1250:
1972:
1234:
58:
2680:. The subshell energies and their order depend on the nuclear charge; 4s is lower than 3d as per the Madelung rule in K with 19 protons, but 3d is lower in Sc with 21 protons. In addition to there being ample experimental evidence to support this view, it makes the explanation of the order of ionization of electrons in this and other
1182:, the electronic configuration can be built up by placing electrons in the lowest available subshell until the total number of electrons added is equal to the atomic number. Thus subshells are filled in the order of increasing energy, using two general rules to help predict electronic configurations:
2651:
In recent years it has been noted that the order of filling subshells in neutral atoms does not always correspond to the order of adding or removing electrons for a given atom. For example, in the fourth row of the periodic table, the
Madelung rule indicates that the 4s subshell is occupied before
1945:
there are not expected to be many more elements that show the expected configuration from
Madelung's rule beyond 120. The general idea that after the two 8s elements, there come regions of chemical activity of 5g, followed by 6f, followed by 7d, and then 8p, does however mostly seem to hold true,
1929:
All these exceptions are not very relevant for chemistry, as the energy differences are quite small and the presence of a nearby atom can change the preferred configuration. The periodic table ignores them and follows idealised configurations. They occur as the result of interelectronic repulsion
1428:
The
Madelung energy ordering rule applies only to neutral atoms in their ground state. There are twenty elements (eleven in the d-block and nine in the f-block) for which the Madelung rule predicts an electron configuration that differs from that determined experimentally, although the
2684:
more intelligible, given that 4s electrons are invariably preferentially ionized. Generally the
Madelung rule should only be used for neutral atoms; however, even for neutral atoms there are exceptions in the d-block and f-block (as shown above).
2106:
proposed this as an empirical rule for the order of filling atomic subshells, and most
English-language sources therefore refer to the Madelung rule. Madelung may have been aware of this pattern as early as 1926. The Russian-American engineer
3065:
2098:
energy ordering rule turned out to be an approximation rather than a perfect fit, although for all elements that are exceptions the regularised configuration is a low-energy excited state, well within reach of chemical bond energies.
1085:, placed in square brackets. For phosphorus, the last previous noble gas is neon, so the configuration is abbreviated to 3s 3p, where signifies the core electrons whose configuration in phosphorus is identical to that of neon.
2089:
values of the elements, since they did not accord with his energy ordering rule, and he considered that the discrepancies involved must have arisen from measurement errors. As it happens, the actual values were correct and the
1941:, starting the g-block, should be an exception in which the expected 5g electron is transferred to 8p (similarly to lawrencium). After this, sources do not agree on the predicted configurations, but due to very strong
1390:, electrons leave approximately in the order 6p, 6s, 5d, 4f, etc. On a related note, writing configurations in this way emphasizes the outermost electrons and their involvement in chemical bonding.
2322:
2486:
3042:
Kragh, Helge, '7 A Theory of the
Chemical Elements', Niels Bohr and the Quantum Atom: The Bohr Model of Atomic Structure 1913–1925 (Oxford, 2012; online edn, Oxford Academic, 24 May 2012),
2190:
3595:
1054:, then fill subshells of higher energy. For example, the 1s subshell is filled before the 2s subshell is occupied. In this way, the electrons of an atom or ion form the most stable
839:
3702:
849:
1355:
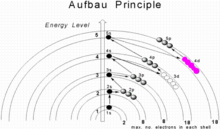
value is filled first. The subshell ordering by this rule is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, 8s, 5g, ... For example, thallium (
899:
873:
1112:
before any are occupied doubly. If double occupation does occur, the Pauli exclusion principle requires that electrons that occupy the same orbital must have different
956:
1432:
One inorganic chemistry textbook describes the
Madelung rule as essentially an approximate empirical rule although with some theoretical justification, based on the
2522:
878:
854:
3409:
2646:
2620:
834:
482:
1995:
to the properties of electrons and explained chemical properties in physical terms. Each added electron is subject to the electric field created by the positive
2594:
2570:
2550:
2409:
2389:
2365:
2345:
172:
167:
162:
3588:
908:
869:
865:
824:
2195:
This formula correctly predicts both the first and second parts of the
Madelung rule (the second part being that for two subshells with the same value of
883:
819:
545:
1950:, which acts like an extra covering shell together with 8s and is slowly drowned into the core across the 5g and 6f series) and a destabilized part (8p
711:
182:
152:
3697:
814:
2864:
Jørgensen, Christian (1973). "The Loose
Connection between Electron Configuration and the Chemical Behavior of the Heavy Elements (Transuranics)".
2573:
2077:
in 1928, and in 1930 he made explicit the quantum basis of this pattern, based on knowledge of atomic ground states determined by the analysis of
2229:
The full
Madelung rule was derived from a similar potential in 1971 by Yury N. Demkov and Valentin N. Ostrovsky. They considered the potential
3723:
3581:
859:
809:
3820:
3430:
2997:
903:
829:
706:
1178:
is equal to 0, 1, 2, and 3 for s, p, d, and f subshells, so that the maximum numbers of electrons are 2, 6, 10, and 14 respectively. In the
949:
1723:
The valence d-subshell often "borrows" one electron (in the case of thorium two electrons) from the valence f-subshell. For example, in
203:
3531:
3289:
3248:
743:
694:
3630:
3362:
2905:
2808:
2774:
2729:
2226:, based on the Thomas–Fermi model of the atom. Many French- and Russian-language sources therefore refer to the Klechkowski rule. '
3465:
Kitagawara, Y.; Barut, A.O. (1984). "On the dynamical symmetry of the periodic table. II. Modified Demkov-Ostrovsky atomic model".
131:
2036:'s model of the atom, including the effects of electron spin, provided a more complete explanation of the empirical aufbau rules.
2207:
fills first). Wiswesser argued for this formula based on the pattern of both angular and radial nodes, the concept now known as
942:
893:
1942:
140:
2235:
845:
698:
114:
3406:
3436:
Ostrovsky, V.N. (2005). "On Recent Discussion Concerning Quantum Justification of the Periodic Table of the Elements".
3021:
2417:
2018:
prior to quantum mechanics, electrons were supposed to occupy classical elliptical orbits. The orbits with the highest
2055:
2022:
are "circular orbits" outside the inner electrons, but orbits with low angular momentum (s- and p-subshell) have high
148:
3379:
3389:
3123:
Karapetoff, Vladimir (1930). "A chart of consecutive sets of electronic orbits within atoms of chemical elements".
2745:
779:
284:
3281:
Introduction to Quantum Mechanics 1: Thermal Radiation and Experimental Facts Regarding the Quantization of Matter
3279:
2124:
305:
263:
3825:
3718:
1097:
687:
277:
270:
298:
3815:
3659:
3650:
3564:
2208:
2027:
1172:
1164:
87:
2894:
Feynman, Richard; Leighton, Robert B.; Sands, Matthew (1964). "19. The Hydrogen Atom and The Periodic Table".
312:
291:
3668:
2525:
2111:
was the first to publish the rule in 1930, though Janet also published an illustration of it the same year.
922:
440:
382:
190:
97:
41:
1433:
3604:
3384:
2529:
1975:
In the old quantum theory, orbits with low angular momentum (s- and p-subshell) get closer to the nucleus.
1055:
756:
450:
220:
61:
Electrons occupy the shells and sub-shells of an atom in approximate accordance with the Aufbau principle.
1707:
Lr, where the 6d electron predicted by the Madelung rule is replaced by a 7p electron: the rule predicts
1448:
d-subshell "borrows" one electron (in the case of palladium two electrons) from the valence s-subshell.
515:
31:
1429:
Madelung-predicted electron configurations are at least close to the ground state even in those cases.
370:
3510:
3474:
3328:
3214:
3159:
3077:
2937:
2833:
2023:
1292:
738:
646:
503:
245:
1958:), and that the 8s shell gets replaced by the 9s shell as the covering s-shell for the 7d elements.
1327: = 0, 1, 2, 3 correspond to the s, p, d, and f subshells, respectively. Subshells with a lower
3677:
2108:
1214:
1101:
1017:
791:
3453:
3183:
2015:
1151:
and one electron are added each time to the neutral atom. The maximum number of electrons in any
786:
662:
639:
634:
2572:
arises at zero energy and then becomes bound, recovering the Madelung order. The application of
2081:. This table came to be referred to as the left-step table. Janet "adjusted" some of the actual
2981:
2969:
1754:, the preceding noble gas. However, the measured electron configuration of the uranium atom is
1249:
3780:
3749:
3625:
3568:
3426:
3418:
3358:
3285:
3175:
3105:
2993:
2921:
2901:
2804:
2770:
2725:
2694:
2368:
2115:
2078:
1992:
1689:, the preceding noble gas. However, the measured electron configuration of the copper atom is
1299:
974:
771:
767:
763:
751:
603:
3794:
3765:
3518:
3495:
3482:
3445:
3222:
3167:
3132:
3095:
3085:
3043:
2985:
2945:
2873:
2841:
2681:
2215:
2019:
1445:
1140:
1070:
984:
929:
731:
676:
510:
433:
426:
419:
412:
405:
398:
391:
318:
311:
304:
126:
121:
3543:
2495:
3413:
2800:
2794:
1996:
1008:
888:
718:
297:
290:
283:
276:
269:
262:
255:
236:
229:
2625:
2599:
2552:
passes through each of these values, a manifold containing all states with that value of
2073:
correspond to the principal and azimuthal quantum numbers respectively) was suggested by
1987:, "building-up principle", rather than being named for a scientist. It was formulated by
3514:
3478:
3332:
3218:
3163:
3081:
2941:
2837:
3642:
3620:
3615:
3316:
3171:
2716:
2579:
2555:
2535:
2394:
2374:
2350:
2330:
2103:
2033:
2007:
there is no energy difference between subshells with the same principal quantum number
1422:
1366:
Other authors write the subshells outside of the noble gas core in order of increasing
1278:
1222:
1152:
1113:
1109:
1105:
1093:
1089:
1082:
1047:
970:
658:
654:
598:
591:
586:
461:
360:
340:
80:
75:
49:
3486:
3136:
3100:
2845:
2026:, so that they get closer to the nucleus and feel on average a less strongly screened
1265:
In neutral atoms, the approximate order in which subshells are filled is given by the
333:
3809:
3770:
3744:
2118:
proposed that the subshells are filled in order of increasing values of the function
2074:
1285:
1144:
1074:
350:
326:
3457:
3187:
246:
3565:
Electron Configurations, the Aufbau Principle, Degenerate Orbitals, and Hund's Rule
1971:
1807:
1694:
1233:
1179:
1031:
622:
610:
498:
332:
235:
2895:
2003:
the negative charge of other electrons that are bound to the nucleus. Although in
1405:= 0) are exceptional: their energy levels are appreciably far from those of their
3202:
3029:. Nobel Symposium NS160 – Chemistry and Physics of Heavy and Superheavy Elements.
1253:
For multielectron atoms the energy spectra of shells interleave resulting in the
3057:
627:
615:
325:
2668:
atom is ionized by removing electrons (only), the configurations differ: Sc is
441:
3789:
3449:
1988:
1934:
1783:
1701:
1537:
1481:
1387:
1063:
522:
57:
17:
3355:
Shattered Symmetry: Group Theory from the Eightfold Way to the Periodic Table
3203:"The Periodic System and Atomic Structure I. An Elementary Physical Approach"
3150:
Ostrovsky, Valentin N. (2003). "Physical Explanation of the Periodic Table".
2970:"Superheavy elements: a prediction of their chemical and physical properties"
1938:
1823:
1767:
1505:
1489:
1078:
568:
535:
369:
349:
339:
319:
3179:
3109:
2877:
1245:
value. The direction of the red arrow indicates the order of state filling.
3090:
1930:
effects; when atoms are positively ionised, most of the anomalies vanish.
1746:= 6 + 2 = 8). The rule then predicts the electron configuration
1677:= 3 + 2 = 5). The rule then predicts the electron configuration
3573:
2665:
2004:
1791:
1521:
1457:
1043:
555:
527:
30:"Atomic build-up" redirects here. For the spread of nuclear weapons, see
3073:
2652:
the 3d. Therefore, the neutral atom ground state configuration for K is
2989:
2950:
2925:
1946:
except that relativity "splits" the 8p shell into a stabilized part (8p
1815:
1799:
1724:
1497:
1473:
1218:
563:
359:
3522:
3226:
256:
2750:
1831:
1775:
1655:
1513:
1465:
1148:
1051:
1186:
Electrons are assigned to subshells in order of increasing value of
2211:, and the influence of the core electrons on the valence orbitals.
3249:"Justification of the Rule for Successive Filling of (n+l) Groups"
1970:
1751:
1686:
1248:
1232:
490:
56:
3317:"n+l Filling Rule in the Periodic System and Focusing Potentials"
2824:
Ostrovsky, V. N. (1981). "Dynamic symmetry of atomic potential".
2218:
proposed a theoretical explanation for the importance of the sum
230:
1529:
1035:
3577:
2717:"Chapter 5: Ground state properties of nuclei: the shell model"
1167:. The maximum number of electrons in a subshell is equal to 2(2
1066:
atom, meaning that the 1s subshell has 2 electrons, and so on.
1039:
2056:
periodic table in which each row corresponds to one value of
1933:
The above exceptions are predicted to be the only ones until
999:
990:
2011:, this is not true for the outer electrons of other atoms.
1738:= 5 + 3 = 8) is occupied before the 6d subshell (
1669:= 4 + 0 = 4) is occupied before the 3d subshell (
1069:
The configuration is often abbreviated by writing only the
1436:
of the atom as a many-electron quantum-mechanical system.
1205:, electrons are assigned first to the subshell with lower
3044:
https://doi.org/10.1093/acprof:oso/9780199654987.003.0007
1693:. By filling the 3d subshell, copper can be in a lower
1088:
Electron behavior is elaborated by other principles of
2528:
for this potential can be described analytically with
1401:
value have similar energies, but the s-orbitals (with
2628:
2602:
2582:
2558:
2538:
2498:
2420:
2397:
2377:
2353:
2333:
2317:{\displaystyle U_{1/2}(r)=-{\frac {2v}{rR(r+R)^{2}}}}
2238:
2127:
1991:
in the early 1920s. This was an early application of
1661:
Cu, according to the Madelung rule, the 4s subshell (
1425:
is usually drawn to begin with the s-block elements.
993:
434:
1730:
U, according to the Madelung rule, the 5f subshell (
1359: = 81) has the ground-state configuration
1217:
is used to predict the configuration of protons and
996:
427:
420:
406:
3779:
3758:
3737:
3711:
3703:
Electron configurations of the elements (data page)
3690:
3641:
987:
413:
399:
2826:Journal of Physics B: Atomic and Molecular Physics
2715:
2640:
2614:
2588:
2564:
2544:
2516:
2480:
2403:
2383:
2359:
2339:
2316:
2184:
2596:have lower energy, and that the s-orbitals (with
2481:{\displaystyle v=v_{N}={\frac {1}{4}}R^{2}N(N+1)}
1077:are replaced by the symbol for the last previous
3315:Demkov, Yury N.; Ostrovsky, Valentin N. (1972).
392:
3321:Journal of Experimental and Theoretical Physics
3253:Journal of Experimental and Theoretical Physics
3058:"The Order of Electron Shells in Ionized Atoms"
2974:Recent Impact of Physics on Inorganic Chemistry
1386:. They do so to emphasize that if this atom is
1237:The states crossed by same red arrow have same
1213:A version of the aufbau principle known as the
1104:are available, electrons will occupy different
1967:The aufbau principle in the new quantum theory
3589:
3357:. Oxford University Press. pp. 360–381.
950:
8:
3496:"Transition Metals and the Aufbau Principle"
2714:Cottingham, W. N.; Greenwood, D. A. (1986).
2185:{\displaystyle W(n,l)=n+l-{\frac {l}{l+1}}.}
1981:
1361:1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p
1319:represents the principal quantum number and
3407:Image: Understanding order of shell filling
3353:Thyssen, Pieter; Ceulemans, Arnout (2017).
3056:Goudsmit, S. A.; Richards, Paul I. (1964).
2859:
2857:
2855:
2769:(2nd ed.). Prentice Hall. p. 38.
2765:Miessler, Gary L.; Tarr, Donald A. (1998).
2622:) have their energies approaching the next
2367:are constant parameters; this approaches a
3596:
3582:
3574:
3152:Annals of the New York Academy of Sciences
3023:Is the Periodic Table all right ("PT OK")?
1979:The principle takes its name from German,
1413:group and are closer to those of the next
1335:value are filled before those with higher
1058:possible. An example is the configuration
957:
943:
36:
3099:
3089:
2963:
2961:
2949:
2627:
2601:
2581:
2557:
2537:
2497:
2454:
2440:
2431:
2419:
2396:
2376:
2352:
2332:
2305:
2272:
2247:
2243:
2237:
2214:In 1961 the Russian agricultural chemist
2161:
2126:
1323:the azimuthal quantum number; the values
3698:Periodic table (electron configurations)
3038:
3036:
2889:
2887:
1954:, which has nearly the same energy as 9p
1760:
1450:
3380:"The Trouble With the Aufbau Principle"
2866:Angewandte Chemie International Edition
2788:
2786:
2706:
48:
2799:(1st ed.). McGraw-Hill. pp.
3310:
3308:
1197:For subshells with the same value of
7:
2203:, the one with the smaller value of
1750:where denotes the configuration of
1711:, but the measured configuration is
1685:where denotes the configuration of
1393:In general, subshells with the same
1363:or in condensed form, 6s 4f 5d 6p.
1102:multiple orbitals of the same energy
3201:Wiswesser, William J. (July 1945).
2524:, the zero-energy solutions to the
1937:, where the 8s shell is completed.
1343:values. In the many cases of equal
3172:10.1111/j.1749-6632.2003.tb06097.x
2722:An introduction to nuclear physics
1351:values, the subshell with a lower
25:
3631:Introduction to quantum mechanics
3425:. Berlin: Springer Science 2008,
3125:Journal of the Franklin Institute
3072:(4): 664–671 (with correction in
3378:Scerri, Eric (7 November 2013).
983:
3494:Vanquickenborne, L. G. (1994).
3423:Chemistry from First Principles
2900:. Vol. 3. Addison–Wesley.
2897:The Feynman Lectures on Physics
2926:"Misapplying the Periodic Law"
2724:. Cambridge University Press.
2576:show that states with smaller
2475:
2463:
2302:
2289:
2263:
2257:
2143:
2131:
2102:In 1936, the German physicist
1143:to another of the next higher
1100:. Hund's rule asserts that if
1:
3503:Journal of Chemical Education
3207:Journal of Chemical Education
3137:10.1016/S0016-0032(30)91131-3
2930:Journal of Chemical Education
1229:Madelung energy ordering rule
546:dividing metals and nonmetals
3821:Foundational quantum physics
3388:. Vol. 50, no. 6.
3691:Ground-state configurations
3487:10.1088/0022-3700/17/21/013
3247:Klechkovskii, V.M. (1962).
2846:10.1088/0022-3700/14/23/008
1171: + 1), where the
214:By periodic table structure
27:Principle of atomic physics
3842:
3660:Azimuthal quantum number (
3651:Principal quantum number (
3390:Royal Society of Chemistry
2796:Modern Inorganic Chemistry
2793:Jolly, William L. (1984).
2114:In 1945, American chemist
1370:, or if equal, increasing
904:expansion coefficient
780:Goldschmidt classification
29:
3719:Pauli exclusion principle
3669:Magnetic quantum number (
3611:
3450:10.1007/s10698-005-2141-y
2976:. Structure and Bonding.
2968:Fricke, Burkhard (1975).
2664:and so on. However, if a
2411:satisfies the condition
1719:Exceptions in the f-block
1440:Exceptions in the d-block
1273:rule, also known as the:
1108:singly and with the same
1098:Pauli exclusion principle
688:List of chemical elements
3438:Foundations of Chemistry
3278:Sakho, Ibrahima (2019).
3046:, accessed 23 Feb. 2024.
2832:(23): 4425–4439 (4429).
2746:"Electron configuration"
1298:Wiswesser's rule (after
1291:Klechkowsky rule (after
1173:azimuthal quantum number
1165:principal quantum number
1050:of the lowest available
1026:'), also called the
581:By other characteristics
191:Systematic element names
1700:A special exception is
1421:group. This is why the
804:Data pages for elements
483:metallic classification
249:(alkaline earth metals)
3605:Electron configuration
3532:"On the Madelung Rule"
3385:Education in Chemistry
3284:. Wiley. p. 115.
3066:Proc. Natl. Acad. Sci.
3020:Pyykkö, Pekka (2016).
2878:10.1002/anie.197300121
2642:
2616:
2590:
2566:
2546:
2530:Gegenbauer polynomials
2518:
2482:
2405:
2385:
2361:
2341:
2318:
2186:
1999:of the atomic nucleus
1982:
1976:
1262:
1246:
1073:explicitly, while the
1056:electron configuration
1012:
840:Electrical resistivity
732:Properties of elements
115:Periodic table history
62:
3759:Bonding participation
3678:Spin quantum number (
3530:Scerri, E.R. (2017).
3091:10.1073/pnas.51.4.664
2643:
2617:
2591:
2567:
2547:
2519:
2517:{\displaystyle N=n+l}
2483:
2406:
2386:
2362:
2342:
2319:
2187:
2065:(where the values of
2024:subshell eccentricity
1974:
1679:1s 2s 2p 3s 3p 3d 4s
1308:(building-up) rule or
1277:Madelung rule (after
1252:
1236:
1030:, states that in the
1024:building-up principle
592:Platinum-group metals
141:Discovery of elements
60:
32:Nuclear proliferation
2754:. 19 September 2013.
2626:
2600:
2580:
2556:
2536:
2526:Schrödinger equation
2496:
2418:
2395:
2375:
2351:
2331:
2236:
2125:
2050:energy ordering rule
1943:relativistic effects
1293:Vsevolod Klechkovsky
874:of vaporization
739:Relative atomic mass
712:by isotope stability
707:by atomic properties
70:Periodic table forms
3542:(3). Archived from
3515:1994JChEd..71..469V
3479:1984JPhB...17.4251K
3333:1972JETP...35...66D
3219:1945JChEd..22..314W
3164:2003NYASA.988..182O
3082:1964PNAS...51..664G
2942:2009JChEd..86.1186J
2838:1981JPhB...14.4425O
2767:Inorganic Chemistry
2641:{\displaystyle n+l}
2615:{\displaystyle l=0}
2574:perturbation-theory
2209:orbital penetration
2109:Vladimir Karapetoff
1215:nuclear shell model
640:Rare-earth elements
635:Main-group elements
3412:2014-11-15 at the
2990:10.1007/BFb0116498
2951:10.1021/ed086p1186
2922:Jensen, William B.
2638:
2612:
2586:
2562:
2542:
2514:
2478:
2401:
2381:
2357:
2337:
2314:
2182:
2016:old quantum theory
1977:
1920:5f 6d 7s
1917:5f 6d 7s
1914:5f 6d 7s
1905:4f 5d 6s
1902:4f 5d 6s
1434:Thomas–Fermi model
1284:Janet rule (after
1263:
1247:
63:
3803:
3802:
3781:Electron counting
3750:Unpaired electron
3626:Quantum mechanics
3569:Purdue University
3523:10.1021/ed071p469
3431:978-1-4020-8546-8
3419:Boeyens, J. C. A.
3392:. pp. 24–26.
3227:10.1021/ed022p314
2999:978-3-540-07109-9
2695:Ionization energy
2682:transition metals
2589:{\displaystyle n}
2565:{\displaystyle N}
2545:{\displaystyle v}
2448:
2404:{\displaystyle v}
2384:{\displaystyle r}
2369:Coulomb potential
2360:{\displaystyle v}
2340:{\displaystyle R}
2312:
2177:
2116:William Wiswesser
1993:quantum mechanics
1927:
1926:
1652:
1651:
1382: = 81)
1300:William Wiswesser
1139:Passing from one
1071:valence electrons
1021:
975:quantum chemistry
967:
966:
879:Ionization energy
855:Electronegativity
764:Electronegativity
744:Crystal structure
604:Refractory metals
453:(s, p, d, f, ...)
16:(Redirected from
3833:
3826:Chemical bonding
3795:18-electron rule
3766:Valence electron
3738:Electron pairing
3729:Aufbau principle
3712:Electron filling
3681:
3672:
3663:
3654:
3598:
3591:
3584:
3575:
3554:
3552:
3551:
3526:
3500:
3490:
3461:
3394:
3393:
3375:
3369:
3368:
3350:
3344:
3343:
3341:
3339:
3312:
3303:
3302:
3300:
3298:
3275:
3269:
3268:
3266:
3264:
3244:
3238:
3237:
3235:
3233:
3198:
3192:
3191:
3147:
3141:
3140:
3120:
3114:
3113:
3103:
3093:
3062:
3053:
3047:
3040:
3031:
3030:
3028:
3017:
3011:
3010:
3008:
3006:
2965:
2956:
2955:
2953:
2918:
2912:
2911:
2891:
2882:
2881:
2861:
2850:
2849:
2821:
2815:
2814:
2790:
2781:
2780:
2762:
2756:
2755:
2742:
2736:
2735:
2719:
2711:
2679:
2675:
2671:
2663:
2659:
2655:
2647:
2645:
2644:
2639:
2621:
2619:
2618:
2613:
2595:
2593:
2592:
2587:
2571:
2569:
2568:
2563:
2551:
2549:
2548:
2543:
2523:
2521:
2520:
2515:
2487:
2485:
2484:
2479:
2459:
2458:
2449:
2441:
2436:
2435:
2410:
2408:
2407:
2402:
2390:
2388:
2387:
2382:
2366:
2364:
2363:
2358:
2346:
2344:
2343:
2338:
2323:
2321:
2320:
2315:
2313:
2311:
2310:
2309:
2281:
2273:
2256:
2255:
2251:
2225:
2216:V.M. Klechkowski
2202:
2191:
2189:
2188:
2183:
2178:
2176:
2162:
2097:
2088:
2072:
2063:
2048:
2020:angular momentum
1985:
1761:
1757:
1749:
1745:
1737:
1714:
1710:
1692:
1684:
1680:
1676:
1668:
1654:For example, in
1451:
1420:
1412:
1404:
1400:
1385:
1362:
1350:
1342:
1334:
1326:
1322:
1272:
1260:
1244:
1204:
1193:
1177:
1170:
1135:
1134:
1130:
1125:
1124:
1120:
1061:
1016:
1006:
1005:
1002:
1001:
998:
995:
992:
989:
979:Aufbau principle
959:
952:
945:
930:Chemistry Portal
564:nonmetal halogen
467:Aufbau principle
443:
436:
429:
422:
415:
408:
401:
394:
375:
365:
355:
345:
335:
328:
321:
314:
307:
300:
293:
286:
279:
272:
265:
258:
251:
240:
232:
204:Sets of elements
132:1869 predictions
102:
96:
92:
37:
21:
3841:
3840:
3836:
3835:
3834:
3832:
3831:
3830:
3816:Electron states
3806:
3805:
3804:
3799:
3775:
3754:
3733:
3707:
3686:
3679:
3670:
3661:
3652:
3643:Quantum numbers
3637:
3607:
3602:
3561:
3549:
3547:
3529:
3498:
3493:
3473:(21): 4251–59.
3464:
3435:
3414:Wayback Machine
3403:
3401:Further reading
3398:
3397:
3377:
3376:
3372:
3365:
3352:
3351:
3347:
3337:
3335:
3314:
3313:
3306:
3296:
3294:
3292:
3277:
3276:
3272:
3262:
3260:
3246:
3245:
3241:
3231:
3229:
3200:
3199:
3195:
3149:
3148:
3144:
3122:
3121:
3117:
3060:
3055:
3054:
3050:
3041:
3034:
3026:
3019:
3018:
3014:
3004:
3002:
3000:
2967:
2966:
2959:
2920:
2919:
2915:
2908:
2893:
2892:
2885:
2863:
2862:
2853:
2823:
2822:
2818:
2811:
2792:
2791:
2784:
2777:
2764:
2763:
2759:
2744:
2743:
2739:
2732:
2713:
2712:
2708:
2703:
2691:
2677:
2673:
2669:
2661:
2657:
2653:
2624:
2623:
2598:
2597:
2578:
2577:
2554:
2553:
2534:
2533:
2494:
2493:
2450:
2427:
2416:
2415:
2393:
2392:
2373:
2372:
2349:
2348:
2329:
2328:
2301:
2282:
2274:
2239:
2234:
2233:
2223:
2200:
2166:
2123:
2122:
2095:
2086:
2070:
2061:
2052:
2046:
1969:
1964:
1957:
1953:
1949:
1841:Core electrons
1834:
1826:
1818:
1810:
1802:
1794:
1786:
1778:
1770:
1755:
1747:
1743:
1735:
1729:
1721:
1712:
1708:
1706:
1690:
1682:
1678:
1674:
1666:
1660:
1547:Core electrons
1540:
1532:
1524:
1516:
1508:
1500:
1492:
1484:
1476:
1468:
1460:
1442:
1418:
1410:
1402:
1398:
1383:
1360:
1348:
1340:
1332:
1324:
1320:
1270:
1258:
1242:
1231:
1202:
1191:
1175:
1168:
1132:
1128:
1127:
1122:
1118:
1117:
1059:
986:
982:
963:
914:
913:
889:Oxidation state
805:
797:
796:
734:
724:
723:
690:
680:
669:
668:
599:Precious metals
582:
574:
573:
516:post-transition
485:
474:
473:
462:Atomic orbitals
215:
207:
196:
195:
117:
107:
106:
100:
94:
90:
71:
35:
28:
23:
22:
15:
12:
11:
5:
3839:
3837:
3829:
3828:
3823:
3818:
3808:
3807:
3801:
3800:
3798:
3797:
3792:
3786:
3784:
3777:
3776:
3774:
3773:
3768:
3762:
3760:
3756:
3755:
3753:
3752:
3747:
3741:
3739:
3735:
3734:
3732:
3731:
3726:
3721:
3715:
3713:
3709:
3708:
3706:
3705:
3700:
3694:
3692:
3688:
3687:
3685:
3684:
3675:
3666:
3657:
3647:
3645:
3639:
3638:
3636:
3635:
3634:
3633:
3623:
3621:Atomic orbital
3618:
3616:Electron shell
3612:
3609:
3608:
3603:
3601:
3600:
3593:
3586:
3578:
3572:
3571:
3560:
3559:External links
3557:
3556:
3555:
3527:
3509:(6): 469–471.
3491:
3462:
3433:
3416:
3402:
3399:
3396:
3395:
3370:
3363:
3345:
3304:
3291:978-1786304872
3290:
3270:
3239:
3213:(7): 314–322.
3193:
3158:(1): 182–192.
3142:
3131:(5): 609–624.
3115:
3074:issue 5, p 906
3048:
3032:
3012:
2998:
2957:
2913:
2906:
2883:
2851:
2816:
2809:
2782:
2775:
2757:
2737:
2730:
2705:
2704:
2702:
2699:
2698:
2697:
2690:
2687:
2637:
2634:
2631:
2611:
2608:
2605:
2585:
2561:
2541:
2513:
2510:
2507:
2504:
2501:
2490:
2489:
2477:
2474:
2471:
2468:
2465:
2462:
2457:
2453:
2447:
2444:
2439:
2434:
2430:
2426:
2423:
2400:
2380:
2356:
2336:
2325:
2324:
2308:
2304:
2300:
2297:
2294:
2291:
2288:
2285:
2280:
2277:
2271:
2268:
2265:
2262:
2259:
2254:
2250:
2246:
2242:
2193:
2192:
2181:
2175:
2172:
2169:
2165:
2160:
2157:
2154:
2151:
2148:
2145:
2142:
2139:
2136:
2133:
2130:
2104:Erwin Madelung
2079:atomic spectra
2051:
2038:
2034:Wolfgang Pauli
2028:nuclear charge
1968:
1965:
1963:
1960:
1955:
1951:
1947:
1925:
1924:
1921:
1918:
1915:
1912:
1909:
1906:
1903:
1900:
1897:
1893:
1892:
1889:
1886:
1883:
1880:
1877:
1874:
1871:
1868:
1865:
1864:Madelung rule
1861:
1860:
1858:
1856:
1854:
1852:
1850:
1848:
1846:
1844:
1842:
1838:
1837:
1832:
1829:
1824:
1821:
1816:
1813:
1808:
1805:
1800:
1797:
1792:
1789:
1784:
1781:
1776:
1773:
1768:
1765:
1727:
1720:
1717:
1704:
1681:, abbreviated
1658:
1650:
1649:
1646:
1643:
1640:
1637:
1634:
1631:
1628:
1625:
1622:
1619:
1616:
1612:
1611:
1608:
1605:
1602:
1599:
1596:
1593:
1590:
1587:
1584:
1581:
1578:
1577:Madelung rule
1574:
1573:
1570:
1567:
1564:
1562:
1560:
1558:
1556:
1554:
1552:
1550:
1548:
1544:
1543:
1538:
1535:
1530:
1527:
1522:
1519:
1514:
1511:
1506:
1503:
1498:
1495:
1490:
1487:
1482:
1479:
1474:
1471:
1466:
1463:
1458:
1455:
1441:
1438:
1423:periodic table
1378:, such as Tl (
1313:
1312:
1309:
1303:
1296:
1289:
1282:
1279:Erwin Madelung
1230:
1227:
1223:atomic nucleus
1211:
1210:
1195:
1090:atomic physics
1083:periodic table
1075:core electrons
1060:1s 2s 2p 3s 3p
971:atomic physics
965:
964:
962:
961:
954:
947:
939:
936:
935:
934:
933:
926:
916:
915:
912:
911:
909:Vapor pressure
906:
896:
894:Speed of sound
891:
886:
881:
876:
870:of fusion
862:
857:
852:
844:Electron
842:
837:
832:
827:
825:Critical point
822:
817:
812:
806:
803:
802:
799:
798:
795:
794:
789:
783:
782:
776:
775:
760:
759:
754:
747:
746:
741:
735:
730:
729:
726:
725:
722:
721:
715:
714:
709:
703:
702:
691:
686:
685:
682:
681:
674:
671:
670:
667:
666:
651:
650:
643:
642:
637:
631:
630:
625:
619:
618:
613:
607:
606:
601:
595:
594:
589:
587:Coinage metals
583:
580:
579:
576:
575:
572:
571:
566:
560:
559:
551:
550:
549:
548:
540:
539:
531:
530:
525:
519:
518:
513:
507:
506:
504:alkaline earth
501:
495:
494:
486:
480:
479:
476:
475:
472:
471:
470:
469:
464:
456:
455:
446:
445:
438:
431:
424:
417:
410:
403:
396:
388:
387:
378:
377:
367:
357:
347:
337:
330:
323:
316:
309:
302:
295:
288:
281:
274:
267:
260:
253:
243:
226:
225:
216:
213:
212:
209:
208:
201:
198:
197:
194:
193:
187:
186:
178:
177:
176:
175:
170:
165:
157:
156:
144:
143:
137:
136:
135:
134:
129:
118:
113:
112:
109:
108:
105:
104:
84:
83:
78:
72:
69:
68:
65:
64:
53:
52:
50:Periodic table
46:
45:
26:
24:
18:Wiswesser rule
14:
13:
10:
9:
6:
4:
3:
2:
3838:
3827:
3824:
3822:
3819:
3817:
3814:
3813:
3811:
3796:
3793:
3791:
3788:
3787:
3785:
3782:
3778:
3772:
3771:Core electron
3769:
3767:
3764:
3763:
3761:
3757:
3751:
3748:
3746:
3745:Electron pair
3743:
3742:
3740:
3736:
3730:
3727:
3725:
3722:
3720:
3717:
3716:
3714:
3710:
3704:
3701:
3699:
3696:
3695:
3693:
3689:
3683:
3676:
3674:
3667:
3665:
3658:
3656:
3649:
3648:
3646:
3644:
3640:
3632:
3629:
3628:
3627:
3624:
3622:
3619:
3617:
3614:
3613:
3610:
3606:
3599:
3594:
3592:
3587:
3585:
3580:
3579:
3576:
3570:
3566:
3563:
3562:
3558:
3546:on 2017-04-12
3545:
3541:
3537:
3533:
3528:
3524:
3520:
3516:
3512:
3508:
3504:
3497:
3492:
3488:
3484:
3480:
3476:
3472:
3468:
3463:
3459:
3455:
3451:
3447:
3444:(3): 235–39.
3443:
3439:
3434:
3432:
3428:
3424:
3420:
3417:
3415:
3411:
3408:
3405:
3404:
3400:
3391:
3387:
3386:
3381:
3374:
3371:
3366:
3364:9780190611392
3360:
3356:
3349:
3346:
3334:
3330:
3326:
3322:
3318:
3311:
3309:
3305:
3293:
3287:
3283:
3282:
3274:
3271:
3258:
3254:
3250:
3243:
3240:
3228:
3224:
3220:
3216:
3212:
3208:
3204:
3197:
3194:
3189:
3185:
3181:
3177:
3173:
3169:
3165:
3161:
3157:
3153:
3146:
3143:
3138:
3134:
3130:
3126:
3119:
3116:
3111:
3107:
3102:
3097:
3092:
3087:
3083:
3079:
3075:
3071:
3068:
3067:
3059:
3052:
3049:
3045:
3039:
3037:
3033:
3025:
3024:
3016:
3013:
3001:
2995:
2991:
2987:
2983:
2979:
2975:
2971:
2964:
2962:
2958:
2952:
2947:
2943:
2939:
2935:
2931:
2927:
2923:
2917:
2914:
2909:
2907:0-201-02115-3
2903:
2899:
2898:
2890:
2888:
2884:
2879:
2875:
2871:
2867:
2860:
2858:
2856:
2852:
2847:
2843:
2839:
2835:
2831:
2827:
2820:
2817:
2812:
2810:0-07-032760-2
2806:
2802:
2798:
2797:
2789:
2787:
2783:
2778:
2776:0-13-841891-8
2772:
2768:
2761:
2758:
2753:
2752:
2747:
2741:
2738:
2733:
2731:0-521-31960-9
2727:
2723:
2718:
2710:
2707:
2700:
2696:
2693:
2692:
2688:
2686:
2683:
2667:
2649:
2635:
2632:
2629:
2609:
2606:
2603:
2583:
2575:
2559:
2539:
2531:
2527:
2511:
2508:
2505:
2502:
2499:
2472:
2469:
2466:
2460:
2455:
2451:
2445:
2442:
2437:
2432:
2428:
2424:
2421:
2414:
2413:
2412:
2398:
2378:
2370:
2354:
2334:
2306:
2298:
2295:
2292:
2286:
2283:
2278:
2275:
2269:
2266:
2260:
2252:
2248:
2244:
2240:
2232:
2231:
2230:
2227:
2222: +
2221:
2217:
2212:
2210:
2206:
2199: +
2198:
2179:
2173:
2170:
2167:
2163:
2158:
2155:
2152:
2149:
2146:
2140:
2137:
2134:
2128:
2121:
2120:
2119:
2117:
2112:
2110:
2105:
2100:
2094: +
2093:
2085: +
2084:
2080:
2076:
2075:Charles Janet
2068:
2064:
2060: +
2059:
2049:
2044: +
2043:
2039:
2037:
2035:
2031:
2029:
2025:
2021:
2017:
2012:
2010:
2006:
2002:
1998:
1994:
1990:
1986:
1984:
1983:Aufbauprinzip
1973:
1966:
1961:
1959:
1944:
1940:
1936:
1931:
1923:5f6d 7s
1922:
1919:
1916:
1913:
1910:
1907:
1904:
1901:
1898:
1896:Experimental
1895:
1894:
1890:
1887:
1884:
1881:
1878:
1875:
1872:
1869:
1866:
1863:
1862:
1859:
1857:
1855:
1853:
1851:
1849:
1847:
1845:
1843:
1840:
1839:
1836:
1830:
1828:
1822:
1820:
1814:
1812:
1806:
1804:
1798:
1796:
1790:
1788:
1782:
1780:
1774:
1772:
1766:
1763:
1762:
1759:
1753:
1742: +
1741:
1734: +
1733:
1726:
1718:
1716:
1703:
1698:
1696:
1688:
1673: +
1672:
1665: +
1664:
1657:
1647:
1644:
1641:
1638:
1635:
1632:
1629:
1626:
1623:
1620:
1617:
1615:Experimental
1614:
1613:
1609:
1606:
1603:
1600:
1597:
1594:
1591:
1588:
1585:
1582:
1579:
1576:
1575:
1571:
1568:
1565:
1563:
1561:
1559:
1557:
1555:
1553:
1551:
1549:
1546:
1545:
1542:
1536:
1534:
1528:
1526:
1520:
1518:
1512:
1510:
1504:
1502:
1496:
1494:
1488:
1486:
1480:
1478:
1472:
1470:
1464:
1462:
1456:
1453:
1452:
1449:
1447:
1439:
1437:
1435:
1430:
1426:
1424:
1417: +
1416:
1409: +
1408:
1397: +
1396:
1391:
1389:
1381:
1377:
1373:
1369:
1364:
1358:
1354:
1347: +
1346:
1339: +
1338:
1331: +
1330:
1318:
1311:diagonal rule
1310:
1307:
1304:
1301:
1297:
1294:
1290:
1287:
1286:Charles Janet
1283:
1280:
1276:
1275:
1274:
1269: +
1268:
1257: +
1256:
1251:
1241: +
1240:
1235:
1228:
1226:
1224:
1220:
1216:
1208:
1201: +
1200:
1196:
1190: +
1189:
1185:
1184:
1183:
1181:
1174:
1166:
1162:
1158:
1154:
1150:
1146:
1145:atomic number
1142:
1137:
1115:
1111:
1107:
1103:
1099:
1095:
1091:
1086:
1084:
1080:
1076:
1072:
1067:
1065:
1057:
1053:
1049:
1045:
1041:
1037:
1033:
1029:
1025:
1019:
1014:
1013:Aufbauprinzip
1010:
1004:
980:
976:
972:
960:
955:
953:
948:
946:
941:
940:
938:
937:
932:
931:
927:
925:
924:
920:
919:
918:
917:
910:
907:
905:
902: /
901:
898:Thermal
897:
895:
892:
890:
887:
885:
884:Melting point
882:
880:
877:
875:
872: /
871:
868: /
867:
863:
861:
858:
856:
853:
851:
850:configuration
848: /
847:
843:
841:
838:
836:
833:
831:
828:
826:
823:
821:
820:Boiling point
818:
816:
815:Atomic radius
813:
811:
808:
807:
801:
800:
793:
790:
788:
785:
784:
781:
778:
777:
773:
769:
765:
762:
761:
758:
757:configuration
755:
753:
749:
748:
745:
742:
740:
737:
736:
733:
728:
727:
720:
717:
716:
713:
710:
708:
705:
704:
700:
699:in human body
696:
693:
692:
689:
684:
683:
679:
678:
673:
672:
664:
660:
656:
653:
652:
648:
645:
644:
641:
638:
636:
633:
632:
629:
626:
624:
623:Native metals
621:
620:
617:
614:
612:
609:
608:
605:
602:
600:
597:
596:
593:
590:
588:
585:
584:
578:
577:
570:
567:
565:
562:
561:
558:
557:
553:
552:
547:
544:
543:
542:
541:
538:
537:
533:
532:
529:
526:
524:
521:
520:
517:
514:
512:
509:
508:
505:
502:
500:
497:
496:
493:
492:
488:
487:
484:
478:
477:
468:
465:
463:
460:
459:
458:
457:
454:
452:
448:
447:
444:
439:
437:
432:
430:
425:
423:
418:
416:
411:
409:
404:
402:
397:
395:
390:
389:
386:
384:
380:
379:
376:
374:
373:(noble gases)
368:
366:
364:
358:
356:
354:
348:
346:
344:
338:
336:
331:
329:
324:
322:
317:
315:
310:
308:
303:
301:
296:
294:
289:
287:
282:
280:
275:
273:
268:
266:
261:
259:
254:
252:
250:
244:
241:
239:
238:alkali metals
233:
228:
227:
224:
222:
218:
217:
211:
210:
206:
205:
200:
199:
192:
189:
188:
184:
180:
179:
174:
173:controversies
171:
169:
166:
164:
161:
160:
159:
158:
155:
154:
150:
146:
145:
142:
139:
138:
133:
130:
128:
125:
124:
123:
120:
119:
116:
111:
110:
99:
89:
86:
85:
82:
79:
77:
74:
73:
67:
66:
59:
55:
54:
51:
47:
43:
39:
38:
33:
19:
3728:
3548:. Retrieved
3544:the original
3539:
3535:
3506:
3502:
3470:
3466:
3441:
3437:
3422:
3383:
3373:
3354:
3348:
3336:. Retrieved
3327:(1): 66–69.
3324:
3320:
3295:. Retrieved
3280:
3273:
3261:. Retrieved
3256:
3252:
3242:
3230:. Retrieved
3210:
3206:
3196:
3155:
3151:
3145:
3128:
3124:
3118:
3069:
3064:
3051:
3022:
3015:
3003:. Retrieved
2977:
2973:
2936:(10): 1186.
2933:
2929:
2916:
2896:
2872:(1): 12–19.
2869:
2865:
2829:
2825:
2819:
2795:
2766:
2760:
2749:
2740:
2721:
2709:
2676:, and Sc is
2650:
2491:
2326:
2228:
2219:
2213:
2204:
2196:
2194:
2113:
2101:
2091:
2082:
2066:
2057:
2053:
2045:
2041:
2032:
2013:
2008:
2000:
1980:
1978:
1932:
1928:
1739:
1731:
1722:
1699:
1695:energy state
1670:
1662:
1653:
1443:
1431:
1427:
1414:
1406:
1394:
1392:
1379:
1375:
1371:
1367:
1365:
1356:
1352:
1344:
1336:
1328:
1316:
1314:
1305:
1266:
1264:
1254:
1238:
1212:
1206:
1198:
1187:
1180:ground state
1160:
1156:
1138:
1087:
1068:
1032:ground state
1027:
1023:
978:
968:
928:
921:
900:conductivity
695:by abundance
675:
647:Transuranium
628:Noble metals
616:Light metals
611:Heavy metals
554:
534:
489:
466:
449:
381:
372:
362:
353:(chalcogens)
352:
343:(pnictogens)
342:
248:
237:
219:
202:
183:in East Asia
147:
122:D. Mendeleev
3724:Hund's rule
3338:25 November
3232:5 September
1939:Element 121
1935:element 120
1891:5f 7s
1648:7s 7p
1610:6d 7s
1384:4f 5d 6s 6p
1094:Hund's rule
1046:first fill
1028:Aufbau rule
88:Alternative
3810:Categories
3790:Octet rule
3550:2018-04-15
3467:J. Phys. B
2701:References
2371:for small
1989:Niels Bohr
1911:6d 7s
1908:6d 7s
1899:5d 6s
1888:5f 7s
1885:5f 7s
1882:5f 7s
1879:5f 7s
1876:5f 7s
1873:4f 6s
1870:4f 6s
1867:4f 6s
1702:lawrencium
1645:5d 6s
1642:5d 6s
1639:4d 5s
1633:4d 5s
1630:4d 5s
1627:4d 5s
1624:4d 5s
1621:3d 4s
1618:3d 4s
1607:5d 6s
1604:5d 6s
1601:4d 5s
1598:4d 5s
1595:4d 5s
1592:4d 5s
1589:4d 5s
1586:4d 5s
1583:3d 4s
1580:3d 4s
1092:, such as
1064:phosphorus
864:Heat
835:Elasticity
536:Metalloids
523:lanthanide
511:transition
385:(1–7, ...)
363:(halogens)
168:for places
163:for people
127:1871 table
3536:Inference
3005:4 October
2270:−
2159:−
1572: 5f
1079:noble gas
1048:subshells
1044:electrons
810:Abundance
787:Nutrition
750:Electron
719:by symbol
665:actinides
569:noble gas
556:Nonmetals
153:etymology
81:32-column
76:18-column
3458:93589189
3410:Archived
3297:11 April
3259:(2): 334
3188:21629328
3180:12796101
3110:16591167
2924:(2009).
2689:See also
2672:, Sc is
2666:scandium
2660:, Sc is
2656:, Ca is
2005:hydrogen
1756:5f 6d 7s
1713:5f 7s 7p
1709:5f 6d 7s
1569: 4f
1566: 4f
1219:neutrons
1159:, where
1106:orbitals
1096:and the
1062:for the
923:Category
866:capacity
860:Hardness
846:affinity
752:affinity
677:Elements
649:elements
528:actinide
98:extended
42:a series
40:Part of
3511:Bibcode
3475:Bibcode
3329:Bibcode
3263:23 June
3215:Bibcode
3160:Bibcode
3078:Bibcode
2938:Bibcode
2834:Bibcode
2648:group.
2391:. When
2014:In the
1962:History
1725:uranium
1446:valence
1388:ionized
1163:is the
1141:element
1131:⁄
1121:⁄
1081:in the
1020:
1007:, from
830:Density
792:Valence
772:Pauling
383:Periods
3456:
3429:
3361:
3288:
3186:
3178:
3108:
3101:300183
3098:
2996:
2982:89–144
2904:
2807:
2773:
2751:WyzAnt
2728:
2492:where
2327:where
1997:charge
1656:copper
1306:aufbau
1221:in an
1149:proton
1147:, one
1052:energy
1034:of an
1009:German
977:, the
663:trans-
499:alkali
491:Metals
451:Blocks
223:(1–18)
221:Groups
149:Naming
101:
95:
91:
44:on the
3783:rules
3567:from
3499:(PDF)
3454:S2CID
3184:S2CID
3061:(PDF)
3027:(PDF)
2801:10–12
2674:4s 3d
2670:4s 3d
2662:4s 3d
2532:. As
1752:radon
1748:5f 7s
1691:3d 4s
1687:argon
1683:3d 4s
1315:Here
1153:shell
1126:and −
1114:spins
1022:'
768:Allen
659:minor
655:Major
103:forms
3427:ISBN
3359:ISBN
3340:2022
3299:2021
3286:ISBN
3265:2022
3234:2020
3176:PMID
3106:PMID
3007:2013
2994:ISBN
2902:ISBN
2805:ISBN
2771:ISBN
2726:ISBN
2347:and
2069:and
2040:The
1764:Atom
1454:Atom
1444:The
1261:rule
1155:is 2
1110:spin
1036:atom
1018:lit.
973:and
661:and
151:and
3519:doi
3483:doi
3446:doi
3223:doi
3168:doi
3156:988
3133:doi
3129:210
3096:PMC
3086:doi
3076:).
2986:doi
2946:doi
2874:doi
2842:doi
2001:and
1956:1/2
1952:3/2
1948:1/2
1705:103
1539:103
1136:).
1040:ion
1038:or
969:In
481:By
371:18
361:17
351:16
341:15
93:and
3812::
3538:.
3534:.
3517:.
3507:71
3505:.
3501:.
3481:.
3471:17
3469:.
3452:.
3440:.
3421::
3382:.
3325:35
3323:.
3319:.
3307:^
3257:14
3255:.
3251:.
3221:.
3211:22
3209:.
3205:.
3182:.
3174:.
3166:.
3154:.
3127:.
3104:.
3094:.
3084:.
3070:51
3063:.
3035:^
2992:.
2984:.
2980::
2978:21
2972:.
2960:^
2944:.
2934:86
2932:.
2928:.
2886:^
2870:12
2868:.
2854:^
2840:.
2830:14
2828:.
2803:.
2785:^
2748:.
2720:.
2678:3d
2658:4s
2654:4s
2054:A
2030:.
1835:Cm
1833:96
1827:Np
1825:93
1817:92
1811:Pa
1809:91
1803:Th
1801:90
1795:Ac
1793:89
1787:Gd
1785:64
1779:Ce
1777:58
1771:La
1769:57
1758:.
1728:92
1715:.
1697:.
1659:29
1636:4d
1541:Lr
1533:Au
1531:79
1525:Pt
1523:78
1517:Ag
1515:47
1509:Pd
1507:46
1501:Rh
1499:45
1493:Ru
1491:44
1485:Mo
1483:42
1477:Nb
1475:41
1469:Cu
1467:29
1461:Cr
1459:24
1374:+
1225:.
1116:(+
1042:,
1015:,
1011::
1000:aʊ
991:aʊ
770:,
657:,
442:8+
334:14
327:13
320:12
313:11
306:10
247:2
3682:)
3680:s
3673:)
3671:m
3664:)
3662:ℓ
3655:)
3653:n
3597:e
3590:t
3583:v
3553:.
3540:1
3525:.
3521::
3513::
3489:.
3485::
3477::
3460:.
3448::
3442:7
3367:.
3342:.
3331::
3301:.
3267:.
3236:.
3225::
3217::
3190:.
3170::
3162::
3139:.
3135::
3112:.
3088::
3080::
3009:.
2988::
2954:.
2948::
2940::
2910:.
2880:.
2876::
2848:.
2844::
2836::
2813:.
2779:.
2734:.
2636:l
2633:+
2630:n
2610:0
2607:=
2604:l
2584:n
2560:N
2540:v
2512:l
2509:+
2506:n
2503:=
2500:N
2488:,
2476:)
2473:1
2470:+
2467:N
2464:(
2461:N
2456:2
2452:R
2446:4
2443:1
2438:=
2433:N
2429:v
2425:=
2422:v
2399:v
2379:r
2355:v
2335:R
2307:2
2303:)
2299:R
2296:+
2293:r
2290:(
2287:R
2284:r
2279:v
2276:2
2267:=
2264:)
2261:r
2258:(
2253:2
2249:/
2245:1
2241:U
2224:l
2220:n
2205:n
2201:l
2197:n
2180:.
2174:1
2171:+
2168:l
2164:l
2156:l
2153:+
2150:n
2147:=
2144:)
2141:l
2138:,
2135:n
2132:(
2129:W
2096:l
2092:n
2087:l
2083:n
2071:l
2067:n
2062:l
2058:n
2047:l
2042:n
2009:n
1819:U
1744:l
1740:n
1736:l
1732:n
1675:l
1671:n
1667:l
1663:n
1419:l
1415:n
1411:l
1407:n
1403:l
1399:l
1395:n
1380:Z
1376:l
1372:n
1368:n
1357:Z
1353:n
1349:l
1345:n
1341:l
1337:n
1333:l
1329:n
1325:l
1321:l
1317:n
1302:)
1295:)
1288:)
1281:)
1271:l
1267:n
1259:l
1255:n
1243:l
1239:n
1209:.
1207:n
1203:l
1199:n
1194:.
1192:l
1188:n
1176:l
1169:l
1161:n
1157:n
1133:2
1129:1
1123:2
1119:1
1003:/
997:b
994:f
988:ˈ
985:/
981:(
958:e
951:t
944:v
774:)
766:(
701:)
697:(
435:7
428:6
421:5
414:4
407:3
400:2
393:1
299:9
292:8
285:7
278:6
271:5
264:4
257:3
242:)
234:(
231:1
185:)
181:(
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.