17:
156:-containing biomolecules. In this process, gold atoms on the nanoparticles' surface react with the thiol, dissolving as gold-thiolate complexes until the dissolution reaction stops; this leaves behind a residual species of thiolate-protected gold clusters that is particularly stable. This type of synthesis is also possible using other non thiol-based ligands.
202:" configuration have indeed been identified as the most stable ones. This electronic shell closure and the resulting gain in stability is responsible for the discrete distribution of a few stable cluster sizes (magic numbers) observed in their synthesis, rather than a quasi-continuous distribution of sizes.
119:
can be used for directed synthesis of clusters. The high affinity of the gold ions to electronegative and (partially) charged atoms of functional groups yields potential seeds for cluster formation. The interface between the metal and the template can act as a stabilizer and steer the final size of
861:
Alfredo
Tlahuice-Flores, Ulises Santiago, Daniel Bahena, Ekaterina Vinogradova, Cecil V Conroy, Tarushee Ahuja, Stephan B. H. Bach, Arturo Ponce, Gangli Wang, Miguel Jose-Yacaman, and Robert L. Whetten: On the Structure of the Thiolated Au130 Cluster, J. Phys. Chem. A. 2013, Volume 117, Number 40,
90:
The reduction process depends on the equilibrium between different oxidation states of the gold and the oxidized or reduced forms of the reducing agent, or thiols. Gold(I)-thiolate polymers have been identified as important in the initial steps of the reaction. Several synthesis recipes exist that
879:
Yuxiang Chen, Chenjie Zeng, Chong Liu, Kristin
Kirschbaum, Chakicherla Gayathri, Roberto R. Gil, Nathaniel L. Rosi, and Rongchao Jin: Crystal Structure of Barrel-Shaped Chiral Au130(p-MBT)50 Nanocluster, Journal of the American Chemical Society 2015, Volume 137, Number 32, pages 10076–10079
914:
Cheng-An J. Lin, Chih-Hsien Lee, Jyun-Tai Hsieh, Hsueh-Hsiao Wang, Jimmy K. Li, Ji-Lin Shen, Wen-Hsiung Chan, Hung-I Yeh, Walter H. Chang: Synthesis of
Fluorescent Metallic Nanoclusters toward Biomedical Application: Recent Progress and Present Challenges, Journal of Medical and Biological
844:
Yael Levi-Kalisman, Pablo D. Jadzinsky, Nir
Kalisman, Hironori Tsunoyama, Tatsuya Tsukuda, David A. Bushnell, and Roger D. Kornberg: Synthesis and Characterization of Au102(p-MBA)44 Nanoparticles, Journal of the American Chemical Society 2011, Volume 133, Number 9, pages 2976–2982
618:. The protected gold particles' stability and fluorescence makes them efficient emitters of electromagnetic radiation that can be tuned by varying the cluster size and the type of ligand used for protection. The protective shell can function (have
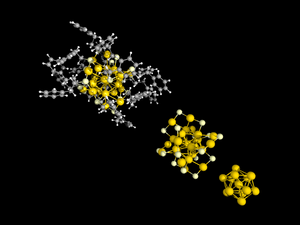
185:
in these species are areas where they behave like molecular, rather than metallic, substances. This molecular optical behavior sharply distinguishes thiolate-protected clusters from gold nanoparticles, whose optical characteristics are driven by
336:
cluster, based on
Density Functional Theory (DFT) was confirmed in 2015. This result represents the maturity of this field where calculations are able to guide the experimental work. The following table features some sizes.
792:
Marcos M. Alvarez, Joseph T. Khoury, T. Gregory
Schaaff, Marat N. Shafigullin, Igor Vezmar, and Robert L. Whetten: Optical Absorption Spectra of Nanocrystal Gold Molecules, J. Phys. Chem. B, 1997, 101 (19), 3706–3712
754:
Xiangming Meng, Zhao Liu, Manzhou Zhu and
Rongchao Jin: Controlled reduction for size selective synthesis of thiolate-protected gold nanoclusters Aun (n = 20, 24, 39, 40), Nanoscale Research Letters,
827:
Manzhou Zhu, Huifeng Qian and
Rongchao Jin: Thiolate-Protected Au20 Clusters with a Large Energy Gap of 2.1 eV, Journal of the American Chemical Society 2009, Volume 131, Number 21, pages 7220-7221 (
669:
Yuichi
Negishi, Katsuyuki Nobusada, Tatsuya Tsukuda: "Glutathione-Protected Gold Clusters Revisited: Bridging the Gap between Gold(I)−Thiolate Complexes and Thiolate-Protected Gold Nanocrystals",
95:, however the mechanism is not yet fully understood. The synthesis produces a mixture of dissolved, thiolate-protected gold clusters of different sizes. These particles can then be separated by
217:
and are end products of the etching procedure after an addition of excess thiols does not lead to further metal dissolution. Some important clusters with magic numbers are (SG:
695:
Y, Negishi (June 1994). "Magic-numbered Au(n) clusters protected by glutathione monolayers (n = 18, 21, 25, 28, 32, 39): isolation and spectroscopic characterization".
79:
The wet chemical synthesis of thiolate-protected gold clusters is achieved by the reduction of gold(III) salt solutions, using a mild reducing agent in the presence of
83:
compounds. This method starts with gold ions and synthesizes larger particles from them, therefore this type of synthesis can be regarded as a "bottom-up approach" in
213:
are connected with the number of metal atoms in those thiolate-protected clusters which display an outstanding stability. Such clusters can be synthesized
177:
gap. This existence of discrete electronic states was first indicated by the discrepancy between their optical absorption and the predictions of classical
173:
of the thiolate-protected gold clusters is characterized by strongly pronounced quantum effects. These result in discrete electronic states, and a nonzero
103:). If the synthesis is performed in a kinetically controlled manner, particularly stable representatives can be obtained with particles of uniform size (
775:
Atomically monodispersed and fluorescent sub-nanometer gold clusters by biomolecule-assisted etching of nanometer-sized gold particles and rods (
919:
622:
added) in a way that selective binding (for example, as a complementary protein receptor of DNA-DNA-interaction) qualifies them for the use as
32:
Ph, white: H, grey: C, dull yellow :S, yellow: Au) single crystal X-ray diffractometry. Top left: full structure; middle : only
729:
Manzhou Zhu, Eric Lanni, Niti Garg, Mark E. Bier, and
Rongchao Jin: Kinetically Controlled, High-Yield Synthesis of Au25 Clusters,
198:, that are labeled S, P, D, F according to their respective angular momentum on the atomic level. Those clusters that have a "
190:. Some of thiolate-protected clusters' properties can be described using a model in which the clusters are treated like "
614:) can be made available for bionanotechnological applications by linking them with biomolecules through the process of
941:
187:
589:
527:
482:
456:
421:
210:
63:
16:
59:
because of their unique stability and electronic properties. They are considered to be stable compounds.
170:
916:
96:
62:
These clusters can range in size up to hundreds of gold atoms, above which they are classified as
712:
619:
607:
182:
116:
56:
898:
881:
863:
846:
828:
811:
794:
776:
759:
738:
704:
678:
650:
451:
447:
538:
514:
923:
763:
133:
125:
148:
Top-down synthesis of the clusters can be achieved by the "etching" of larger metallic
615:
178:
92:
84:
935:
530:
519:
149:
48:
316:
with the para-mercaptobenzoice (para-mercapto-benzoic acid, p-MBA) produced ligand.
611:
559:
195:
563:
535:
524:
511:
492:
487:
477:
426:
417:
409:
383:
378:
218:
214:
104:
815:
623:
191:
174:
121:
780:
716:
885:
810:
A unified view of ligand-protected gold clusters as superatom complexes (
897:
Synthesis and Bioconjugation of 2 and 3 nm-diameter Gold Nanoparticles (
654:
137:
129:
902:
867:
850:
832:
798:
742:
708:
682:
153:
641:
Rongchao Jin: Quantum sized, thiolate-protected gold nanoclusters;
80:
324:
Worthy of note is that in 2013, a structural prediction of the Au
100:
52:
33:
308:
is also well-known. It was greater than representatives Au
55:
ions and thin layer compounds that play a special role in
115:
Rather than starting from "naked" gold ions in solution,
610:, intrinsic properties of the clusters (for example,
181:. The discrete optical transitions and occurrence of
194:". According to this model they exhibit atomic-like
36:
core and Au-S shell displayed, bottom right: only Au
8:
120:the cluster. Some potential templates are
344:
15:
634:
91:are similar to the Brust synthesis of
107:), avoiding further separation steps.
764:10.1186/1556-276X-7-277-3479.48780458
665:
663:
7:
915:Engineering, (2009) Vol 29, No 6, (
87:to the synthesis of nanoparticles.
14:
165:Electronic and optical properties
45:Thiolate-protected gold clusters
47:are a type of ligand-protected
1:
111:Template-mediated synthesis
958:
452:Angew. Chem Int. Ed. 2015
448:Angew. Chem Int. Ed. 2015
200:closed superatomic shell
816:10.1073/pnas.0801001105
677:, 127 (14), 5261–5270 (
781:10.1002/chem.200802743
737:, 130 (4), 1138–1139 (
75:Wet chemical synthesis
41:
564:J. Phys. Chem. A 2013
19:
886:10.1021/jacs.5b05378
341:Composition database
320:Structure prediction
171:electronic structure
66:gold nanoparticles.
862:pages 10470–10476 (
152:with redox-active,
97:gel electrophoresis
51:, synthesized from
922:2015-06-10 at the
655:10.1039/B9NR00160C
117:template reactions
42:
942:Cluster chemistry
903:10.1021/bc900135d
868:10.1021/jp406665m
851:10.1021/ja109131w
833:10.1021/ja902208h
799:10.1021/jp962922n
743:10.1021/ja0782448
731:J. Am. Chem. Soc.
709:10.1021/ja0483589
703:(21): 6518–6519.
683:10.1021/ja042218h
671:J. Am. Chem. Soc.
620:functional groups
608:bionanotechnology
599:
598:
354:Crystal Structure
196:electronic states
188:Plasmon resonance
183:photoluminescence
144:Etching synthesis
949:
927:
912:
906:
895:
889:
877:
871:
859:
853:
842:
836:
825:
819:
808:
802:
790:
784:
773:
767:
752:
746:
727:
721:
720:
692:
686:
667:
658:
639:
536:Anal. Chem. 2013
363:Exp. powder XRD
345:
134:polyelectrolytes
126:oligonucleotides
957:
956:
952:
951:
950:
948:
947:
946:
932:
931:
930:
924:Wayback Machine
913:
909:
896:
892:
878:
874:
860:
856:
843:
839:
826:
822:
809:
805:
791:
787:
774:
770:
753:
749:
728:
724:
694:
693:
689:
668:
661:
649:, 2, 343–362l (
640:
636:
632:
604:
581:
577:
554:
550:
508:
504:
474:
470:
442:
438:
406:
402:
375:
371:
343:
335:
331:
327:
322:
315:
311:
307:
303:
299:
292:
288:
284:
280:
276:
272:
268:
264:
260:
256:
252:
248:
244:
240:
236:
232:
228:
224:
208:
167:
162:
146:
113:
77:
72:
57:cluster physics
40:-core displayed
39:
31:
27:
23:
20:Structure of Au
12:
11:
5:
955:
953:
945:
944:
934:
933:
929:
928:
907:
890:
872:
854:
837:
820:
803:
785:
768:
747:
722:
687:
659:
633:
631:
628:
616:bioconjugation
603:
600:
597:
596:
594:
592:
587:
584:
582:
579:
575:
571:
570:
568:
566:
561:
557:
555:
552:
548:
544:
543:
541:
539:Nano Lett 2015
533:
528:Nanoscale 2013
522:
517:
515:Nano Lett 2015
509:
506:
502:
498:
497:
495:
490:
485:
483:Nanoscale 2014
480:
475:
472:
468:
464:
463:
461:
459:
454:
445:
443:
440:
436:
432:
431:
429:
424:
415:
412:
407:
404:
400:
396:
395:
392:
389:
386:
381:
376:
373:
369:
365:
364:
361:
358:
355:
352:
349:
342:
339:
333:
329:
325:
321:
318:
313:
309:
305:
301:
297:
290:
286:
282:
278:
274:
270:
266:
262:
258:
254:
250:
246:
242:
238:
234:
230:
226:
222:
215:monodispersely
207:
204:
179:Mie scattering
166:
163:
161:
158:
145:
142:
112:
109:
105:monodispersely
93:colloidal gold
85:nanotechnology
76:
73:
71:
68:
37:
29:
25:
21:
13:
10:
9:
6:
4:
3:
2:
954:
943:
940:
939:
937:
925:
921:
918:
911:
908:
904:
900:
894:
891:
887:
883:
876:
873:
869:
865:
858:
855:
852:
848:
841:
838:
834:
830:
824:
821:
817:
813:
807:
804:
800:
796:
789:
786:
782:
778:
772:
769:
765:
761:
757:
751:
748:
744:
740:
736:
732:
726:
723:
718:
714:
710:
706:
702:
698:
697:J Am Chem Soc
691:
688:
684:
680:
676:
672:
666:
664:
660:
656:
652:
648:
644:
638:
635:
629:
627:
625:
621:
617:
613:
609:
601:
595:
593:
591:
588:
585:
583:
573:
572:
569:
567:
565:
562:
560:
558:
556:
546:
545:
542:
540:
537:
534:
532:
529:
526:
523:
521:
518:
516:
513:
510:
500:
499:
496:
494:
491:
489:
486:
484:
481:
479:
476:
466:
465:
462:
460:
458:
455:
453:
449:
446:
444:
434:
433:
430:
428:
425:
423:
419:
416:
413:
411:
408:
398:
397:
393:
390:
387:
385:
382:
380:
377:
367:
366:
362:
359:
356:
353:
350:
347:
346:
340:
338:
319:
317:
294:
220:
216:
212:
211:Magic numbers
206:Magic numbers
205:
203:
201:
197:
193:
189:
184:
180:
176:
172:
164:
159:
157:
155:
151:
150:nanoparticles
143:
141:
139:
135:
131:
127:
123:
118:
110:
108:
106:
102:
98:
94:
88:
86:
82:
74:
69:
67:
65:
60:
58:
54:
50:
49:metal cluster
46:
35:
18:
910:
893:
875:
857:
840:
823:
806:
788:
771:
755:
750:
734:
730:
725:
700:
696:
690:
674:
670:
646:
642:
637:
612:fluorescence
605:
602:Applications
531:Sci Adv 2015
520:Sci Adv 2015
323:
295:
209:
199:
168:
147:
114:
89:
78:
61:
44:
43:
360:Exp. UV-Vis
348:Composition
219:Glutathione
758:, 7, 277 (
630:References
624:biosensors
357:DFT models
351:Mass Spec.
192:superatoms
160:Properties
122:dendrimers
64:passivated
643:Nanoscale
590:PCCP 2015
586:not known
525:JACS 2012
512:JACS 2010
493:JPCL 2010
488:JACS 2012
478:JPCL 2010
457:PCCP 2012
427:JACS 2005
422:PCCP 2013
418:JACS 2013
414:Not known
410:JACS 2005
384:JACS 2000
379:JACS 2005
175:HOMO/LUMO
70:Synthesis
936:Category
920:Archived
917:Abstract
717:15161256
394:Example
285:, and Au
138:polymers
130:proteins
28:-,(R=SCH
391:Example
312:(p-MBA)
715:
221:): Au
154:thiol
81:thiol
756:2012
735:2008
713:PMID
675:2005
647:2010
578:(SR)
551:(SR)
505:(SR)
471:(SR)
439:(SR)
403:(SR)
372:(SR)
328:(SCH
300:(SCH
289:(SG)
281:(SG)
277:, Au
273:(SG)
269:, Au
265:(SG)
261:, Au
257:(SG)
253:, Au
249:(SG)
245:, Au
241:(SG)
237:, Au
233:(SG)
229:, Au
225:(SG)
169:The
136:and
101:PAGE
53:gold
34:gold
899:doi
882:doi
864:doi
847:doi
829:doi
812:doi
795:doi
777:doi
760:doi
739:doi
705:doi
701:126
679:doi
651:doi
606:In
576:187
549:130
326:130
310:102
304:Ph)
938::
926:).
905:).
888:).
870:).
835:).
818:).
801:).
783:).
766:).
745:).
733:,
711:.
699:.
685:).
673:,
662:^
657:).
645:,
626:.
580:68
574:Au
553:50
547:Au
507:24
503:40
501:Au
473:20
469:24
467:Au
450:,
441:14
437:18
435:Au
420:,
405:13
401:15
399:Au
374:10
370:10
368:Au
334:50
314:44
306:16
298:20
296:Au
293:.
291:24
287:39
283:22
279:33
275:20
271:29
267:18
263:25
259:17
255:22
251:16
247:22
243:14
239:18
235:13
231:15
227:10
223:10
140:.
132:,
128:,
124:,
38:13
26:18
22:25
901::
884::
880:(
866::
849::
831::
814::
797::
793:(
779::
762::
741::
719:.
707::
681::
653::
388:-
332:)
330:3
302:2
99:(
30:2
24:R
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.