536:
alcohol are on the same side of the seven-membered ring to give 1,1-Difluorocyclopropane
Dibenzosuberol & (3). This is halogenated with 48% HBr to give the product where both groups are now positioned anti (4). Displacement of the bromide with pyrazine gives the quat (5). Sodium borohydride was able to reduce the aromaticity in the sidechain giving the corresponding piperazine, i.e. Fb= HCl=PC9799090 (6). The reaction of 5-hydroxyquinoline (7) with (R)-glycidyl nosylate (8) affords (R)-1-(5-Quinolinyloxy)-2,3-epoxypropane (8). The convergent synthesis between 6 & 9 gives Zosuquidar in good yield.
304:
281:
528:
29:
535:
When dibenzosuberone (1) is treated with difluorocarbene (generated in situ from lithium chlorodifluoroacetate), a cyclopropanation occurs to give 10,11-difluoromethanodibenzosuberone (2). Reduction of the ketone with borohydride proceeds to afford the derivative wherein the fused cyclpropyl and
709:
Barnett, Charles J.; Huff, Bret; Kobierski, Michael E.; Letourneau, Michael; Wilson, Thomas M. (2004). "Stereochemistry of C-6 Nucleophilic
Displacements on 1,1-Difluorocyclopropyldibenzosuberanyl Substrates. An Improved Synthesis of Multidrug Resistance Modulator LY335979 Trihydrochloride". The
491:
dependent fashion. Cancers overexpressing P-glycoproteins are able to pump out therapeutic molecules before they are able to reach their target, effectively making the cancer multi-drug resistant. Zosuquidar inhibits P-glycoproteins, inhibiting the efflux pump and restoring sensitivity to
684:
Pfister, J.R.; Makra, F.; Muehldorf, A.V.; Wu, H.; Nelson, J.T.; Cheung, P.; Bruno, N.A.; Casey, S.M.; Zutshi, N.; Slate, D.L. (1995). "Methanodibenzosuberylpiperazines as potent multidrug resistance reversal agents". Bioorganic & Medicinal
Chemistry Letters 5 (21): 2473–2476.
591:"Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: a randomized, placebo-controlled trial of the Eastern Cooperative Oncology Group 3999"
379:
421:
InChI=1S/C32H31F2N3O2/c33-32(34)29-22-7-1-3-9-24(22)31(25-10-4-2-8-23(25)30(29)32)37-17-15-36(16-18-37)19-21(38)20-39-28-13-5-12-27-26(28)11-6-14-35-27/h1-14,21,29-31,38H,15-20H2/t21-,29-,30+,31-/m1/s1
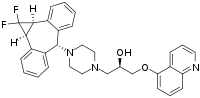
49:
437:
565:
393:
759:
643:
for "Daunorubicin & Cytarabine +/- Zosuquidar inTreating Older
Patients With Newly Diagnosed Acute Myeloid Leukemia or Refractory Anemia" at
764:
413:
553:
94:
70:
664:
200:
260:
561:
789:
769:
516:
508:
299:
774:
512:
488:
249:
784:
779:
276:
115:
644:
504:
589:
Cripe LD, Uno H, Paietta EM, Litzow MR, Ketterling RP, Bennett JM, et al. (November 2010).
620:
496:
469:
189:
610:
602:
316:
124:
209:
303:
280:
615:
590:
472:
462:
753:
743:"b,b-DIFLUOROSTYRENE". Organic Syntheses. 47: 49. 1967. doi:10.15227/orgsyn.047.0049.
292:
527:
656:
495:
Zosuqidar was initially characterized by Syntex
Corporation, which was acquired by
149:
639:
606:
721:
696:
519:
did not meet its primary endpoint and Eli Lilly discontinued its development.
480:
476:
355:
180:
500:
624:
20:
160:
169:
710:
Journal of
Organic Chemistry 69 (22): 7653–7660. doi:10.1021/jo049051v.
484:
135:
28:
401:
Cl.Cl.Cl.FC4(F)3c1ccccc1C(c2c(cccc2)34)N5CCN(CC5)C(O)COc7c6cccnc6ccc7
240:
229:
526:
378:
369:
465:
220:
106:)-1-{4-cyclopropa annulen-6-yl}-3-(quinolin-5-yloxy)propan-2-ol
507:
by the FDA in 2006 for AML. In 2010, it was announced that a
265:
444:
367:
354:
315:
310:
291:
259:
239:
219:
199:
179:
159:
134:
114:
85:
69:
64:
48:
40:
35:
694:WO9424107 idem Jurg R. Pfister, Doris L. Slate,
487:that pump foreign substances out of cells in an
148:
123:
8:
531:Original: Improved: Also: Starting material:
19:
475:. Other drugs with this mechanism include
302:
279:
188:
614:
208:
584:
582:
545:
418:
398:
275:
168:
99:
293:
18:
657:"Zosuquidar - Kanisa Pharmaceuticals"
483:. P-glycoproteins are trans-membrane
248:
7:
499:in 1990. Roche licensed the drug to
228:
139:
663:. Springer Nature Switzerland AG.
14:
685:doi:10.1016/0960-894X(95)00426-T.
725:(2003 to Eli Lilly And Company).
339:
333:
327:
27:
700:(1997 to Syntex (U.S.A.) Inc.).
667:from the original on 2023-02-10
568:from the original on 2023-02-10
426:Key:IHOVFYSQUDPMCN-DBEBIPAYSA-N
760:Drugs not assigned an ATC code
345:
321:
1:
554:"Zosuquidar trihydrochloride"
607:10.1182/blood-2010-04-277269
806:
311:Chemical and physical data
765:Experimental cancer drugs
562:National Cancer Institute
492:chemotherapeutic agents.
434:
409:
389:
90:
26:
719:Bret Eugene Huff et al.
517:myelodysplastic syndrome
509:phase III clinical trial
503:in 1997. It was granted
637:Clinical trial number
532:
513:acute myeloid leukemia
722:U.S. patent 6,521,755
697:U.S. patent 5,654,304
530:
511:for the treatment of
461:) is an experimental
734:US6624304, US6570016
558:NCI Drug Dictionary
23:
645:ClinicalTrials.gov
533:
505:orphan drug status
457:(development code
601:(20): 4077–4085.
452:
451:
380:Interactive image
261:CompTox Dashboard
16:Chemical compound
797:
744:
741:
735:
732:
726:
724:
717:
711:
707:
701:
699:
692:
686:
682:
676:
675:
673:
672:
653:
647:
635:
629:
628:
618:
586:
577:
576:
574:
573:
550:
448:
447:
440:
382:
362:
347:
341:
335:
329:
323:
306:
295:
284:
283:
269:
267:
252:
232:
212:
192:
172:
152:
142:
141:
127:
31:
24:
22:
805:
804:
800:
799:
798:
796:
795:
794:
790:Abandoned drugs
770:Organofluorides
750:
749:
748:
747:
742:
738:
733:
729:
720:
718:
714:
708:
704:
695:
693:
689:
683:
679:
670:
668:
655:
654:
650:
636:
632:
588:
587:
580:
571:
569:
552:
551:
547:
542:
525:
473:P-glycoproteins
443:
441:
438:(what is this?)
435:
430:
427:
422:
417:
416:
405:
402:
397:
396:
385:
360:
350:
344:
338:
332:
326:
287:
263:
255:
235:
215:
195:
175:
155:
138:
130:
110:
107:
98:
97:
81:
78:Investigational
60:
17:
12:
11:
5:
803:
801:
793:
792:
787:
782:
777:
772:
767:
762:
752:
751:
746:
745:
736:
727:
712:
702:
687:
677:
648:
630:
578:
544:
543:
541:
538:
524:
521:
463:antineoplastic
450:
449:
432:
431:
429:
428:
425:
423:
420:
412:
411:
410:
407:
406:
404:
403:
400:
392:
391:
390:
387:
386:
384:
383:
375:
373:
365:
364:
358:
352:
351:
348:
342:
336:
330:
324:
319:
313:
312:
308:
307:
297:
289:
288:
286:
285:
272:
270:
257:
256:
254:
253:
245:
243:
237:
236:
234:
233:
225:
223:
217:
216:
214:
213:
205:
203:
197:
196:
194:
193:
185:
183:
177:
176:
174:
173:
165:
163:
157:
156:
154:
153:
145:
143:
132:
131:
129:
128:
120:
118:
112:
111:
109:
108:
101:
93:
92:
91:
88:
87:
83:
82:
80:
79:
75:
73:
67:
66:
62:
61:
59:
58:
54:
52:
46:
45:
42:
38:
37:
33:
32:
15:
13:
10:
9:
6:
4:
3:
2:
802:
791:
788:
786:
783:
781:
778:
776:
775:Cyclopropanes
773:
771:
768:
766:
763:
761:
758:
757:
755:
740:
737:
731:
728:
723:
716:
713:
706:
703:
698:
691:
688:
681:
678:
666:
662:
658:
652:
649:
646:
642:
641:
634:
631:
626:
622:
617:
612:
608:
604:
600:
596:
592:
585:
583:
579:
567:
563:
559:
555:
549:
546:
539:
537:
529:
522:
520:
518:
514:
510:
506:
502:
498:
493:
490:
486:
482:
478:
474:
471:
468:. Zosquidir
467:
464:
460:
456:
446:
439:
433:
424:
419:
415:
408:
399:
395:
388:
381:
377:
376:
374:
371:
366:
359:
357:
353:
320:
318:
314:
309:
305:
301:
298:
296:
294:ECHA InfoCard
290:
282:
278:
277:DTXSID9057894
274:
273:
271:
262:
258:
251:
247:
246:
244:
242:
238:
231:
227:
226:
224:
222:
218:
211:
207:
206:
204:
202:
198:
191:
187:
186:
184:
182:
178:
171:
167:
166:
164:
162:
158:
151:
147:
146:
144:
137:
133:
126:
122:
121:
119:
117:
113:
105:
100:
96:
89:
84:
77:
76:
74:
72:
68:
63:
56:
55:
53:
51:
47:
43:
39:
36:Clinical data
34:
30:
25:
739:
730:
715:
705:
690:
680:
669:. Retrieved
661:Adis Insight
660:
651:
638:
633:
598:
594:
570:. Retrieved
557:
548:
534:
494:
458:
454:
453:
442:
436:
250:ChEMBL444172
103:
71:Legal status
65:Legal status
785:Piperazines
640:NCT00046930
363: g·mol
300:100.236.552
125:167354-41-8
86:Identifiers
41:Other names
780:Quinolines
754:Categories
671:2024-05-27
572:2024-05-27
540:References
515:(AML) and
481:laniquidar
477:tariquidar
455:Zosuquidar
368:3D model (
356:Molar mass
210:AB5K82X98Y
181:ChemSpider
116:CAS Number
95:IUPAC name
21:Zosuquidar
523:Synthesis
501:Eli Lilly
459:LY-335979
44:LY-335979
665:Archived
625:20716770
566:Archived
485:proteins
470:inhibits
445:(verify)
190:24599682
161:DrugBank
50:ATC code
616:2993615
361:527.616
317:Formula
170:DB06191
136:PubChem
623:
613:
394:SMILES
241:ChEMBL
230:D06387
150:153997
595:Blood
497:Roche
414:InChI
370:JSmol
621:PMID
479:and
466:drug
221:KEGG
201:UNII
57:None
611:PMC
603:doi
599:116
489:ATP
266:EPA
140:CID
756::
659:.
619:.
609:.
597:.
593:.
581:^
564:.
560:.
556:.
331:31
325:32
102:(2
674:.
627:.
605::
575:.
372:)
349:2
346:O
343:3
340:N
337:2
334:F
328:H
322:C
268:)
264:(
104:R
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.