38:
17:
31:
24:
83:
Stereochemistry demands special attention because three-dimensionality is the most difficult part of a structure to visualize. Techniques for presenting 3-dimensional structures reflect the tastes of the artist. Three dimensionality is best highlighted by the depictions of bonds, using wedges,
75:
Depictions of molecular compounds is well accomplished using ChemDraw and related software. Cations and anions are also typically discrete and can be depicted unambiguously. For simple structures, say <10 atoms, it is helpful to depict all atoms explicitly. For more complex molecules, most
84:
bolding, and hashed formats. Some artists highlight three-dimensionality by varying fonts sizes, e.g. slightly larger fonts for the "front" atoms. In organic chemistry, double bonds and C-H bonds are shorter than most single bonds.
118:, adopt low-dimensional structures, in which case the layers (2-D) or chains (1-D) should be shown. Some inorganic solids dissociate - or crack - into molecular species heating or upon dissolving, e.g.
147:
95:. It is a matter of taste whether one includes the lone pair in a drawing. Lone pairs of electrons are more common for depictions that emphasize bonding, as in simple gaseous molecules, such as
142:
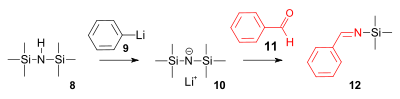
64:. The structures of many reagents are often misunderstood because simplified formulas are presented in reaction schemes whereas the actual structures are more complex. Examples are
130:
76:
hydrogen atoms attached to carbon are omitted, and carbon atoms are represented by vertices. For ease of readability,
44:
Chemical structures are presented to help readers understand the nature of the titled material. One can subdivide
69:
57:
72:. Readers of Knowledge (XXG) often comment (complain) that structures shown are incorrect for this reason.
119:
61:
37:
16:
126:
45:
49:
125:
Some important chemical species cannot be easily represented with simple pictures, e.g.
122:. In such cases it is helpful to depict both the molecular and the nonmolecular forms.
107:
65:
111:
80:
fonts are preferred. Many artists employ color to highlight parts of the molecules.
100:
92:
30:
77:
88:
115:
96:
23:
36:
29:
22:
15:
148:
Knowledge (XXG):WikiProject
Chemistry/Structure drawing workgroup
110:, are best represented with colour-coded spheres that emphasise
53:
143:
Knowledge (XXG):Manual of Style/Chemistry/Structure drawing
60:, and nonmolecular species, which includes most purely
48:
into two main groups: molecules, which includes most
8:
7:
91:of electrons, which are sometimes
14:
1:
106:Nonmolecular compounds, e.g.
131:non-stoichiometric compounds
164:
70:lithium diisopropylamide
58:organometallic compounds
93:stereochemically active
41:
34:
27:
20:
40:
33:
26:
19:
114:. Many solids, e.g.
87:Most molecules have
62:inorganic compounds
120:Aluminium chloride
46:chemical compounds
42:
35:
28:
21:
127:hydrochloric acid
155:
163:
162:
158:
157:
156:
154:
153:
152:
139:
12:
11:
5:
161:
159:
151:
150:
145:
138:
135:
108:sodium hydride
66:methyl lithium
13:
10:
9:
6:
4:
3:
2:
160:
149:
146:
144:
141:
140:
136:
134:
132:
128:
123:
121:
117:
113:
109:
104:
102:
98:
94:
90:
85:
81:
79:
73:
71:
67:
63:
59:
55:
52:, polyatomic
51:
47:
39:
32:
25:
18:
124:
105:
101:nitric oxide
86:
82:
74:
43:
89:lone pairs
78:sans-serif
137:See also
116:graphite
112:packing
97:ammonia
50:organic
56:, and
54:gases
129:and
99:and
68:and
133:.
103:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.