2008:
1929:
1799:
1776:
1732:
1675:
1598:
1573:
1438:
1036:
661:Various thionoesters may be prepared through the
140:Another common route entails the displacement of
1351:palladium-catalyzed C–N cleavage of thioamides"
98:, the best-known thioesters are derivatives of
894:Ullmann's Encyclopedia of Industrial Chemistry
27:Organosulfur compounds of the form R–SC(=O)–R’
1416:
8:
1043:(3rd ed.). New York: Worth Publishing.
509:Oxidation of the sulfur atom in thioesters (
403:, in which the thioester is coupled with an
466:, precursor to steroids. Examples include
1796:
1595:
1423:
1409:
1401:
506:, which tags the protein for degradation.
407:by a palladium catalyst to give a ketone.
1232:An Introduction to Organosulfur Chemistry
1206:
951:
646:They can also be made by the reaction of
391:
387:
383:
379:
375:
371:
362:
358:
354:
350:
346:
342:
230:
226:
208:
204:
200:
196:
192:
188:
184:
166:
162:
1071:Archives of Biochemistry and Biophysics
726:
724:
722:
718:
399:A reaction unique to thioesters is the
926:McGrath, N. A.; Raines, R. T. (2011).
7:
1355:Organic & Biomolecular Chemistry
1064:Mansuy, D.; Dansette, P. M. (2011).
1234:. Chichester: John Wiley and Sons.
549:, including those found in complex
25:
1144:"The Beginnings of Life on Earth"
529:Thioesters and the origin of life
438:Category:Thioesters of coenzyme A
423:Thioesters are components of the
1257:The Journal of Organic Chemistry
411:
316:
74:prefix. They are the product of
1108:Chemical Research in Toxicology
238:A typical dehydration agent is
135:RSNa + R'COCl → R'COSR + NaCl
490:proceeds via the formation of
324:This reaction is exploited in
144:by the alkali metal salt of a
1:
903:10.1002/14356007.a05_217.pub2
878:10.1016/S0040-4039(01)81842-6
427:method for peptide synthesis.
178:and the thiocarboxylic acid:
58:with the molecular structure
1316:10.1021/acs.orglett.1c04074
815:, vol. 11, p. 281
794:Mori, Y.; Seki, M. (2007).
783:. Organic Chemistry Portal.
749:Topics in Current Chemistry
650:with esters or by treating
284:in the presence of thiols.
2396:
1039:Principles of Biochemistry
1012:10.1002/047084289X.rn00855
736:10.1002/9780470771099.ch15
435:
2318:
1084:10.1016/j.abb.2010.09.015
781:"Synthesis of thioesters"
702:Liebeskind–Srogl coupling
106:. The R and R' represent
989:10.15227/orgsyn.061.0048
613:of methyl thionobenzoate
425:native chemical ligation
326:native chemical ligation
62:. They are analogous to
2329:chemical classification
1269:10.1021/acs.joc.8b02260
1230:Cremlyn, R. J. (1996).
897:. Weinheim: Wiley-VCH.
672:
643:
614:
603:
579:
494:. The biosynthesis of
454:Thioesters are common
451:
428:
56:organosulfur compounds
43:
2336:chemical nomenclature
671:
642:
609:
593:
539:
445:
422:
154:potassium thioacetate
33:
1142:de Duve, C. (1995).
484:acyl carrier protein
272:They also arise via
246:and greener solvent
1792:not C, H or O)
1263:(20): 12784–12792.
1191:2016NatSR...629883C
865:Tetrahedron Letters
692:Thiocarboxylic acid
663:transesterification
486:(ACP) thioesters.
146:thiocarboxylic acid
2234:Hypervalent iodine
1367:10.1039/d1ob02349g
1179:Scientific Reports
1148:American Scientist
841:10.1039/C9GC00355J
673:
648:Lawesson's reagent
644:
615:
604:
452:
429:
263:Mitsunobu reaction
229:H → RSC(O)R' + H
218:dehydrating agents
114:in the case of R.
64:carboxylate esters
44:
2370:Functional groups
2357:
2356:
2295:Sulfenyl chloride
2273:
2272:
1772:
1771:
1591:(only C, H and O)
1432:Functional groups
1199:10.1038/srep29883
1121:10.1021/tx3000279
1021:978-0-471-93623-7
977:Organic Syntheses
944:10.1021/ar200081s
872:(33): 3119–3122.
813:Collected Volumes
801:Organic Syntheses
766:978-3-540-23012-0
636:with an alcohol.
634:thioacyl chloride
535:Christian de Duve
448:acetyl coenzyme A
405:organozinc halide
401:Fukuyama coupling
330:peptide synthesis
328:, a protocol for
130:salt of a thiol:
48:organic chemistry
42:in the case of R.
16:(Redirected from
2387:
2324:
2229:Trifluoromethoxy
1797:
1793:
1596:
1592:
1445:
1425:
1418:
1411:
1402:
1395:
1394:
1361:(7): 1532–1537.
1342:
1336:
1335:
1295:
1289:
1288:
1252:
1246:
1245:
1227:
1221:
1220:
1210:
1185:(29883): 29883.
1170:
1164:
1163:
1139:
1133:
1132:
1115:(5): 1058–1065.
1102:
1096:
1095:
1061:
1055:
1054:
1042:
1032:
1026:
1025:
999:
993:
992:
972:
966:
965:
955:
923:
917:
916:
888:
882:
881:
859:
853:
852:
835:(8): 1900–1906.
824:
818:
816:
809:
791:
785:
784:
777:
771:
770:
744:
738:
728:
656:hydrogen sulfide
611:Skeletal formula
602:in the case of R
575:phosphate groups
415:
395:
382:SAc + HSMe → H
366:
353:OMs + KSAc → H
320:
234:
212:
170:
165:COSK + RX → CH
136:
93:
85:
69:
61:
21:
2395:
2394:
2390:
2389:
2388:
2386:
2385:
2384:
2360:
2359:
2358:
2353:
2322:
2314:
2269:
2224:Trichloromethyl
2219:Trifluoromethyl
2193:
2170:
2132:
2109:
2004:
1973:Phosphine oxide
1925:
1791:
1789:
1788:
1786:
1784:
1782:
1780:
1778:
1768:
1728:
1671:
1590:
1589:
1584:
1579:
1569:
1443:
1442:
1434:
1429:
1399:
1398:
1344:
1343:
1339:
1304:Organic Letters
1297:
1296:
1292:
1254:
1253:
1249:
1242:
1229:
1228:
1224:
1172:
1171:
1167:
1141:
1140:
1136:
1104:
1103:
1099:
1063:
1062:
1058:
1051:
1034:
1033:
1029:
1022:
1001:
1000:
996:
974:
973:
969:
925:
924:
920:
913:
890:
889:
885:
861:
860:
856:
829:Green Chemistry
826:
825:
821:
811:
793:
792:
788:
779:
778:
774:
767:
757:10.1007/b101007
746:
745:
741:
729:
720:
715:
688:
631:
627:
623:
588:
531:
472:acetoacetyl-CoA
440:
434:
393:
389:
385:
381:
377:
373:
369:
364:
360:
356:
352:
348:
344:
340:
303:
299:
290:
267:thioacetic acid
252:Acid anhydrides
232:
228:
224:
210:
206:
202:
198:
194:
190:
186:
182:
168:
164:
160:
134:
120:
91:
83:
80:carboxylic acid
67:
59:
28:
23:
22:
15:
12:
11:
5:
2393:
2391:
2383:
2382:
2377:
2375:Origin of life
2372:
2362:
2361:
2355:
2354:
2352:
2351:
2350:
2349:
2344:
2332:
2325:
2319:
2316:
2315:
2313:
2312:
2310:Sulfinylamines
2307:
2302:
2297:
2292:
2290:Phosphoramides
2287:
2285:Isothiocyanate
2281:
2279:
2275:
2274:
2271:
2270:
2268:
2267:
2262:
2261:
2260:
2250:
2249:
2248:
2238:
2237:
2236:
2231:
2226:
2221:
2216:
2205:
2203:
2195:
2194:
2192:
2191:
2186:
2180:
2178:
2172:
2171:
2169:
2168:
2163:
2161:Selenenic acid
2158:
2156:Seleninic acid
2153:
2151:Selenonic acid
2148:
2142:
2140:
2134:
2133:
2131:
2130:
2125:
2119:
2117:
2111:
2110:
2108:
2107:
2102:
2097:
2092:
2087:
2082:
2077:
2072:
2067:
2062:
2057:
2052:
2047:
2042:
2037:
2032:
2031:
2030:
2020:
2014:
2012:
2006:
2005:
2003:
2002:
1997:
1992:
1987:
1986:
1985:
1975:
1970:
1965:
1960:
1959:
1958:
1948:
1947:
1946:
1944:Phosphodiester
1935:
1933:
1927:
1926:
1924:
1923:
1918:
1913:
1908:
1903:
1898:
1893:
1888:
1883:
1878:
1873:
1868:
1863:
1858:
1853:
1848:
1843:
1838:
1833:
1828:
1823:
1822:
1821:
1816:
1805:
1803:
1794:
1790:(one element,
1774:
1773:
1770:
1769:
1767:
1766:
1765:
1764:
1754:
1753:
1752:
1747:
1736:
1734:
1730:
1729:
1727:
1726:
1721:
1716:
1715:
1714:
1704:
1703:
1702:
1697:
1692:
1681:
1679:
1673:
1672:
1670:
1669:
1667:Methylenedioxy
1664:
1659:
1658:
1657:
1652:
1642:
1641:
1640:
1635:
1625:
1624:
1623:
1613:
1608:
1602:
1600:
1593:
1571:
1570:
1568:
1567:
1562:
1557:
1556:
1555:
1550:
1540:
1539:
1538:
1533:
1528:
1523:
1518:
1513:
1503:
1502:
1501:
1496:
1486:
1485:
1484:
1479:
1474:
1469:
1464:
1459:
1448:
1446:
1444:(only C and H)
1436:
1435:
1430:
1428:
1427:
1420:
1413:
1405:
1397:
1396:
1337:
1310:(2): 619–624.
1290:
1247:
1240:
1222:
1165:
1154:(5): 428–437.
1134:
1097:
1078:(1): 174–185.
1056:
1049:
1027:
1020:
994:
967:
938:(9): 752–761.
932:Acc. Chem. Res
918:
912:978-3527306732
911:
883:
854:
819:
786:
772:
765:
739:
717:
716:
714:
711:
710:
709:
704:
699:
694:
687:
684:
629:
625:
621:
587:
584:
530:
527:
433:
430:
417:
416:
397:
396:
367:
322:
321:
306:
305:
301:
297:
296:RC(O)SR' + H
289:
286:
248:cyclopentanone
236:
235:
214:
213:
172:
171:
138:
137:
119:
116:
76:esterification
26:
24:
18:Thioester bond
14:
13:
10:
9:
6:
4:
3:
2:
2392:
2381:
2378:
2376:
2373:
2371:
2368:
2367:
2365:
2348:
2345:
2343:
2340:
2339:
2338:
2337:
2333:
2331:
2330:
2326:
2321:
2320:
2317:
2311:
2308:
2306:
2303:
2301:
2298:
2296:
2293:
2291:
2288:
2286:
2283:
2282:
2280:
2276:
2266:
2263:
2259:
2256:
2255:
2254:
2251:
2247:
2244:
2243:
2242:
2239:
2235:
2232:
2230:
2227:
2225:
2222:
2220:
2217:
2215:
2212:
2211:
2210:
2207:
2206:
2204:
2202:
2201:
2196:
2190:
2189:Telluroketone
2187:
2185:
2182:
2181:
2179:
2177:
2173:
2167:
2164:
2162:
2159:
2157:
2154:
2152:
2149:
2147:
2144:
2143:
2141:
2139:
2135:
2129:
2126:
2124:
2121:
2120:
2118:
2116:
2112:
2106:
2103:
2101:
2098:
2096:
2093:
2091:
2088:
2086:
2083:
2081:
2078:
2076:
2075:Sulfonic acid
2073:
2071:
2068:
2066:
2065:Sulfinic acid
2063:
2061:
2060:Thiosulfonate
2058:
2056:
2053:
2051:
2050:Thiosulfinate
2048:
2046:
2045:Sulfenic acid
2043:
2041:
2038:
2036:
2033:
2029:
2026:
2025:
2024:
2021:
2019:
2016:
2015:
2013:
2011:
2007:
2001:
2000:Phosphaallene
1998:
1996:
1995:Phosphaalkyne
1993:
1991:
1990:Phosphaalkene
1988:
1984:
1981:
1980:
1979:
1976:
1974:
1971:
1969:
1966:
1964:
1961:
1957:
1954:
1953:
1952:
1949:
1945:
1942:
1941:
1940:
1937:
1936:
1934:
1932:
1928:
1922:
1919:
1917:
1914:
1912:
1909:
1907:
1904:
1902:
1899:
1897:
1894:
1892:
1889:
1887:
1884:
1882:
1879:
1877:
1874:
1872:
1869:
1867:
1864:
1862:
1859:
1857:
1854:
1852:
1849:
1847:
1844:
1842:
1839:
1837:
1834:
1832:
1829:
1827:
1824:
1820:
1817:
1815:
1812:
1811:
1810:
1807:
1806:
1804:
1802:
1798:
1795:
1775:
1763:
1760:
1759:
1758:
1755:
1751:
1748:
1746:
1743:
1742:
1741:
1738:
1737:
1735:
1731:
1725:
1722:
1720:
1717:
1713:
1710:
1709:
1708:
1705:
1701:
1698:
1696:
1693:
1691:
1688:
1687:
1686:
1683:
1682:
1680:
1678:
1674:
1668:
1665:
1663:
1662:Ethylenedioxy
1660:
1656:
1653:
1651:
1648:
1647:
1646:
1643:
1639:
1636:
1634:
1631:
1630:
1629:
1626:
1622:
1619:
1618:
1617:
1614:
1612:
1609:
1607:
1604:
1603:
1601:
1597:
1594:
1588:
1582:
1577:
1572:
1566:
1563:
1561:
1558:
1554:
1551:
1549:
1546:
1545:
1544:
1541:
1537:
1534:
1532:
1529:
1527:
1524:
1522:
1519:
1517:
1514:
1512:
1509:
1508:
1507:
1504:
1500:
1497:
1495:
1492:
1491:
1490:
1487:
1483:
1480:
1478:
1475:
1473:
1470:
1468:
1465:
1463:
1460:
1458:
1455:
1454:
1453:
1450:
1449:
1447:
1441:
1437:
1433:
1426:
1421:
1419:
1414:
1412:
1407:
1406:
1403:
1392:
1388:
1384:
1380:
1376:
1372:
1368:
1364:
1360:
1356:
1352:
1350:
1341:
1338:
1333:
1329:
1325:
1321:
1317:
1313:
1309:
1305:
1301:
1294:
1291:
1286:
1282:
1278:
1274:
1270:
1266:
1262:
1258:
1251:
1248:
1243:
1241:0-471-95512-4
1237:
1233:
1226:
1223:
1218:
1214:
1209:
1204:
1200:
1196:
1192:
1188:
1184:
1180:
1176:
1169:
1166:
1161:
1157:
1153:
1149:
1145:
1138:
1135:
1130:
1126:
1122:
1118:
1114:
1110:
1109:
1101:
1098:
1093:
1089:
1085:
1081:
1077:
1073:
1072:
1067:
1060:
1057:
1052:
1050:1-57259-153-6
1046:
1041:
1040:
1031:
1028:
1023:
1017:
1013:
1009:
1005:
998:
995:
990:
986:
982:
978:
971:
968:
963:
959:
954:
949:
945:
941:
937:
933:
929:
922:
919:
914:
908:
904:
900:
896:
895:
887:
884:
879:
875:
871:
867:
866:
858:
855:
850:
846:
842:
838:
834:
830:
823:
820:
814:
807:
803:
802:
797:
790:
787:
782:
776:
773:
768:
762:
758:
754:
750:
743:
740:
737:
733:
727:
725:
723:
719:
712:
708:
705:
703:
700:
698:
697:Thiocarbonate
695:
693:
690:
689:
685:
683:
681:
677:
670:
666:
664:
659:
657:
653:
649:
641:
637:
635:
619:
612:
608:
601:
597:
592:
585:
583:
578:
576:
572:
568:
564:
560:
556:
552:
548:
544:
538:
536:
528:
526:
524:
520:
516:
512:
507:
505:
501:
497:
493:
489:
485:
481:
480:cinnamoyl-CoA
477:
476:propionyl-CoA
473:
469:
465:
461:
457:
456:intermediates
449:
446:Structure of
444:
439:
431:
426:
421:
414:
410:
409:
408:
406:
402:
368:
339:
338:
337:
333:
331:
327:
319:
315:
314:
313:
311:
295:
294:
293:
287:
285:
283:
279:
275:
274:carbonylation
270:
268:
264:
259:
257:
253:
249:
245:
241:
223:
222:
221:
219:
181:
180:
179:
177:
176:Mannich bases
159:
158:
157:
155:
151:
147:
143:
133:
132:
131:
129:
125:
124:acid chloride
117:
115:
113:
109:
105:
101:
97:
89:
81:
77:
73:
65:
57:
53:
49:
41:
37:
32:
19:
2334:
2327:
2241:Vinyl halide
2198:
2128:Borinic acid
2123:Boronic acid
2100:Thioxanthate
2089:
1440:Hydrocarbons
1358:
1354:
1348:
1340:
1307:
1303:
1293:
1260:
1256:
1250:
1231:
1225:
1182:
1178:
1168:
1151:
1147:
1137:
1112:
1106:
1100:
1075:
1069:
1059:
1038:
1030:
1003:
997:
980:
976:
970:
935:
931:
921:
892:
886:
869:
863:
857:
832:
828:
822:
812:
805:
799:
789:
775:
748:
742:
707:Aldrithiol-2
674:
660:
652:pinner salts
645:
618:Thionoesters
617:
616:
586:Thionoesters
580:
540:
532:
511:thiolactones
508:
500:caffeic acid
488:Acetogenesis
453:
432:Biochemistry
398:
334:
323:
307:
291:
271:
260:
237:
215:
173:
139:
128:alkali metal
121:
96:biochemistry
68:R−C(=O)−O−R’
60:R−C(=O)−S−R’
51:
45:
2305:Thiocyanate
2300:Sulfonamide
2265:Perchlorate
2253:Acyl halide
2214:Fluoroethyl
2095:Thionoester
1983:Phosphonium
1968:Phosphinate
1963:Phosphonous
1951:Phosphonate
1650:Hydroperoxy
1472:Cyclopropyl
598:groups, or
559:fatty acids
519:clopidogrel
515:ticlopidine
468:malonyl-CoA
460:fatty acids
394:SH + MeSAc
365:SAc + KOMs
110:groups, or
84:R−C(=O)−O−H
38:groups, or
2380:Thioesters
2364:Categories
2209:Haloalkane
2080:Thioketone
2035:Persulfide
1931:Phosphorus
1896:Isocyanate
1886:Isonitrile
1787:or oxygen
1785:hydrogen,
1781:not being
1762:Orthoester
1655:Dioxiranes
1633:Enol ether
1521:1-Propenyl
713:References
680:thioamides
571:porphyrins
537:explains:
492:acetyl-CoA
464:mevalonate
436:See also:
225:RSH + R'CO
150:alkylation
104:acetyl-CoA
100:coenzyme A
52:thioesters
2342:inorganic
2176:Tellurium
2090:Thioester
2055:Sulfoxide
2040:Disulfide
2028:Sulfonium
1978:Phosphine
1956:Phosphite
1939:Phosphate
1871:Carbamate
1846:Hydrazone
1779:element,
1777:Only one
1750:Anhydride
1489:Methylene
1391:246418140
1375:1477-0520
1332:245669904
849:107391323
676:Xanthates
523:prasugrel
504:ubiquitin
304:H + RSH
300:O → RCO
288:Reactions
254:and some
195:OH → CH
187:COSH + R'
169:COSR + KX
118:Synthesis
86:) with a
2323:See also
2258:Chloride
2184:Tellurol
2138:Selenium
2105:Xanthate
1819:Ammonium
1801:Nitrogen
1783:carbon,
1740:Carboxyl
1707:Aldehyde
1695:Acryloyl
1677:carbonyl
1581:hydrogen
1536:Cumulene
1383:35129563
1324:34978834
1285:52309850
1277:30235418
1217:27443234
1160:29775520
1129:22482514
1092:20869346
962:21639109
686:See also
567:terpenes
555:peptides
265:, using
256:lactones
126:with an
102:, e.g.,
2347:organic
2146:Selenol
2070:Sulfone
2023:Sulfide
1921:NONOate
1916:Nitroso
1906:Nitrite
1901:Nitrate
1891:Cyanate
1881:Nitrile
1866:Amidine
1861:Imidate
1831:Nitrene
1826:Hydrazo
1814:Enamine
1745:Acetoxy
1733:carboxy
1700:Benzoyl
1638:Epoxide
1621:Methoxy
1611:Alcohol
1565:Carbene
1499:Methine
1208:4956751
1187:Bibcode
953:3242736
628:C(S)OCH
596:organyl
563:sterols
282:alkenes
278:alkynes
142:halides
108:organyl
36:organyl
2246:Iodide
2166:Selone
2010:Sulfur
1719:Ketone
1712:Ketene
1690:Acetyl
1645:Peroxy
1616:Alkoxy
1606:Acetal
1587:oxygen
1576:carbon
1560:Alkyne
1553:Benzyl
1548:Phenyl
1531:Allene
1526:Crotyl
1506:Alkene
1494:Bridge
1482:Pentyl
1467:Propyl
1457:Methyl
1389:
1381:
1373:
1330:
1322:
1283:
1275:
1238:
1215:
1205:
1158:
1127:
1090:
1047:
1018:
983:: 48.
960:
950:
909:
847:
763:
551:lipids
547:esters
521:, and
496:lignin
482:, and
310:amides
94:). In
92:R'−S−H
2278:Other
2115:Boron
2085:Thial
2018:Thiol
1911:Nitro
1876:Imide
1856:Amide
1841:Oxime
1836:Imine
1809:Amine
1757:Ester
1724:Ynone
1628:Ether
1599:R-O-R
1574:Only
1516:Allyl
1511:Vinyl
1477:Butyl
1462:Ethyl
1452:Alkyl
1387:S2CID
1328:S2CID
1281:S2CID
1156:JSTOR
1004:EEROS
845:S2CID
808:: 285
654:with
199:COSCH
88:thiol
78:of a
72:thio-
2200:Halo
1685:Acyl
1585:and
1543:Aryl
1379:PMID
1371:ISSN
1320:PMID
1273:PMID
1236:ISBN
1213:PMID
1125:PMID
1088:PMID
1045:ISBN
1016:ISBN
958:PMID
907:ISBN
761:ISBN
678:and
462:and
386:C(CH
374:C(CH
357:C(CH
345:C(CH
280:and
54:are
1851:Azo
1363:doi
1349:via
1312:doi
1265:doi
1203:PMC
1195:doi
1117:doi
1080:doi
1076:507
1008:doi
985:doi
948:PMC
940:doi
899:doi
874:doi
837:doi
753:doi
732:doi
543:ATP
276:of
244:T3P
240:DCC
207:+ H
203:NR'
191:NCH
152:of
50:,
46:In
2366::
1583:,
1578:,
1385:.
1377:.
1369:.
1359:20
1357:.
1353:.
1326:.
1318:.
1308:24
1306:.
1302:.
1279:.
1271:.
1261:83
1259:.
1211:.
1201:.
1193:.
1181:.
1177:.
1152:83
1150:.
1146:.
1123:.
1113:25
1111:.
1086:.
1074:.
1068:.
1014:.
1006:.
981:61
979:.
956:.
946:.
936:44
934:.
930:.
905:.
870:22
868:.
843:.
833:21
831:.
810:;
806:84
804:.
798:.
759:.
721:^
658:.
569:,
565:,
561:,
557:,
525:.
517:,
478:,
474:,
470:,
332:.
312::
269:.
250:.
220::
183:CH
161:CH
156::
1424:e
1417:t
1410:v
1393:.
1365::
1334:.
1314::
1287:.
1267::
1244:.
1219:.
1197::
1189::
1183:6
1162:.
1131:.
1119::
1094:.
1082::
1053:.
1024:.
1010::
991:.
987::
964:.
942::
915:.
901::
880:.
876::
851:.
839::
817:.
769:.
755::
734::
630:3
626:5
624:H
622:6
600:H
577:.
392:3
390:)
388:2
384:3
380:3
378:)
376:2
372:3
370:H
363:3
361:)
359:2
355:3
351:3
349:)
347:2
343:3
341:H
302:2
298:2
233:O
231:2
227:2
211:O
209:2
205:2
201:2
197:3
193:2
189:2
185:3
167:3
163:3
112:H
90:(
82:(
66:(
40:H
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.