220:
153:
116:
670:
31:
201:
because larger molecules can participate in more intermolecular bonding, although other factors such as structure and polarity play a significant role. The effect of molecular mass can be partially isolated by comparing chemicals of similar structure (i.e. esters, alkanes, etc.). For instance, linear
292:
come in contact with receptors in the nose. Ingredients that vaporize quickly after being applied will produce fragrant vapors for a short time before the oils evaporate. Slow-evaporating ingredients can stay on the skin for weeks or even months, but may not produce enough vapors to produce a strong
255:
to vaporize. These vapors move up the tower and eventually come in contact with cold surfaces, which causes them to condense and be collected. The most volatile chemical condense at the top of the column while the least volatile chemicals to vaporize condense in the lowest portion. On the right is a
138:
is the temperature at which the vapor pressure of a liquid is equal to the surrounding pressure, causing the liquid to rapidly evaporate, or boil. It is closely related to vapor pressure, but is dependent on pressure. The normal boiling point is the boiling point at atmospheric pressure, but it can
106:
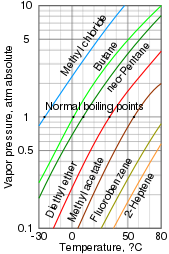
Volatility itself has no defined numerical value, but it is often described using vapor pressures or boiling points (for liquids). High vapor pressures indicate a high volatility, while high boiling points indicate low volatility. Vapor pressures and boiling points are often presented in tables and
227:
Knowledge of volatility is often useful in the separation of components from a mixture. When a mixture of condensed substances contains multiple substances with different levels of volatility, its temperature and pressure can be manipulated such that the more volatile components change to a vapor
127:
condensation, the vapor pressure can be measured. Increasing the temperature increases the amount of vapor that is formed and thus the vapor pressure. In a mixture, each substance contributes to the overall vapor pressure of the mixture, with more volatile compounds making a larger contribution.
126:
is a measurement of how readily a condensed phase forms a vapor at a given temperature. A substance enclosed in a sealed vessel initially at vacuum (no air inside) will quickly fill any empty space with vapor. After the system reaches equilibrium and the rate of evaporation matches the rate of
271:
in the product, alcohol makers would heat the initial alcohol mixture to a temperature where most of the ethanol vaporizes while most of the water remains liquid. The ethanol vapor is then collected and condensed in a separate container, resulting in a much more concentrated product.
293:
aroma. To prevent these problems, perfume designers carefully consider the volatility of essential oils and other ingredients in their perfumes. Appropriate evaporation rates are achieved by modifying the amount of highly volatile and non-volatile ingredients used.
77:
into a liquid or solid; less volatile substances will more readily condense from a vapor than highly volatile ones. Differences in volatility can be observed by comparing how fast substances within a group evaporate (or
164:
An important factor influencing a substance's volatility is the strength of the interactions between its molecules. Attractive forces between molecules are what holds materials together, and materials with stronger
228:
while the less volatile substances remain in the liquid or solid phase. The newly formed vapor can then be discarded or condensed into a separate container. When the vapors are collected, this process is known as
247:
entering a refinery is composed of many useful chemicals that need to be separated. The crude oil flows into a distillation tower and is heated up, which allows the more volatile components such as
90:
will remain condensed. In general, solids are much less volatile than liquids, but there are some exceptions. Solids that sublimate (change directly from solid to vapor) such as dry ice (solid
189:
while dimethyl ether molecules are not. The result in an overall stronger attractive force between the ethanol molecules, making it the less volatile substance of the two.
107:
charts that can be used to compare chemicals of interest. Volatility data is typically found through experimentation over a range of temperatures and pressures.
185:
O), have different volatilities due to the different interactions that occur between their molecules in the liquid phase: ethanol molecules are capable of
607:
556:
504:
445:
420:
369:
461:
1127:
1122:
302:
874:
944:
869:
600:
79:
1056:
884:
939:
684:
332:
1117:
1066:
236:
1112:
1132:
593:
337:
1091:
990:
620:
985:
312:
240:
82:
in the case of solids) when exposed to the atmosphere. A highly volatile substance such as rubbing alcohol (
745:
1000:
750:
263:
The difference in volatility between water and ethanol has traditionally been used in the refinement of
929:
689:
166:
904:
796:
786:
699:
654:
327:
1051:
980:
814:
257:
548:
465:
412:
1081:
1076:
1046:
1005:
894:
846:
831:
724:
694:
552:
500:
496:
441:
416:
365:
83:
1036:
659:
540:
488:
404:
317:
264:
186:
521:
219:
152:
1026:
879:
616:
522:"Alcohol Distillation: Basic Principles, Equipment, Performance Relationships, and Safety"
322:
243:, which allows several chemicals of varying volatility to be separated in a single step.
1137:
824:
819:
776:
709:
704:
289:
198:
174:
123:
91:
1106:
1061:
1041:
964:
924:
859:
791:
714:
541:
489:
405:
135:
115:
17:
1086:
959:
954:
949:
914:
864:
781:
307:
229:
50:
37:
liquid readily transitions to vapor at room temperature, indicating high volatility
995:
889:
801:
669:
54:
30:
575:
206:
exhibit decreasing volatility as the number of carbons in the chain increases.
934:
909:
836:
806:
740:
719:
87:
580:
244:
42:
585:
98:
can vaporize at a similar rate as some liquids under standard conditions.
1071:
899:
252:
74:
58:
86:) will quickly evaporate, while a substance with low volatility such as
1031:
919:
854:
771:
766:
281:
268:
170:
34:
640:
248:
203:
157:
95:
66:
62:
649:
635:
218:
151:
70:
29:
285:
61:, a substance with high volatility is more likely to exist as a
589:
65:, while a substance with low volatility is more likely to be a
645:
156:
Normal boiling point (red) and melting point (blue) of linear
49:
is a material quality which describes how readily a substance
73:. Volatility can also describe the tendency of a vapor to
197:
In general, volatility tends to decrease with increasing
440:. New York: W.H. Freeman and Company. pp. 368–369.
169:, such as most solids, are typically not very volatile.
280:
Volatility is an important consideration when crafting
1019:
973:
845:
759:
733:
677:
628:
119:A log-lin vapor pressure chart for various liquids
139:also be reported at higher and lower pressures.
547:. UK: The Royal Society of Chemistry. pp.
601:
8:
267:. In order to increase the concentration of
364:. John Wiley & Sons. pp. 279–281.
362:Elementary Principles of Chemical Processes
608:
594:
586:
389:. John Wiley & Sons. pp. 639–641.
177:, two chemicals with the same formula (C
114:
387:Engineering and Chemical Thermodynamics
349:
581:Definition of volatile from Wiktionary
256:picture illustrating the design of a
7:
491:Purification of Laboratory Chemicals
398:
396:
355:
353:
27:Tendency of a substance to vaporize
25:
668:
487:Armarego, Wilfred L. F. (2009).
223:A crude oil distillation column.
239:utilizes a technique known as
1:
1057:Macroscopic quantum phenomena
411:. Houghton Mifflin. pp.
1067:Order and disorder (physics)
462:"Hydrocarbon boiling points"
543:The Chemistry of Fragrances
403:Zumdahl, Steven S. (2007).
303:Clausius–Clapeyron relation
160:vs. number of carbon atoms.
1154:
1128:Engineering thermodynamics
385:Koretsky, Milo D. (2013).
666:
338:Volatile organic compound
1123:Thermodynamic properties
1092:Thermo-dielectric effect
991:Enthalpy of vaporization
685:Bose–Einstein condensate
576:Volatility from ilpi.com
360:Felder, Richard (2015).
333:Vapor–liquid equilibrium
986:Enthalpy of sublimation
313:Fractional distillation
241:fractional distillation
1001:Latent internal energy
751:Color-glass condensate
539:Sell, Charles (2006).
436:Atkins, Peter (2013).
224:
161:
120:
38:
811:Magnetically ordered
495:. Elsevier. pp.
222:
167:intermolecular forces
155:
148:Intermolecular forces
118:
33:
690:Fermionic condensate
237:petroleum refinement
143:Contributing factors
18:Volatility (physics)
1118:Chemical properties
905:Chemical ionization
797:Programmable matter
787:Quantum spin liquid
655:Supercritical fluid
438:Chemical Principles
328:Relative volatility
1113:Physical chemistry
1052:Leidenfrost effect
981:Enthalpy of fusion
746:Quark–gluon plasma
468:on 7 February 2023
258:distillation tower
225:
162:
121:
39:
1133:Phase transitions
1100:
1099:
1082:Superheated vapor
1077:Superconductivity
1047:Equation of state
895:Flash evaporation
847:Phase transitions
832:String-net liquid
725:Photonic molecule
695:Degenerate matter
558:978-0-85404-824-3
506:978-1-85617-567-8
447:978-1-319-07903-1
422:978-0-618-52844-8
371:978-1-119-17764-7
84:isopropyl alcohol
16:(Redirected from
1145:
1037:Compressed fluid
672:
617:States of matter
610:
603:
596:
587:
563:
562:
546:
536:
530:
529:
517:
511:
510:
494:
484:
478:
477:
475:
473:
464:. Archived from
458:
452:
451:
433:
427:
426:
410:
400:
391:
390:
382:
376:
375:
357:
318:Partial pressure
284:. Humans detect
265:drinking alcohol
193:Molecular weight
187:hydrogen bonding
21:
1153:
1152:
1148:
1147:
1146:
1144:
1143:
1142:
1103:
1102:
1101:
1096:
1027:Baryonic matter
1015:
969:
940:Saturated fluid
880:Crystallization
841:
815:Antiferromagnet
755:
729:
673:
664:
624:
614:
572:
567:
566:
559:
538:
537:
533:
520:Kvaalen, Eric.
519:
518:
514:
507:
486:
485:
481:
471:
469:
460:
459:
455:
448:
435:
434:
430:
423:
402:
401:
394:
384:
383:
379:
372:
359:
358:
351:
346:
299:
290:aromatic vapors
278:
235:The process of
217:
212:
195:
184:
180:
150:
145:
133:
113:
104:
28:
23:
22:
15:
12:
11:
5:
1151:
1149:
1141:
1140:
1135:
1130:
1125:
1120:
1115:
1105:
1104:
1098:
1097:
1095:
1094:
1089:
1084:
1079:
1074:
1069:
1064:
1059:
1054:
1049:
1044:
1039:
1034:
1029:
1023:
1021:
1017:
1016:
1014:
1013:
1008:
1006:Trouton's rule
1003:
998:
993:
988:
983:
977:
975:
971:
970:
968:
967:
962:
957:
952:
947:
942:
937:
932:
927:
922:
917:
912:
907:
902:
897:
892:
887:
882:
877:
875:Critical point
872:
867:
862:
857:
851:
849:
843:
842:
840:
839:
834:
829:
828:
827:
822:
817:
809:
804:
799:
794:
789:
784:
779:
777:Liquid crystal
774:
769:
763:
761:
757:
756:
754:
753:
748:
743:
737:
735:
731:
730:
728:
727:
722:
717:
712:
710:Strange matter
707:
705:Rydberg matter
702:
697:
692:
687:
681:
679:
675:
674:
667:
665:
663:
662:
657:
652:
643:
638:
632:
630:
626:
625:
615:
613:
612:
605:
598:
590:
584:
583:
578:
571:
570:External links
568:
565:
564:
557:
531:
512:
505:
479:
453:
446:
428:
421:
392:
377:
370:
348:
347:
345:
342:
341:
340:
335:
330:
325:
320:
315:
310:
305:
298:
295:
277:
274:
216:
213:
211:
208:
199:molecular mass
194:
191:
182:
178:
175:dimethyl ether
149:
146:
144:
141:
132:
129:
124:Vapor pressure
112:
111:Vapor pressure
109:
103:
100:
92:carbon dioxide
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1150:
1139:
1136:
1134:
1131:
1129:
1126:
1124:
1121:
1119:
1116:
1114:
1111:
1110:
1108:
1093:
1090:
1088:
1085:
1083:
1080:
1078:
1075:
1073:
1070:
1068:
1065:
1063:
1062:Mpemba effect
1060:
1058:
1055:
1053:
1050:
1048:
1045:
1043:
1042:Cooling curve
1040:
1038:
1035:
1033:
1030:
1028:
1025:
1024:
1022:
1018:
1012:
1009:
1007:
1004:
1002:
999:
997:
994:
992:
989:
987:
984:
982:
979:
978:
976:
972:
966:
965:Vitrification
963:
961:
958:
956:
953:
951:
948:
946:
943:
941:
938:
936:
933:
931:
930:Recombination
928:
926:
925:Melting point
923:
921:
918:
916:
913:
911:
908:
906:
903:
901:
898:
896:
893:
891:
888:
886:
883:
881:
878:
876:
873:
871:
870:Critical line
868:
866:
863:
861:
860:Boiling point
858:
856:
853:
852:
850:
848:
844:
838:
835:
833:
830:
826:
823:
821:
818:
816:
813:
812:
810:
808:
805:
803:
800:
798:
795:
793:
792:Exotic matter
790:
788:
785:
783:
780:
778:
775:
773:
770:
768:
765:
764:
762:
758:
752:
749:
747:
744:
742:
739:
738:
736:
732:
726:
723:
721:
718:
716:
713:
711:
708:
706:
703:
701:
698:
696:
693:
691:
688:
686:
683:
682:
680:
676:
671:
661:
658:
656:
653:
651:
647:
644:
642:
639:
637:
634:
633:
631:
627:
622:
618:
611:
606:
604:
599:
597:
592:
591:
588:
582:
579:
577:
574:
573:
569:
560:
554:
550:
545:
544:
535:
532:
527:
523:
516:
513:
508:
502:
498:
493:
492:
483:
480:
467:
463:
457:
454:
449:
443:
439:
432:
429:
424:
418:
414:
409:
408:
399:
397:
393:
388:
381:
378:
373:
367:
363:
356:
354:
350:
343:
339:
336:
334:
331:
329:
326:
324:
321:
319:
316:
314:
311:
309:
306:
304:
301:
300:
296:
294:
291:
287:
283:
275:
273:
270:
266:
261:
259:
254:
250:
246:
242:
238:
233:
231:
221:
214:
209:
207:
205:
200:
192:
190:
188:
176:
172:
168:
159:
154:
147:
142:
140:
137:
136:Boiling point
131:Boiling point
130:
128:
125:
117:
110:
108:
101:
99:
97:
93:
89:
88:vegetable oil
85:
81:
76:
72:
68:
64:
60:
56:
53:. At a given
52:
48:
44:
36:
32:
19:
1087:Superheating
1010:
960:Vaporization
955:Triple point
950:Supercooling
915:Lambda point
865:Condensation
782:Time crystal
760:Other states
700:Quantum Hall
542:
534:
525:
515:
490:
482:
470:. Retrieved
466:the original
456:
437:
431:
406:
386:
380:
361:
323:Raoult's law
308:Distillation
279:
262:
234:
230:distillation
226:
215:Distillation
210:Applications
196:
163:
134:
122:
105:
46:
40:
996:Latent heat
945:Sublimation
890:Evaporation
825:Ferromagnet
820:Ferrimagnet
802:Dark matter
734:High energy
102:Description
55:temperature
1107:Categories
1011:Volatility
974:Quantities
935:Regelation
910:Ionization
885:Deposition
837:Superglass
807:Antimatter
741:QCD matter
720:Supersolid
715:Superfluid
678:Low energy
344:References
47:volatility
407:Chemistry
245:Crude oil
80:sublimate
51:vaporizes
43:chemistry
1072:Spinodal
1020:Concepts
900:Freezing
472:28 April
297:See also
282:perfumes
253:kerosene
75:condense
59:pressure
1032:Binodal
920:Melting
855:Boiling
772:Crystal
767:Colloid
276:Perfume
269:ethanol
204:alkanes
171:Ethanol
158:alkanes
35:Bromine
660:Plasma
641:Liquid
555:
551:-202.
526:Purdue
503:
444:
419:
415:-466.
368:
249:butane
96:iodine
67:liquid
63:vapour
1138:Gases
650:Vapor
636:Solid
629:State
499:-12.
288:when
286:odors
94:) or
71:solid
621:list
553:ISBN
501:ISBN
474:2021
442:ISBN
417:ISBN
366:ISBN
251:and
173:and
57:and
646:Gas
549:200
413:460
69:or
41:In
1109::
648:/
524:.
395:^
352:^
260:.
232:.
45:,
623:)
619:(
609:e
602:t
595:v
561:.
528:.
509:.
497:9
476:.
450:.
425:.
374:.
183:6
181:H
179:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.