24:
33:
312:
195:
519:
514:
42:
650:
814:
737:
The C–P–C bond angles are consistent with the notion that phosphorus predominantly uses the 3p orbitals for forming bonds and that there is little sp hybridization of the phosphorus atom. The latter is a common feature of the chemistry of phosphorus. As a result, the lone pair of trimethylphosphine
23:
527:
494:
581:
663:
1197:
1075:
Sattler, A.; Parkin, G. (2011). "Formation of a
Cationic Alkylidene Complex via Formal Hydride Abstraction: Synthesis and Structural Characterization of X (X = Br, I)".
351:
32:
573:
1181:
1127:
1059:
896:
is 118°. This angle is an indication of the amount of steric protection that this ligand provides to the metal that to which it is bound.
1151:
1009:
326:
1233:
899:
Being a relatively compact phosphine, several can bind to a single transition metal, as illustrated by the existence of Pt(PEt
877:
670:
1228:
567:
259:
701:. This colorless liquid has a strongly unpleasant odor, characteristic of alkylphosphines. The compound is a common
290:
518:
226:
1238:
992:
Schier, Annette; Schmidbaur, Hubert (2006). "P-Donor
Ligands". In Charles A. McAuliffe, Anthony G. Mackie (ed.).
686:
202:
754:
220:
513:
190:
854:
706:
541:
506:
466:
66:
54:
307:
1043:
1035:
936:
750:
86:
853:
reacts with strong acids to give salts X. This reaction is reversible. With strong bases, such as
629:
577:
132:
1177:
1147:
1123:
1092:
1055:
1005:
940:
893:
608:
120:
1169:
1115:
1084:
1047:
997:
374:
268:
172:
41:
932:
311:
194:
96:
963:
152:
1110:
H. F. Klein (1978). "Trimethylphosphonium
Methylide (Trimethyl Methylenephosphorane)".
1024:
E. Fluck, The
Chemistry of Phosphine, Topics in Current Chemistry Vol. 35, 64 pp, 1973.
873:
641:
615:
450:
1222:
928:
798:
440:
430:
183:
1164:
T. Yoshida; T. Matsuda; S. Otsuka (1990). "Tetrakis(Triethylphosphine)Platinum(0)".
547:
248:
731:
588:
559:
1173:
1119:
1051:
399:
163:
1001:
555:
718:
622:
279:
1096:
892:
that forms complexes with most metals. As a ligand, trimethylphosphine's
1088:
1034:
Leutkens Jr., M. L.; Sattelberger, A. P.; Murray, H. H.; Basil, J. D.;
420:
235:
203:
872:
is easily oxidised to the phosphine oxide with oxygen. It reacts with
551:
889:
702:
478:
813:
640:
Except where otherwise noted, data are given for materials in their
958:
956:
812:
143:
119:
109:
970:. UK: European Bioinformatics Institute. 6 June 2006. IUPAC Names
738:
has predominantly s-character as is the case for phosphine, PH
295:
445:
38 to 39 °C (100 to 102 °F; 311 to 312 K)
658:
857:, a methyl group undergoes deprotonation to give PMe
994:Encyclopedia of Inorganic Chemistry, First Edition
734:. The C–P–C bond angles are approximately 98.6°.
968:Chemical Entities of Biological Interest (ChEBI)
247:
95:
8:
987:
985:
1146:, 3rd Ed, Pearson/Prentice Hall publisher,
911:) is an air-stable solid that releases PMe
310:
193:
171:
15:
907:. Its complex with silver iodide, AgI(PMe
267:
952:
435:−86 °C (−123 °F; 187 K)
356:
331:
306:
225:
184:
1114:. Vol. XVIII. pp. 138–140.
888:Trimethylphosphine is a highly basic
593:−19 °C (−2 °F; 254 K)
338:Key: YWWDBCBWQNCYNR-UHFFFAOYSA-N
151:
7:
749:can be prepared by the treatment of
238:
1168:. Vol. 28. pp. 122–123.
1046:. Vol. 28. pp. 305–310.
964:"Trimethylphosphine (CHEBI:35890)"
801:, from which the more volatile PMe
392:
71:Trimethylphosphane (substitutive)
14:
648:
517:
512:
386:
40:
31:
22:
644:(at 25 °C , 100 kPa).
1142:G. L. Miessler and D. A. Tarr
1038:(1990). "Trimethylphosphine".
931:. It converts to a much safer
878:tetramethylphosphonium bromide
797:The synthesis is conducted in
380:
335:InChI=1S/C3H9P/c1-4(2)3/h1-3H3
73:Trimethylphosphorus (additive)
1:
1198:"Trimethylphosphine solution"
697:, commonly abbreviated as PMe
1255:
1174:10.1002/9780470132593.ch32
1120:10.1002/9780470132494.ch23
1052:10.1002/9780470132593.ch76
721:molecule with approximate
687:organophosphorus compound
638:
597:
493:
488:
459:
455:49.9 kPa (at 20 °C)
367:
347:
322:
79:
65:
53:
48:
39:
30:
21:
1002:10.1002/0470862106.ia177
755:methylmagnesium chloride
568:Precautionary statements
1234:Foul-smelling chemicals
1077:Chemical Communications
855:alkyl lithium compounds
884:Coordination chemistry
842:
707:coordination chemistry
816:
713:Structure and bonding
689:with the formula P(CH
467:Coordination geometry
67:Systematic IUPAC name
935:upon treatment with
55:Preferred IUPAC name
1229:Tertiary phosphines
1166:Inorganic Syntheses
1144:Inorganic Chemistry
1112:Inorganic Syntheses
1083:(48): 12828–12830.
1044:Inorganic Syntheses
1040:Inorganic Syntheses
937:sodium hypochlorite
817:Structure of HW(PMe
751:triphenyl phosphite
472:Trigonal pyramidal
407: g·mol
227:trimethyl+phosphine
133:Beilstein Reference
18:
17:Trimethylphosphine
1089:10.1039/C1CC15457E
843:
805:can be distilled.
683:Trimethylphosphine
671:Infobox references
598:Related compounds
59:Trimethylphosphane
16:
1183:978-0-470-13259-3
1129:978-0-470-13249-4
1061:978-0-470-13259-3
1036:Fackler J. P. Jr.
941:hydrogen peroxide
894:Tolman cone angle
679:Chemical compound
677:
676:
604:Related compounds
542:Hazard statements
415:Colorless liquid
291:CompTox Dashboard
121:Interactive image
1246:
1239:Methyl compounds
1213:
1212:
1210:
1208:
1202:sigmaaldrich.com
1194:
1188:
1187:
1161:
1155:
1140:
1134:
1133:
1107:
1101:
1100:
1072:
1066:
1065:
1031:
1025:
1022:
1016:
1015:
989:
980:
979:
977:
975:
960:
661:
655:
652:
651:
583:
579:
575:
561:
557:
553:
549:
521:
516:
406:
394:
388:
382:
375:Chemical formula
315:
314:
299:
297:
271:
251:
240:
229:
205:
197:
186:
175:
155:
123:
99:
44:
35:
26:
19:
1254:
1253:
1249:
1248:
1247:
1245:
1244:
1243:
1219:
1218:
1217:
1216:
1206:
1204:
1196:
1195:
1191:
1184:
1163:
1162:
1158:
1141:
1137:
1130:
1109:
1108:
1104:
1074:
1073:
1069:
1062:
1033:
1032:
1028:
1023:
1019:
1012:
991:
990:
983:
973:
971:
962:
961:
954:
949:
933:phosphine oxide
926:
921:
914:
910:
906:
902:
886:
871:
864:
860:
852:
848:
840:
836:
832:
828:
824:
820:
811:
804:
792:
788:
784:
780:
776:
772:
768:
764:
748:
741:
730:
715:
700:
696:
692:
680:
673:
668:
667:
666: ?)
657:
653:
649:
645:
633:
628:
626:
621:
619:
614:
612:
605:
570:
544:
530:
509:
481:
469:
404:
391:
385:
377:
363:
360:
355:
354:
343:
340:
339:
336:
330:
329:
318:
300:
293:
274:
254:
241:
215:
178:
158:
135:
126:
113:
102:
89:
75:
74:
72:
61:
60:
12:
11:
5:
1252:
1250:
1242:
1241:
1236:
1231:
1221:
1220:
1215:
1214:
1189:
1182:
1156:
1135:
1128:
1102:
1067:
1060:
1026:
1017:
1010:
981:
951:
950:
948:
945:
924:
920:
917:
915:upon heating.
912:
908:
904:
900:
885:
882:
874:methyl bromide
869:
862:
858:
850:
846:
838:
834:
830:
826:
822:
818:
810:
807:
802:
795:
794:
790:
786:
782:
778:
774:
770:
766:
762:
746:
739:
725:
714:
711:
698:
694:
690:
678:
675:
674:
669:
647:
646:
642:standard state
639:
636:
635:
631:
624:
617:
610:
606:
603:
600:
599:
595:
594:
591:
585:
584:
582:P305+P351+P338
571:
566:
563:
562:
545:
540:
537:
536:
531:
526:
523:
522:
510:
505:
502:
501:
491:
490:
486:
485:
482:
477:
474:
473:
470:
465:
462:
461:
457:
456:
453:
451:Vapor pressure
447:
446:
443:
437:
436:
433:
427:
426:
423:
417:
416:
413:
409:
408:
402:
396:
395:
389:
383:
378:
373:
370:
369:
365:
364:
362:
361:
358:
350:
349:
348:
345:
344:
342:
341:
337:
334:
333:
325:
324:
323:
320:
319:
317:
316:
308:DTXSID00208120
303:
301:
289:
286:
285:
282:
276:
275:
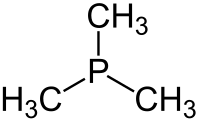
273:
272:
264:
262:
256:
255:
253:
252:
244:
242:
234:
231:
230:
223:
217:
216:
214:
213:
209:
207:
199:
198:
188:
180:
179:
177:
176:
168:
166:
160:
159:
157:
156:
148:
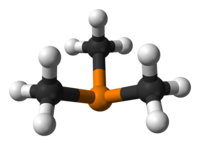
146:
140:
139:
136:
131:
128:
127:
125:
124:
116:
114:
107:
104:
103:
101:
100:
92:
90:
85:
82:
81:
77:
76:
70:
69:
63:
62:
58:
57:
51:
50:
46:
45:
37:
36:
28:
27:
13:
10:
9:
6:
4:
3:
2:
1251:
1240:
1237:
1235:
1232:
1230:
1227:
1226:
1224:
1203:
1199:
1193:
1190:
1185:
1179:
1175:
1171:
1167:
1160:
1157:
1153:
1152:0-13-035471-6
1149:
1145:
1139:
1136:
1131:
1125:
1121:
1117:
1113:
1106:
1103:
1098:
1094:
1090:
1086:
1082:
1078:
1071:
1068:
1063:
1057:
1053:
1049:
1045:
1041:
1037:
1030:
1027:
1021:
1018:
1013:
1011:0-470-86078-2
1007:
1003:
999:
995:
988:
986:
982:
969:
965:
959:
957:
953:
946:
944:
942:
938:
934:
930:
927:is toxic and
918:
916:
897:
895:
891:
883:
881:
879:
875:
866:
856:
815:
808:
806:
800:
799:dibutyl ether
760:
759:
758:
756:
752:
743:
735:
733:
729:
724:
720:
712:
710:
708:
704:
688:
684:
672:
665:
660:
643:
637:
634:
627:
620:
613:
607:
602:
601:
596:
592:
590:
587:
586:
572:
569:
565:
564:
546:
543:
539:
538:
535:
532:
529:
525:
524:
520:
515:
511:
508:
504:
503:
499:
497:
492:
487:
483:
480:
479:Dipole moment
476:
475:
471:
468:
464:
463:
458:
454:
452:
449:
448:
444:
442:
441:Boiling point
439:
438:
434:
432:
431:Melting point
429:
428:
424:
422:
419:
418:
414:
411:
410:
403:
401:
398:
397:
379:
376:
372:
371:
366:
357:
353:
346:
332:
328:
321:
313:
309:
305:
304:
302:
292:
288:
287:
283:
281:
278:
277:
270:
266:
265:
263:
261:
258:
257:
250:
246:
245:
243:
237:
233:
232:
228:
224:
222:
219:
218:
211:
210:
208:
206:
201:
200:
196:
192:
189:
187:
185:ECHA InfoCard
182:
181:
174:
170:
169:
167:
165:
162:
161:
154:
150:
149:
147:
145:
142:
141:
137:
134:
130:
129:
122:
118:
117:
115:
111:
106:
105:
98:
94:
93:
91:
88:
84:
83:
78:
68:
64:
56:
52:
47:
43:
38:
34:
29:
25:
20:
1205:. Retrieved
1201:
1192:
1165:
1159:
1143:
1138:
1111:
1105:
1080:
1076:
1070:
1039:
1029:
1020:
993:
974:25 September
972:. Retrieved
967:
922:
898:
887:
867:
849:of 8.65, PMe
844:
796:
744:
736:
727:
722:
716:
682:
681:
533:
495:
80:Identifiers
765:MgCl + P(OC
589:Flash point
528:Signal word
484:1.19 Debye
412:Appearance
368:Properties
191:100.008.932
153:CHEBI:35890
1223:Categories
947:References
929:pyrophoric
507:Pictograms
460:Structure
425:735 mg cm
400:Molar mass
269:5FL6SQK9H3
164:ChemSpider
108:3D model (
87:CAS Number
845:With a pK
833:), "W(PMe
809:Reactions
719:pyramidal
498:labelling
280:UN number
212:209-823-1
204:EC Number
1207:1 August
1097:22048609
876:to give
732:symmetry
717:It is a
489:Hazards
97:594-09-2
664:what is
662: (
421:Density
236:PubChem
138:969138
1180:
1150:
1126:
1095:
1058:
1008:
919:Safety
890:ligand
777:→ P(CH
703:ligand
685:is an
659:verify
656:
534:Danger
405:76.079
359:CP(C)C
352:SMILES
49:Names
793:OMgCl
785:+ 3 C
753:with
327:InChI
284:1993
249:68983
173:62205
144:ChEBI
110:JSmol
1209:2023
1178:ISBN
1148:ISBN
1124:ISBN
1093:PMID
1056:ISBN
1006:ISBN
976:2011
865:Li.
761:3 CH
578:P261
574:P210
560:H335
556:H319
552:H315
548:H225
260:UNII
221:MeSH
1170:doi
1116:doi
1085:doi
1048:doi
998:doi
939:or
923:PMe
868:PMe
829:PCH
825:(Me
745:PMe
705:in
630:PPh
616:NMe
609:PEt
496:GHS
296:EPA
239:CID
1225::
1200:.
1176:.
1122:.
1091:.
1081:47
1079:.
1054:.
1042:.
1004:.
996:.
984:^
966:.
955:^
943:.
880:.
861:CH
841:".
757::
742:.
709:.
623:PH
580:,
576:,
558:,
554:,
550:,
500::
1211:.
1186:.
1172::
1154:.
1132:.
1118::
1099:.
1087::
1064:.
1050::
1014:.
1000::
978:.
925:3
913:3
909:3
905:4
903:)
901:3
870:3
863:2
859:2
851:3
847:a
839:5
837:)
835:3
831:2
827:2
823:4
821:)
819:3
803:3
791:5
789:H
787:6
783:3
781:)
779:3
775:3
773:)
771:5
769:H
767:6
763:3
747:3
740:3
728:v
726:3
723:C
699:3
695:3
693:)
691:3
654:N
632:3
625:3
618:3
611:3
393:P
390:9
387:H
384:3
381:C
298:)
294:(
112:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.