667:
753:
200:
127:
361:
356:
24:
687:(209 nm) region and exhibits a very weak emission of 735 nm at room temperature in the solid state or in glasses of toluene. On the basis of octahedral symmetry, the lower energy absorption is suggested to be a spin-forbidden d-d transition, while the higher energy absorption is proposed to be a ligand-to-metal
1490:
1149:"Organoplatinum compounds VII1For part VI see Ref. ([4]b).1: Trimethylplatinum fluoride [(CH3)3PtF]4, the missing link in organoplatinum cluster chemistry: its synthesis, crystal structure and a comparison to the crystal structure of [(CH3)3PtOH]4"
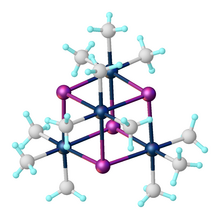
651:. Each Pt atom is coordinated in pseudo-octahedral geometry, while the tetramer unit is nearly cubic in the Pt-I core. The average Pt-I bond distance is 2.83 Å, while the average Pt-C bond distance is 2.04 Å. The fluoride, bromide, chloride, and pseudohalide (OH, N
1133:
577:. Due to its stability, it is often utilized as a precursor en route to the synthesis of other organoplatinum compound, such as hydrosilylation catalysts. It is also used as a precursor for forming platinum layers for electronics.
589:
with excess methylmagnesium iodide. The trimethylated Pt(IV) complex could be isolated as a yellow crystalline solid, which contained iodine impurities. Improvements on the synthesis in later years utilized the more facile
369:
336:
732:
The cubane structure can undergo decomposition by ligand substitution and breaking of the bridging iodine, to form a variety of octahedral organoplatinum complexes. Derived compounds include those with
717:
The tetramer decomposes, sometime explosively, when heated below at 175 - 200°C, resulting in platinum metal, ethane, and iodomethane. When exposed to UV light in solution, the tetramer can undergo
1488:, Ziche, Wolfgang & Köllnberger, Andreas, "Platinum Complexes and Their Use in Compounds That Can Be Cross-Linked by a Hydrosilylation Reaction", issued 2016-03-03
479:
239:
1259:
Ghosh, Biswa Nath; Topić, Filip; Sahoo, Prasit Kumar; Mal, Prasenjit; Linnera, Jarno; Kalenius, Elina; Tuononen, Heikki M.; Rissanen, Kari (2014-12-02).
475:
1261:"Synthesis, structure and photophysical properties of a highly luminescent terpyridine-diphenylacetylene hybrid fluorophore and its metal complexes"
1410:"An amino acid bioconjugate of an organoplatinum tris(pyrazolyl)borate complex: Synthesis and structure of [p-(tBuO–Phe–CO)C6H4Tp]PtMe3"
1049:"Characterization of (methylcyclopentadienyl)trimethylplatinum and low-temperature organometallic chemical vapor deposition of platinum metal"
666:
427:
947:
752:
1047:
Xue, Ziling; Strouse, M. Jane; Shuh, David K.; Knobler, Carolyn B.; Kaesz, Herbert D.; Hicks, Robert F.; Williams, R. Stanley (1989).
1509:
1015:"Studies on the thermal activation of iodotrimethylplatinum(IV) tetramer: hydrosilation catalyst generation from an inert precursor"
709:
spectroscopy have also been used to indicate the tetramer structure with cubic breathing modes at very low energies of < 250 cm.
214:
863:
738:
463:
557:. It is a white, air-stable solid that was one of the first σ-alkyl metal complexes reported. It arises from the reaction of
471:
84:
558:
459:
534:
1131:, Doppiu, Angelino; Karch, Ralf & Woerner, Eileen, "Trimethylplatinum(iv) iodide", issued 2021-09-23
421:
178:
360:
766:
648:
134:
1147:
Donath, H; Avtomonov, E. V; Sarraje, I; von Dahlen, K. -H; El-Essawi, M; Lorberth, J; Seo, B. -S (1998-05-29).
926:
Clegg, D. E.; Hall, J. R.; Brubaker, C. H.; Gilbert, G. L.; Dyke, M. (2007-01-05), Muetterties, Earl L. (ed.),
435:
503:
499:
355:
1514:
770:
483:
122:
742:
660:
562:
550:
1447:
Hiratani, Masahiko; Nabatame, Toshihide; Matsui, Yuichi; Imagawa, Kazushige; Kimura, Shinichiro (2001).
722:
702:
383:
348:
1486:
765:
Trimethylplatinum iodide has been utilized as a precursor to volatile Pt complexes for possible use in
1409:
1221:
1148:
413:
1460:
984:
734:
688:
1315:
1260:
195:
746:
591:
586:
570:
447:
50:
1449:"Platinum Film Growth by Chemical Vapor Deposition Based on Autocatalytic Oxidative Decomposition"
756:
Various octahedral organoplatinum complexes that can be synthesized from trimethylplatinum iodide.
431:
1390:
1296:
927:
706:
439:
1190:
1014:
451:
1429:
1382:
1343:
1335:
1288:
1280:
1241:
1168:
1110:
1068:
943:
908:
718:
104:
729:)I species. This reactivity is attributed to the ligand-to-metal charge transfer excitation.
598:
to reduce the amount of methylmagnesium that needed to use, which generated more byproducts.
1468:
1421:
1374:
1327:
1272:
1233:
1202:
1160:
1102:
1060:
1026:
992:
935:
900:
607:
515:
262:
972:
487:
60:
851:
692:
574:
491:
467:
199:
126:
1464:
988:
511:
1448:
1363:"Crystal structure of volatile pyridine adducts of trimethylplatinum(IV) β-diketonates"
528:
1237:
1164:
1128:
1503:
1425:
1206:
1030:
321:
166:
115:
1394:
519:
1090:
1048:
696:
603:
443:
1300:
977:
455:
973:"Trimethylplatinum(IV) iodide and its misrepresentation as hexamethyldiplatinum"
684:
595:
1361:
Zharkova, G. I.; Baidina, I. A.; Naumov, D. Yu.; Igumenov, I. K. (2011-06-01).
939:
507:
1378:
996:
850:
Like other platinum complexes, the trimethylplatinum iodide complex catalyzes
296:
95:
1433:
1386:
1339:
1316:"Phosphido pincer complexes of platinum: synthesis, structure and reactivity"
1284:
1245:
1172:
1114:
1072:
912:
405:
1362:
397:
1347:
1314:
Mazzeo, Mina; Strianese, Maria; Kühl, Olaf; Peters, Jonas C. (2011-08-23).
1292:
971:
Donnay, G.; Coleman, L. B.; Krieghoff, N. G.; Cowan, D. O. (1968-01-23).
904:
888:
566:
1408:
Kuchta, Matthew C.; Gemel, Christian; Metzler-Nolte, Nils (2007-02-15).
1191:"Electronic spectra and photochemistry of methyl platinum(IV) complexes"
1106:
1064:
1331:
1276:
691:
due to high oxidation state on the Pt. The emission is suggested to be
153:
135:
1472:
1222:"Vibrational spectra of chloro-, bromo- and iodotrimethylplatinum(IV)"
401:
573:
with four octahedral Pt(IV) centers linked by four iodides as triply
527:
Except where otherwise noted, data are given for materials in their
393:
854:
of alkenes. Catalysis requires heating to decompose the tetramer.
751:
665:
409:
83:
73:
23:
934:, Hoboken, NJ, USA: John Wiley & Sons, Inc., pp. 71–74,
670:
Pt-I bond distance and I-Pt-I bond angles of the cubic tetramer,
610:, starting from other trimethylplatinum(IV) complexes such as (CH
1416:. Third International Symposium on Bioorganometallic Chemistry.
389:
683:
The tetramer absorbs in weakly at 436 nm and strongly in the
1091:"Some properties of several trimethylplatinum(IV) compounds"
495:
183:
647:
The compound exists as a tetramer, crystallizing in a
887:
Pope, William
Jackson; Peachey, Stanley John (1909).
594:, potassium hexachloroplatinate, and the addition of
695:, with metal-metal interactions leading to a large
602:Trimethylplatinum iodide could also be formed from
165:
1013:Pratt, Sandra L.; Faltynek, Roberta A. (1984).
59:
8:
223:InChI=1S/3CH3.HI.Pt/h3*1H3;1H;/q3*-1;;+1/p-1
198:
125:
103:
15:
889:"LXXIII.—The alkyl compounds of platinum"
1220:Clegg, D. E.; Hall, J. R. (1970-04-01).
1053:Journal of the American Chemical Society
842:
838:
834:
830:
826:
822:
818:
814:
810:
806:
802:
796:, which sublimes near room temperature:
792:
788:
784:
780:
776:
874:
244:
219:
194:
1453:Journal of the Electrochemical Society
116:
1184:
1182:
1089:Hoff, G. R.; Brubaker, C. H. (1968).
1042:
1040:
1008:
1006:
882:
880:
878:
659:also exist as tetramers, all forming
226:Key: ZCSQPOLLUOLHHF-UHFFFAOYSA-M
7:
1084:
1082:
966:
964:
1414:Journal of Organometallic Chemistry
1226:Journal of Organometallic Chemistry
1153:Journal of Organometallic Chemistry
156:
14:
1426:10.1016/j.jorganchem.2006.10.028
1189:Kunkely, H.; Vogler, A. (1991).
864:Transition metal alkyl complexes
359:
354:
286:
274:
22:
1367:Journal of Structural Chemistry
531:(at 25 °C , 100 kPa).
1195:Coordination Chemistry Reviews
1019:Journal of Molecular Catalysis
280:
268:
1:
1238:10.1016/S0022-328X(00)86069-6
1165:10.1016/S0022-328X(98)00481-1
928:"Iodo(trimethyl)platinum(IV)"
725:, forming the brief Pt(II)(CH
559:potassium hexachloroplatinate
1207:10.1016/0010-8545(91)84006-Q
1031:10.1016/0304-5102(84)85038-5
592:hexachloroplatinate reagent
1531:
940:10.1002/9780470132418.ch13
721:of two methyl radicals by
565:. The complex exists as a
1379:10.1134/S0022476611030152
997:10.1107/S056774086800186X
773:. One such derivative is
767:chemical vapor deposition
585:Pope and Peachey treated
525:
335:
330:
255:
235:
210:
43:
38:Iodotrimethylplatinum(IV)
35:
30:
21:
17:Trimethylplatinum iodide
1510:Organoplatinum compounds
547:Trimethylplatinum iodide
422:Precautionary statements
771:atomic layer deposition
757:
675:
655:, SCN, SMe) analogues
563:methylmagnesium iodide
551:organoplatinum complex
755:
723:reductive elimination
669:
905:10.1039/CT9099500571
893:J. Chem. Soc., Trans
689:charge transfer band
649:monoclinic unit cell
1465:2001JElS..148C.524H
1320:Dalton Transactions
1265:Dalton Transactions
1107:10.1021/ic50066a041
1095:Inorganic Chemistry
1065:10.1021/ja00206a002
989:1968AcCrB..24..157D
932:Inorganic Syntheses
747:trispyrazolylborate
587:chloroplatinic acid
571:cubane-type cluster
308: g·mol
18:
1332:10.1039/C1DT10825E
1277:10.1039/C4DT02728K
758:
676:
535:Infobox references
16:
1473:10.1149/1.1381389
1326:(35): 9026–9033.
1059:(24): 8779–8784.
949:978-0-470-13241-8
553:with the formula
543:Chemical compound
541:
540:
384:Hazard statements
179:CompTox Dashboard
85:Interactive image
1522:
1495:
1494:
1493:
1489:
1483:
1477:
1476:
1444:
1438:
1437:
1420:(6): 1310–1314.
1405:
1399:
1398:
1358:
1352:
1351:
1311:
1305:
1304:
1256:
1250:
1249:
1217:
1211:
1210:
1186:
1177:
1176:
1144:
1138:
1137:
1136:
1132:
1125:
1119:
1118:
1101:(8): 1655–1656.
1086:
1077:
1076:
1044:
1035:
1034:
1010:
1001:
1000:
968:
959:
958:
957:
956:
923:
917:
916:
884:
846:
795:
608:potassium iodide
575:bridging ligands
521:
517:
513:
509:
505:
501:
497:
493:
489:
485:
481:
477:
473:
469:
465:
461:
457:
453:
449:
445:
441:
437:
433:
429:
415:
411:
407:
403:
399:
395:
391:
363:
358:
307:
305:
288:
282:
276:
270:
263:Chemical formula
203:
202:
187:
185:
169:
158:
137:
129:
118:
107:
87:
63:
26:
19:
1530:
1529:
1525:
1524:
1523:
1521:
1520:
1519:
1500:
1499:
1498:
1491:
1485:
1484:
1480:
1446:
1445:
1441:
1407:
1406:
1402:
1360:
1359:
1355:
1313:
1312:
1308:
1258:
1257:
1253:
1219:
1218:
1214:
1188:
1187:
1180:
1146:
1145:
1141:
1134:
1127:
1126:
1122:
1088:
1087:
1080:
1046:
1045:
1038:
1012:
1011:
1004:
970:
969:
962:
954:
952:
950:
925:
924:
920:
886:
885:
876:
872:
860:
852:hydrosilylation
844:
840:
836:
832:
828:
824:
820:
816:
812:
808:
804:
800:
794:
790:
786:
782:
778:
774:
763:
743:acetylacetonate
728:
715:
693:phosphorescence
681:
673:
661:cubane clusters
658:
654:
645:
643:Crystallography
640:
633:
629:
625:
621:
617:
613:
601:
583:
556:
544:
537:
532:
424:
386:
372:
351:
303:
301:
291:
285:
279:
273:
265:
251:
248:
243:
242:
231:
228:
227:
224:
218:
217:
206:
188:
181:
172:
159:
147:
110:
90:
77:
66:
53:
39:
12:
11:
5:
1528:
1526:
1518:
1517:
1515:Iodo complexes
1512:
1502:
1501:
1497:
1496:
1478:
1439:
1400:
1373:(3): 550–555.
1353:
1306:
1271:(1): 254–267.
1251:
1232:(2): 491–496.
1212:
1178:
1159:(1): 191–196.
1139:
1129:WO2021186087A1
1120:
1078:
1036:
1002:
983:(1): 157–159.
960:
948:
918:
873:
871:
868:
867:
866:
859:
856:
848:
847:
762:
759:
726:
714:
711:
680:
677:
671:
656:
652:
644:
641:
639:
636:
631:
627:
623:
619:
615:
611:
582:
579:
554:
542:
539:
538:
533:
529:standard state
526:
523:
522:
480:P305+P351+P338
425:
420:
417:
416:
387:
382:
379:
378:
373:
368:
365:
364:
352:
347:
344:
343:
333:
332:
328:
327:
324:
318:
317:
314:
310:
309:
299:
293:
292:
289:
283:
277:
271:
266:
261:
258:
257:
253:
252:
250:
249:
246:
238:
237:
236:
233:
232:
230:
229:
225:
222:
221:
213:
212:
211:
208:
207:
205:
204:
196:DTXSID30473900
191:
189:
177:
174:
173:
171:
170:
162:
160:
152:
149:
148:
146:
145:
141:
139:
131:
130:
120:
112:
111:
109:
108:
100:
98:
92:
91:
89:
88:
80:
78:
71:
68:
67:
65:
64:
56:
54:
49:
46:
45:
41:
40:
37:
33:
32:
28:
27:
13:
10:
9:
6:
4:
3:
2:
1527:
1516:
1513:
1511:
1508:
1507:
1505:
1487:
1482:
1479:
1474:
1470:
1466:
1462:
1458:
1454:
1450:
1443:
1440:
1435:
1431:
1427:
1423:
1419:
1415:
1411:
1404:
1401:
1396:
1392:
1388:
1384:
1380:
1376:
1372:
1368:
1364:
1357:
1354:
1349:
1345:
1341:
1337:
1333:
1329:
1325:
1321:
1317:
1310:
1307:
1302:
1298:
1294:
1290:
1286:
1282:
1278:
1274:
1270:
1266:
1262:
1255:
1252:
1247:
1243:
1239:
1235:
1231:
1227:
1223:
1216:
1213:
1208:
1204:
1200:
1196:
1192:
1185:
1183:
1179:
1174:
1170:
1166:
1162:
1158:
1154:
1150:
1143:
1140:
1130:
1124:
1121:
1116:
1112:
1108:
1104:
1100:
1096:
1092:
1085:
1083:
1079:
1074:
1070:
1066:
1062:
1058:
1054:
1050:
1043:
1041:
1037:
1032:
1028:
1024:
1020:
1016:
1009:
1007:
1003:
998:
994:
990:
986:
982:
978:
974:
967:
965:
961:
951:
945:
941:
937:
933:
929:
922:
919:
914:
910:
906:
902:
898:
894:
890:
883:
881:
879:
875:
869:
865:
862:
861:
857:
855:
853:
799:
798:
797:
772:
768:
760:
754:
750:
748:
744:
740:
736:
730:
724:
720:
712:
710:
708:
704:
700:
698:
694:
690:
686:
678:
668:
664:
662:
650:
642:
637:
635:
609:
605:
599:
597:
593:
588:
580:
578:
576:
572:
568:
564:
560:
552:
548:
536:
530:
524:
426:
423:
419:
418:
388:
385:
381:
380:
377:
374:
371:
367:
366:
362:
357:
353:
350:
346:
345:
341:
339:
334:
329:
325:
323:
322:Melting point
320:
319:
315:
312:
311:
300:
298:
295:
294:
267:
264:
260:
259:
254:
245:
241:
234:
220:
216:
209:
201:
197:
193:
192:
190:
180:
176:
175:
168:
164:
163:
161:
155:
151:
150:
143:
142:
140:
138:
133:
132:
128:
124:
121:
119:
117:ECHA InfoCard
114:
113:
106:
102:
101:
99:
97:
94:
93:
86:
82:
81:
79:
75:
70:
69:
62:
58:
57:
55:
52:
48:
47:
42:
34:
29:
25:
20:
1481:
1456:
1452:
1442:
1417:
1413:
1403:
1370:
1366:
1356:
1323:
1319:
1309:
1268:
1264:
1254:
1229:
1225:
1215:
1198:
1194:
1156:
1152:
1142:
1123:
1098:
1094:
1056:
1052:
1025:(1): 47–58.
1022:
1018:
980:
976:
953:, retrieved
931:
921:
896:
892:
849:
764:
761:Applications
735:polypyridine
731:
716:
701:
697:Stokes shift
682:
646:
604:ion exchange
600:
584:
546:
545:
375:
337:
316:white solid
44:Identifiers
36:Other names
1459:(8): C524.
899:: 571–576.
596:iodomethane
370:Signal word
326:190-195 °C
313:Appearance
256:Properties
123:100.206.221
1504:Categories
955:2023-03-11
870:References
813:+ 4 Na(CH
801:[Pt(CH
719:photolysis
713:Reactivity
679:Electronic
349:Pictograms
297:Molar mass
96:ChemSpider
72:3D model (
61:14364-93-3
51:CAS Number
1434:0022-328X
1387:1573-8779
1340:1477-9234
1285:1477-9234
1246:0022-328X
1201:: 15–25.
1173:0022-328X
1115:0020-1669
1073:0002-7863
913:0368-1645
749:ligands.
638:Structure
581:Synthesis
516:P370+P378
504:P337+P313
500:P332+P313
476:P304+P340
472:P304+P312
468:P302+P352
464:P301+P312
340:labelling
144:681-046-4
136:EC Number
1395:93708060
1348:21826355
1293:25373423
858:See also
825:→ 4(CH
703:Infrared
622:) or (CH
567:tetramer
331:Hazards
167:11824720
105:11522740
1461:Bibcode
985:Bibcode
845:+ 4NaI
549:is the
376:Warning
154:PubChem
1492:
1432:
1393:
1385:
1346:
1338:
1301:331480
1299:
1291:
1283:
1244:
1171:
1135:
1113:
1071:
946:
911:
837:)Pt(CH
787:)Pt(CH
745:, and
739:pincer
606:using
240:SMILES
31:Names
1391:S2CID
1297:S2CID
707:Raman
630:Pt(SO
618:Pt(NO
561:with
215:InChI
74:JSmol
1430:ISSN
1383:ISSN
1344:PMID
1336:ISSN
1289:PMID
1281:ISSN
1242:ISSN
1169:ISSN
1111:ISSN
1069:ISSN
944:ISBN
909:ISSN
705:and
569:: a
520:P501
512:P363
508:P362
496:P330
492:P322
488:P321
484:P312
460:P280
456:P273
452:P271
448:P270
444:P264
440:P261
436:P241
432:P240
428:P210
414:H413
410:H332
406:H319
402:H315
398:H312
394:H302
390:H228
306:.374
247:...I
1469:doi
1457:148
1422:doi
1418:692
1375:doi
1328:doi
1273:doi
1234:doi
1203:doi
1199:111
1161:doi
1157:559
1103:doi
1061:doi
1057:111
1027:doi
993:doi
936:doi
901:doi
775:(CH
769:or
634:).
338:GHS
304:468
184:EPA
157:CID
1506::
1467:.
1455:.
1451:.
1428:.
1412:.
1389:.
1381:.
1371:52
1369:.
1365:.
1342:.
1334:.
1324:40
1322:.
1318:.
1295:.
1287:.
1279:.
1269:44
1267:.
1263:.
1240:.
1230:22
1228:.
1224:.
1197:.
1193:.
1181:^
1167:.
1155:.
1151:.
1109:.
1097:.
1093:.
1081:^
1067:.
1055:.
1051:.
1039:^
1023:24
1021:.
1017:.
1005:^
991:.
981:24
979:.
975:.
963:^
942:,
930:,
907:.
897:95
895:.
891:.
877:^
809:I]
741:,
737:,
699:.
685:UV
663:.
518:,
514:,
510:,
506:,
502:,
498:,
494:,
490:,
486:,
482:,
478:,
474:,
470:,
466:,
462:,
458:,
454:,
450:,
446:,
442:,
438:,
434:,
430:,
412:,
408:,
404:,
400:,
396:,
392:,
342::
287:Pt
278:36
272:12
1475:.
1471::
1463::
1436:.
1424::
1397:.
1377::
1350:.
1330::
1303:.
1275::
1248:.
1236::
1209:.
1205::
1175:.
1163::
1117:.
1105::
1099:7
1075:.
1063::
1033:.
1029::
999:.
995::
987::
938::
915:.
903::
843:3
841:)
839:3
835:4
833:H
831:5
829:C
827:3
823:4
821:H
819:5
817:C
815:3
811:4
807:3
805:)
803:3
793:3
791:)
789:3
785:4
783:H
781:5
779:C
777:3
727:3
674:.
672:4
657:4
653:3
632:4
628:3
626:)
624:3
620:3
616:3
614:)
612:3
555:4
302:1
290:4
284:4
281:I
275:H
269:C
186:)
182:(
76:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.