531:. The antibody selectively binds to protein A/G, so a high level of purity (generally >80%) is obtained. The generally harsh conditions of this method may damage easily damaged antibodies. A low pH can break the bonds to remove the antibody from the column. In addition to possibly affecting the product, low pH can cause protein A/G itself to leak off the column and appear in the eluted sample. Gentle elution buffer systems that employ high salt concentrations are available to avoid exposing sensitive antibodies to low pH. Cost is also an important consideration with this method because immobilized protein A/G is a more expensive resin.
691:
8494:
8283:
831:
265:
241:
233:
225:
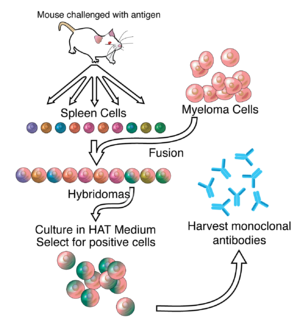
352:) cells. Unfused myeloma cells cannot grow because they lack HGPRT and thus cannot replicate their DNA. Unfused spleen cells cannot grow indefinitely because of their limited life span. Only fused hybrid cells referred to as hybridomas, are able to grow indefinitely in the medium because the spleen cell partner supplies HGPRT and the myeloma partner has traits that make it immortal (similar to a cancer cell).
401:, single B cell culture, single cell amplification from various B cell populations and single plasma cell interrogation technologies. Different from traditional hybridoma technology, the newer technologies use molecular biology techniques to amplify the heavy and light chains of the antibody genes by PCR and produce in either bacterial or mammalian systems with
22:
8482:
4123:
4080:
4037:
517:. This method is one of the more reliable chromatography techniques. Since we are dealing with proteins, properties such as charge and affinity are not consistent and vary with pH as molecules are protonated and deprotonated, while size stays relatively constant. Nonetheless, it has drawbacks such as low resolution, low capacity and low
216:
laboratory. He is a true translational investigator, since he used these monoclonal antibodies to classify human B-cell leukemia and lymphomas as well as to create therapeutic agents for patients. . . More importantly, he was the first in the world to administer a monoclonal antibody to a human (a patient with B-cell lymphoma)."
718:
Monoclonal antibodies are more expensive to manufacture than small molecules due to the complex processes involved and the general size of the molecules, all in addition to the enormous research and development costs involved in bringing a new chemical entity to patients. They are priced to enable
384:
selection to further favour hybridoma growth. This can be achieved by the use of a layer of feeder fibrocyte cells or supplement medium such as briclone. Culture-media conditioned by macrophages can be used. Production in cell culture is usually preferred as the ascites technique is painful to the
647:
sequences from which antibodies with desired specificities can be selected. The phage antibody libraries are a variant of phage antigen libraries. These techniques can be used to enhance the specificity with which antibodies recognize antigens, their stability in various environmental conditions,
509:
has a pI of 4.8, which is significantly lower than that of most monoclonal antibodies, which have a pI of 6.1. Thus, at a pH between 4.8 and 6.1, the average charge of albumin molecules is likely to be more negative, while mAbs molecules are positively charged and hence it is possible to separate
551:
during development and to support purification. The antibody-containing medium is then incubated with the immobilized antigen, either in batch or as the antibody is passed through a column, where it selectively binds and can be retained while impurities are washed away. An elution with a low pH
215:
The translational work needed to implement these ideas is credited to Lee Nadler. As explained in an NIH article, "He was the first to discover monoclonal antibodies directed against human B-cell–specific antigens and, in fact, all the known human B-cell–specific antigens were discovered in his
723:
researchers concluded, "The annual price of mAb therapies is about $ 100,000 higher in oncology and hematology than in other disease states", comparing them on a per patient basis, to those for cardiovascular or metabolic disorders, immunology, infectious diseases, allergy, and ophthalmology.
6634:
1769:
found insufficient evidence for using neutralizing monoclonal antibodies to treat COVID-19 infections. The reviews applied only to people who were unvaccinated against COVID‐19, and only to the COVID-19 variants existing during the studies, not to newer variants, such as
Omicron.
673:
Recombinant DNA has been explored since the late 1980s to increase residence times. In one approach called "CDR grafting", mouse DNA encoding the binding portion of a monoclonal antibody was merged with human antibody-producing DNA in living cells. The expression of this
6627:
6620:
571:
variants, oxidized amino acid side chains, as well as amino and carboxyl terminal amino acid additions. These seemingly minute structural changes can affect preclinical stability and process optimization as well as therapeutic product potency,
602:
studies. Knowledge gained during the preclinical development phase is critical for enhanced product quality understanding and provides a basis for risk management and increased regulatory flexibility. The recent Food and Drug
Administration's
505:(pI). In proteins, the isoelectric point (pI) is defined as the pH at which a protein has no net charge. When the pH > pI, a protein has a net negative charge, and when the pH < pI, a protein has a net positive charge. For example,
465:
in later purification steps. In addition, the concentration of product in the sample may not be sufficient, especially in cases where the desired antibody is produced by a low-secreting cell line. The sample is therefore concentrated by
283:
Much of the work behind production of monoclonal antibodies is rooted in the production of hybridomas, which involves identifying antigen-specific plasma/plasmablast cells that produce antibodies specific to an antigen of interest and
355:
This mixture of cells is then diluted and clones are grown from single parent cells on microtitre wells. The antibodies secreted by the different clones are then assayed for their ability to bind to the antigen (with a test such as
534:
To achieve maximum purity in a single step, affinity purification can be performed, using the antigen to provide specificity for the antibody. In this method, the antigen used to generate the antibody is covalently attached to an
413:
After obtaining either a media sample of cultured hybridomas or a sample of ascites fluid, the desired antibodies must be extracted. Cell culture sample contaminants consist primarily of media components such as growth factors,
137:", conceived of as a compound which selectively targeted a disease-causing organism, and could deliver a toxin for that organism. This underpinned the concept of monoclonal antibodies and monoclonal drug conjugates. Ehrlich and
6110:
300:
is used to fuse adjacent plasma membranes, but the success rate is low, so a selective medium in which only fused cells can grow is used. This is possible because myeloma cells have lost the ability to synthesize
203:
monoclonal antibodies, eliminating the reactions that many monoclonal antibodies caused in some patients. By the 1990s research was making progress in using monoclonal antibodies therapeutically, and in 2018,
244:
Hand-filling wells with a liquid for a research test. This test involves preparation of cultures in which hybrids are grown in large quantities to produce desired antibody. This is effected by fusing a
90:
It is possible to produce monoclonal antibodies that specifically bind to almost any suitable substance; they can then serve to detect or purify it. This capability has become an investigative tool in
560:
Product heterogeneity is common in monoclonal antibodies and other recombinant biological products and is typically introduced either upstream during expression or downstream during manufacturing.
212:
received the Nobel Prize in
Physiology or Medicine for their discovery of cancer therapy by inhibition of negative immune regulation, using monoclonal antibodies that prevent inhibitory linkages.
6103:
607:
initiative attempts to provide guidance on development and to facilitate design of products and processes that maximizes efficacy and safety profile while enhancing product manufacturability.
3969:. Edinburgh: Churchill Livingstone. pp. 241, for the examples infliximab, basiliximab, abciximab, daclizumab, palivusamab, gemtuzumab, alemtuzumab and rituximab, and mechanism and mode.
719:
manufacturers to recoup the typically large investment costs, and where there are no price controls, such as the United States, prices can be higher if they provide great value. Seven
5045:
6096:
405:
technology. One of the advantages of the new technologies is applicable to multiple animals, such as rabbit, llama, chicken and other common experimental animals in the laboratory.
501:
is used at a high enough pH that the desired antibody flows through the column while anions bind to it. Various proteins can also be separated along with the anions based on their
184:
succeeded in making fusions of myeloma cell lines with B cells to create hybridomas that could produce antibodies, specific to known antigens and that were immortalized. They and
4587:
510:
them. Transferrin, on the other hand, has a pI of 5.9, so it cannot be easily separated by this method. A difference in pI of at least 1 is necessary for a good separation.
4057:
3736:
Hernandez I, Bott SW, Patel AS, Wolf CG, Hospodar AR, Sampathkumar S, et al. (February 2018). "Pricing of monoclonal antibody therapies: higher if used for cancer?".
457:
The sample is first conditioned, or prepared for purification. Cells, cell debris, lipids, and clotted material are first removed, typically by centrifugation followed by
4014:
7232:
3323:
Zhang T, Bourret J, Cano T (August 2011). "Isolation and characterization of therapeutic antibody charge variants using cation exchange displacement chromatography".
3493:
598:
has been used to identify and characterize these often unseen variants in quantities that are suitable for subsequent preclinical evaluation regimens such as animal
8235:
4421:
737:
Once monoclonal antibodies for a given substance have been produced, they can be used to detect the presence of this substance. Proteins can be detected using the
302:
666:
monoclonal antibodies were injected into humans, resulting in their rapid removal from the blood, as well as systemic inflammatory effects and the production of
841:
5038:
648:
their therapeutic efficacy and their detectability in diagnostic applications. Fermentation chambers have been used for large scale antibody production.
446:
that are secreted by the bacteria. Depending on the complexity of the media required in cell culture and thus the contaminants, one or the other method (
4367:
643:, rather than mice. These techniques rely on rapid cloning of immunoglobulin gene segments to create libraries of antibodies with slightly different
7641:
4580:
1014:
Monoclonal antibodies for research applications can be found directly from antibody suppliers, or through use of a specialist search engine like
172:
described the production of monoclonal antibodies using human–mouse hybrid cells. This work remains widely cited among those using human-derived
5031:
142:
8173:
7842:
7176:
6578:
6054:
189:
8062:
7225:
4538:
662:
While mouse and human antibodies are structurally similar, the differences between them were sufficient to invoke an immune response when
8228:
823:
both to target antigen and to a conjugate or effector cell. Every intact antibody can bind to cell receptors or other proteins with its
624:
2723:"A robust high throughput platform to generate functional recombinant monoclonal antibodies using rabbit B cells from peripheral blood"
8539:
8345:
7699:
7626:
7531:
4573:
3974:
2603:
1501:
580:. The generally accepted purification method of process streams for monoclonal antibodies includes capture of the product target with
7556:
6188:
2571:
845:
3523:
Schmitz U, Versmold A, Kaufmann P, Frank HG (2000). "Phage display: a molecular tool for the generation of antibodies – a review".
2499:"New high affinity monoclonal antibodies recognize non-overlapping epitopes on mesothelin for monitoring and treating mesothelioma"
4392:
4345:
4919:
1470:
1444:
1414:
702:
human products to reduce the side effects of humanised or chimeric antibodies. Several successful approaches have been proposed:
84:
3930:
3501:
3231:
Beck A, Wurch T, Bailly C, Corvaia N (May 2010). "Strategies and challenges for the next generation of therapeutic antibodies".
2166:
Schwaber J, Cohen EP (August 1973). "Human x mouse somatic cell hybrid clone secreting immunoglobulins of both parental types".
164:
were used to study the structure of antibodies, but it was not yet possible to produce identical antibodies specific to a given
8544:
7218:
1937:
2937:"Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors"
8514:
8351:
8335:
8309:
8264:
8221:
7541:
6612:
6026:
4914:
824:
779:
Therapeutic monoclonal antibodies act through multiple mechanisms, such as blocking of targeted molecule functions, inducing
7646:
4857:
1916:
1002:
589:
514:
498:
8472:
1797:, can cause different kinds of side effects. These side effects can be categorized into common and serious side effects.
7505:
6707:
6036:
4521:, open-access virtual repository publishing data and commentary on any antibodies available to the scientific community.
4106:
4063:
4020:
1898:
1733:
367:
The hybridomas can be grown indefinitely in a suitable cell culture medium. They can also be injected into mice (in the
8190:
7193:
6643:
6595:
6071:
1729:
1664:
1009:
774:
595:
482:
107:
4527:
3767:
1971:
3033:
Vlasak J, Ionescu R (December 2008). "Heterogeneity of monoclonal antibodies revealed by charge-sensitive methods".
8524:
8357:
7284:
4743:
4141:
703:
690:
7749:
5620:
2258:
1342:
698:
Ever since the discovery that monoclonal antibodies could be generated, scientists have targeted the creation of
667:
548:
3276:"Charge variants in IgG1: Isolation, characterization, in vitro binding properties and pharmacokinetics in rats"
8299:
8291:
6832:
6827:
4557:
4512:
1778:
1433:
1403:
820:
720:
552:
buffer or a more gentle, high salt elution buffer is then used to recover purified antibody from the support.
3923:
8178:
8145:
8086:
8037:
7952:
7576:
7181:
6583:
6203:
6059:
4923:
4681:
1766:
1721:
1584:
528:
471:
322:
177:
134:
8529:
8519:
8340:
8270:
8116:
8106:
8076:
7867:
7812:
7739:
7729:
7606:
7088:
6323:
6228:
6088:
4945:
4663:
4599:
1861:
1759:
1279:
1108:
1068:
954:
87:
can also be engineered, by increasing the therapeutic targets of one monoclonal antibody to two epitopes.
8213:
8315:
8091:
8080:
8042:
7932:
7917:
7847:
7827:
7822:
7601:
7355:
6258:
6218:
6119:
5132:
5054:
4892:
4748:
4726:
3401:
van der Schoot JM, Fennemann FL, Valente M, Dolen Y, Hagemans IM, Becker AM, et al. (August 2019).
2011:"The history of monoclonal antibody development – Progress, remaining challenges and future innovations"
1725:
1548:
877:
76:
3615:
Boulianne GL, Hozumi N, Shulman MJ (1984). "Production of functional chimaeric mouse/human antibody".
3275:
7942:
7802:
7571:
7536:
5336:
4909:
4842:
4753:
4677:
4460:
3624:
3571:
3414:
2793:
2734:
2510:
2451:
2296:
2209:
Cambrosio A, Keating P (1992). "Between fact and technique: the beginnings of hybridoma technology".
2075:
1692:
1463:
1437:
1093:
1053:
942:
800:
746:
278:
8282:
4565:
1972:"Inhibitory monoclonal antibodies to human cytochrome P450 enzymes: a new avenue for drug discovery"
8534:
8391:
8057:
8027:
8022:
8007:
7837:
7684:
7636:
7596:
5006:
4969:
4904:
4887:
4639:
1148:
816:
762:
657:
297:
138:
64:
46:
7295:
7779:
7330:
7290:
7064:
6966:
6865:
6736:
6372:
6194:
6123:
5521:
5480:
5320:
5112:
4789:
4508:
4213:
Kreuzberger N, Hirsch C, Chai KL, Tomlinson E, Khosravi Z, Popp M, et al. (September 2021).
4195:
3905:
3795:
3648:
3383:
3256:
2819:
2322:
2234:
2191:
2099:
1777:, a monoclonal antibody drug, received an emergency use authorization from the US FDA for use as
1534:
1138:
1103:
1063:
950:
930:
750:
585:
443:
200:
8493:
1133:
1098:
1058:
946:
761:
Antibodies can also be used to purify their target compounds from mixtures, using the method of
749:, monoclonal antibodies can be used to detect antigens in fixed tissue sections, and similarly,
4015:"Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19"
3403:"Functional diversification of hybridoma-produced antibodies by CRISPR/HDR genomic engineering"
2935:
Duty JA, Szodoray P, Zheng NY, Koelsch KA, Zhang Q, Swiatkowski M, et al. (January 2009).
2440:"Rabbit monoclonal antibodies: generating a fusion partner to produce rabbit-rabbit hybridomas"
2342:"A Nobel Prize-worthy pursuit: cancer immunology and harnessing immunity to tumour neoantigens"
8409:
7666:
7485:
6465:
6339:
5412:
5082:
5023:
4837:
4603:
4476:
4296:
4244:
4187:
4149:
3970:
3897:
3787:
3745:
3718:
3683:
3640:
3597:
3540:
3475:
3440:
3375:
3340:
3305:
3248:
3213:
3195:
3154:
3136:
3097:
3089:
3050:
3015:
2966:
2917:
2868:
2811:
2762:
2703:
2651:
2599:
2577:
2567:
2536:
2479:
2420:
2371:
2314:
2226:
2183:
2148:
2091:
2048:
2030:
1991:
1633:
1598:
1563:
1492:
604:
502:
310:
181:
95:
4174:
Kozlov M (December 2021). "Omicron overpowers key COVID antibody treatments in early tests".
3814:
3172:
Beck A, Nowak C, Meshulam D, Reynolds K, Chen D, Pacardo DB, et al. (20 November 2022).
8401:
8249:
6873:
5402:
5392:
4957:
4897:
4869:
4864:
4832:
4819:
4809:
4468:
4315:
4286:
4278:
4234:
4226:
4179:
3889:
3859:
3826:
3779:
3710:
3675:
3632:
3587:
3579:
3532:
3467:
3430:
3422:
3367:
3332:
3295:
3287:
3240:
3203:
3185:
3144:
3128:
3081:
3042:
3005:
2997:
2956:
2948:
2907:
2899:
2858:
2850:
2801:
2752:
2742:
2693:
2685:
2641:
2633:
2559:
2526:
2518:
2469:
2459:
2410:
2402:
2361:
2353:
2304:
2218:
2175:
2138:
2130:
2083:
2038:
2022:
1983:
1257:
1143:
986:
837:
462:
293:
289:
246:
205:
169:
153:
54:
3560:"Humanization of high-affinity antibodies targeting glypican-3 in hepatocellular carcinoma"
1747:
billion worth of
Regeneron monoclonal antibodies at $ 2,100 per dose to curb the shortage.
371:, surrounding the gut). There, they produce tumors secreting an antibody-rich fluid called
8498:
8486:
5820:
5596:
4852:
4552:
4531:
3934:
3763:
2886:
Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, Ahmed R, et al. (1 January 2009).
1781:
to protect certain moderately to severely immunocompromised individuals against COVID-19.
1309:
1212:
1181:
978:
675:
635:
technologies. Recombinant antibody engineering involves antibody production by the use of
616:
599:
573:
467:
402:
306:
185:
4265:
Hirsch C, Park YS, Piechotta V, Chai KL, Estcourt LJ, Monsef I, et al. (June 2022).
2888:"Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen"
2780:
Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC (September 2003).
2598:: Monoclonal Antibody Production". Washington (DC): National Academies Press (US); 1999.
4464:
3628:
3575:
3418:
2797:
2738:
2594:
National
Research Council (US) Committee on Methods of Producing Monoclonal Antibodies.
2514:
2455:
2300:
2079:
8184:
8150:
7817:
7187:
6842:
6589:
6065:
4879:
4824:
4765:
4685:
4658:
4341:
4291:
4266:
4239:
4214:
3880:
Carter P (November 2001). "Improving the efficacy of antibody-based cancer therapies".
3592:
3559:
3435:
3402:
3300:
3208:
3173:
3149:
3116:
3010:
2985:
2961:
2936:
2912:
2887:
2863:
2838:
2757:
2722:
2698:
2673:
2646:
2621:
2531:
2498:
2415:
2390:
2366:
2341:
2143:
2118:
2043:
2010:
1866:
1736:
to reduce the number of hospitalizations, emergency room visits, and deaths because of
1163:
1159:
577:
490:
264:
240:
232:
224:
196:
79:
bind to multiple epitopes and are usually made by several different antibody-secreting
3783:
3679:
3471:
2984:
Huang J, Doria-Rose NA, Longo NS, Laub L, Lin CL, Turk E, et al. (October 2013).
1987:
830:
8508:
8449:
8375:
8032:
7887:
7440:
7275:
7158:
7117:
6927:
6761:
6544:
5975:
5776:
5721:
5646:
5606:
5526:
5420:
5066:
5058:
4847:
4736:
4199:
4127:
4084:
4041:
3927:
2474:
2439:
1967:
1938:"Cytochrome P450 Mediated Drug and Carcinogen Metabolism using Monoclonal Antibodies"
1893:
1640:
1605:
1570:
1530:
1357:
1325:
1321:
1221:
1190:
970:
902:
707:
632:
628:
568:
398:
330:
4489:
3909:
3799:
3260:
2837:
Koelsch K, Zheng NY, Zhang Q, Duty A, Helms C, Mathias MD, et al. (June 2007).
2823:
2238:
2103:
1945:
8047:
8012:
7997:
7992:
7987:
7982:
7957:
7912:
7907:
7902:
7897:
7882:
7769:
7744:
7724:
7586:
7551:
7490:
7470:
7280:
7265:
7210:
7153:
7044:
7039:
7024:
6981:
6922:
6912:
6897:
6892:
6528:
6523:
6209:
6199:
5965:
5950:
5930:
5920:
5899:
5815:
5756:
5741:
5711:
5691:
5686:
5661:
5641:
5636:
5631:
5581:
5571:
5561:
5511:
5506:
5501:
5430:
5315:
5299:
5202:
5192:
5187:
5162:
5102:
4814:
4799:
4794:
4731:
4629:
4282:
4230:
3652:
3387:
2406:
2326:
2195:
1754:
neutralization tests indicate monoclonal antibody therapies (with the exception of
808:
738:
679:
341:
309:
of nucleic acids. The absence of HGPRT is not a problem for these cells unless the
250:
209:
126:
123:
111:
91:
3993:
3274:
Khawli LA, Goswami S, Hutchinson R, Kwong ZW, Yang J, Wang X, et al. (2010).
3174:"Risk-Based Control Strategies of Recombinant Monoclonal Antibody Charge Variants"
3132:
3117:"Structure, heterogeneity and developability assessment of therapeutic antibodies"
3115:
Xu Y, Wang D, Mason B, Rossomando T, Li N, Liu D, et al. (17 December 2018).
2721:
Seeber S, Ros F, Thorey I, Tiefenthaler G, Kaluza K, Lifke V, et al. (2014).
3831:
3358:
Rathore AS, Winkle H (January 2009). "Quality by design for biopharmaceuticals".
3336:
3070:"Impact of cell culture on recombinant monoclonal antibody product heterogeneity"
2747:
753:
can be used to detect a substance in either frozen tissue section or live cells.
57:. All subsequent antibodies derived this way trace back to a unique parent cell.
8155:
8129:
8124:
8096:
8072:
8067:
8002:
7977:
7967:
7937:
7927:
7892:
7877:
7862:
7852:
7832:
7807:
7792:
7764:
7714:
7709:
7704:
7689:
7679:
7656:
7651:
7621:
7566:
7510:
7500:
7475:
7465:
7460:
7455:
7430:
7405:
7395:
7390:
7385:
7375:
7360:
7345:
7340:
7325:
7320:
7310:
7305:
7300:
7270:
7137:
7103:
7069:
7059:
7034:
6937:
6932:
6887:
6882:
6878:
6855:
6806:
6766:
6693:
6560:
6489:
6473:
6439:
6434:
6395:
6390:
6385:
6362:
6319:
6299:
6294:
6289:
6264:
6249:
6244:
6234:
6214:
6184:
6031:
6021:
5960:
5945:
5935:
5925:
5904:
5889:
5874:
5869:
5864:
5839:
5792:
5771:
5766:
5761:
5751:
5746:
5731:
5706:
5696:
5676:
5656:
5651:
5616:
5611:
5601:
5591:
5586:
5556:
5546:
5516:
5470:
5465:
5455:
5440:
5425:
5397:
5382:
5367:
5362:
5357:
5325:
5268:
5263:
5253:
5233:
5212:
5207:
5177:
5097:
3666:
Chadd HE, Chamow SM (April 2001). "Therapeutic antibody expression technology".
2595:
1850:
1790:
1688:
1657:
1629:
1594:
1559:
1522:
1514:
1485:
1481:
1455:
1395:
1367:
1302:
1173:
1156:
1126:
962:
917:
907:
897:
872:
862:
857:
584:, elution, acidification to inactivate potential mammalian viruses, followed by
564:
525:
461:
with a 0.45 μm filter. These large particles can cause a phenomenon called
419:
326:
314:
285:
161:
80:
4472:
4183:
3046:
2563:
2444:
Proceedings of the
National Academy of Sciences of the United States of America
2026:
397:
Several monoclonal antibody technologies have been developed recently, such as
8101:
8052:
8017:
7947:
7922:
7872:
7857:
7797:
7787:
7759:
7734:
7674:
7616:
7611:
7546:
7526:
7480:
7450:
7445:
7435:
7425:
7420:
7410:
7400:
7370:
7365:
7335:
7315:
7132:
7049:
7029:
7008:
7003:
6971:
6907:
6902:
6796:
6791:
6781:
6776:
6771:
6756:
6751:
6722:
6688:
6455:
6380:
6314:
6309:
6284:
6279:
6274:
6269:
6239:
6224:
5991:
5970:
5955:
5940:
5884:
5879:
5859:
5854:
5825:
5799:
5736:
5716:
5701:
5681:
5671:
5626:
5576:
5566:
5541:
5536:
5531:
5496:
5491:
5460:
5445:
5387:
5377:
5372:
5352:
5289:
5284:
5273:
5243:
5238:
5182:
5157:
5142:
5127:
5122:
5092:
4937:
4690:
4596:
3714:
3500:. MRC Laboratory of Molecular Biology (LMB). 17 September 2009. Archived from
2839:"Mature B cells class switched to IgD are autoreactive in healthy individuals"
2497:
Zhang YF, Phung Y, Gao W, Kawa S, Hassan R, Pastan I, et al. (May 2015).
2134:
1888:
1774:
1755:
1700:
1644:
1626:
1619:
1609:
1591:
1574:
1556:
1271:
1235:
1204:
1086:
1046:
982:
966:
938:
934:
892:
882:
644:
458:
368:
337:
318:
253:
173:
149:
60:
4153:
3199:
3140:
3093:
2034:
8459:
8434:
8429:
7972:
7962:
7754:
7719:
7694:
7631:
7591:
7581:
7561:
7495:
7415:
7380:
7350:
7074:
7054:
6998:
6986:
6976:
6951:
6917:
6850:
6811:
6801:
6786:
6672:
6667:
6518:
6429:
6414:
6400:
6329:
6304:
6254:
6179:
6150:
5894:
5726:
5551:
5450:
5435:
5294:
5258:
5248:
5222:
5217:
5197:
5172:
5167:
5152:
5147:
5117:
5087:
4995:
4700:
4451:
Rajewsky K (November 2019). "The advent and rise of monoclonal antibodies".
4142:"Biden administration moves to stave off shortages of monoclonal antibodies"
4097:
2806:
2781:
2464:
2087:
1903:
1794:
1704:
1680:
1425:
1335:
1249:
912:
887:
867:
803:
against the target cancer cell. Such mAbs can be modified for delivery of a
780:
581:
497:
that the desired antibody binds to the column while anions flow through, or
442:
may be present. There may also be bacterial contamination and, as a result,
349:
345:
257:
25:
A general representation of the method used to produce monoclonal antibodies
4480:
4300:
4248:
4191:
3901:
3848:"Monoclonal War: The Antibody Arsenal and Targets for Expanded Application"
3791:
3749:
3687:
3601:
3544:
3536:
3479:
3444:
3426:
3379:
3344:
3309:
3291:
3252:
3217:
3158:
3101:
3069:
3054:
3019:
3001:
2970:
2921:
2872:
2815:
2766:
2707:
2655:
2581:
2540:
2424:
2375:
2230:
2066:
Waldmann TA (June 1991). "Monoclonal antibodies in diagnosis and therapy".
2052:
1995:
4524:
4422:"Monoclonal Antibodies: List, Types, Side Effects & FDA Uses (Cancer)"
3864:
3847:
3722:
3644:
3190:
2483:
2318:
2187:
2152:
2119:"Monoclonal antibodies: a witness seminar in contemporary medical history"
2095:
8454:
8327:
7250:
6993:
5304:
5227:
5137:
5107:
4985:
4770:
4758:
4716:
4670:
4634:
4215:"SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19"
2952:
2903:
2637:
1814:
1737:
1384:
1375:
1291:
1116:
1076:
958:
812:
783:
in cells which express the target, or by modulating signalling pathways.
742:
544:
439:
431:
427:
380:
361:
99:
42:
3458:
Siegel DL (January 2002). "Recombinant monoclonal antibody technology".
2986:"Isolation of human monoclonal antibodies from peripheral blood B cells"
364:. The most productive and stable clone is then selected for future use.
21:
8424:
8419:
8414:
5781:
5666:
4990:
4804:
4653:
4624:
3371:
2222:
1225:
1194:
974:
796:
620:
540:
536:
518:
506:
415:
372:
165:
102:. Monoclonal antibodies are used in the diagnosis of illnesses such as
72:
68:
50:
3583:
3085:
2782:"Predominant autoantibody production by early human B cell precursors"
2689:
2522:
2357:
1010:
Monoclonal antibody therapy § FDA-approved therapeutic antibodies
795:
involves monoclonal antibodies that bind only to cancer-cell-specific
694:
Approaches have been developed to isolate human monoclonal antibodies.
228:
Looking at slides of cultures of cells that make monoclonal antibodies
8444:
8439:
6135:
6006:
4962:
4950:
4695:
4646:
3893:
3701:
Lonberg N, Huszar D (1995). "Human antibodies from transgenic mice".
3636:
2854:
2309:
2284:
2179:
1880:
1243:
1015:
990:
792:
663:
478:
386:
236:
Monoclonal antibodies can be grown in unlimited quantities in flasks.
157:
103:
3244:
2554:
Yang J, Shen MH (2006). "Polyethylene glycol-mediated cell fusion".
1018:. Below are examples of clinically important monoclonal antibodies.
4267:"SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19"
2438:
Spieker-Polet H, Sethupathi P, Yam PC, Knight KL (September 1995).
6503:
6357:
6352:
6347:
6164:
6127:
804:
689:
640:
636:
547:, which allows selective attachment to a carrier protein, such as
435:
357:
263:
239:
231:
223:
20:
4126:
This article incorporates text from this source, which is in the
4083:
This article incorporates text from this source, which is in the
4040:
This article incorporates text from this source, which is in the
6652:
4058:"FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19"
1353:
1317:
1287:
481:
such as nucleic acids and endotoxins. These can be separated by
385:
animal. Where alternate techniques exist, ascites is considered
325:) makes them unable to use the de novo pathway and become fully
8217:
7214:
6616:
6092:
5027:
5010:
4569:
4518:
2674:"A novel high-affinity human monoclonal antibody to mesothelin"
6646:
for bone, musculoskeletal, circulatory, and neurologic systems
486:
3068:
Liu H, Nowak C, Shao M, Ponniah G, Neill A (September 2016).
1762:) were not likely to be active against the Omicron variant.
989:(IgE) and is useful in treating moderate-to-severe allergic
438:. In both cases, other secretions by the hybridomas such as
4316:"FDA Authorizes COVID Drug Pemgarda for High-Risk Patients"
848:; MAb: monoclonal antibody; scFv, single-chain Fv fragment.
494:
2391:"Lee Marshall Nadler, MD: a conversation with the editor"
2283:
Riechmann L, Clark M, Waldmann H, Winter G (March 1988).
3926:
in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds)
1740:. In September 2021, the Biden administration purchased
3815:"Monoclonal antibody therapy for non-malignant disease"
2672:
Ho M, Feng M, Fisher RJ, Rader C, Pastan I (May 2011).
2259:"The Story of César Milstein and Monoclonal Antibodies"
152:
producing a single antibody were known, in the form of
844:: antibody dependent cell-mediated cytotoxicity; CDC:
8470:
4393:"Monoclonal antibody drugs for cancer: How they work"
524:
A much quicker, single-step method of separation is
145:
for providing the theoretical basis for immunology.
8400:
8384:
8368:
8336:
Single-chain variable fragment / di-scFv / tri-scFv
8326:
8290:
8256:
8138:
8115:
7778:
7665:
7519:
7258:
7249:
7146:
7125:
7116:
7096:
7087:
7017:
6959:
6950:
6864:
6841:
6820:
6744:
6735:
6715:
6706:
6681:
6660:
6651:
6553:
6537:
6511:
6502:
6482:
6464:
6448:
6422:
6413:
6371:
6338:
6172:
6163:
6143:
6134:
6014:
6004:
5984:
5913:
5847:
5838:
5807:
5479:
5411:
5345:
5074:
5065:
4978:
4936:
4878:
4779:
4709:
4617:
4610:
2558:. Methods Mol Biol. Vol. 325. pp. 59–66.
1970:, Krausz KW, Gonzalez FJ, Yang TJ (November 1999).
4052:
4050:
4009:
4007:
2622:"Inaugural Editorial: Searching for Magic Bullets"
75:that is recognized by the antibody). In contrast,
303:hypoxanthine-guanine-phosphoribosyl transferase
1409:clinical trials for other indications underway
8229:
7226:
6628:
6104:
5039:
4581:
2401:(4). National Institutes of Health: 381–389.
1845:Among the possible serious side effects are:
997:Examples of therapeutic monoclonal antibodies
852:MAbs approved by the FDA for cancer include:
543:, it is commonly synthesized with a terminal
333:, thus requiring supplementation to survive.
313:pathway is also disrupted. Exposing cells to
16:Antibodies from clones of the same blood cell
8:
840:, antibody directed enzyme prodrug therapy;
292:cells. Rabbit B-cells can be used to form a
4420:Ogbru O (12 October 2022). Davis CP (ed.).
4271:The Cochrane Database of Systematic Reviews
4219:The Cochrane Database of Systematic Reviews
1720:In 2020, the monoclonal antibody therapies
682:yielded part-mouse, part-human antibodies.
477:Most of the charged impurities are usually
8236:
8222:
8214:
7255:
7233:
7219:
7211:
7122:
7093:
6956:
6741:
6712:
6657:
6635:
6621:
6613:
6508:
6419:
6169:
6140:
6111:
6097:
6089:
6011:
5844:
5071:
5046:
5032:
5024:
5007:
4614:
4588:
4574:
4566:
2615:
2613:
2611:
619:monoclonal antibodies involves repertoire
426:sample is likely to have host antibodies,
4511:at the U.S. National Library of Medicine
4290:
4260:
4258:
4238:
3924:"Principles of Oncologic Pharmacotherapy"
3863:
3830:
3591:
3434:
3299:
3207:
3189:
3148:
3009:
2960:
2911:
2862:
2805:
2756:
2746:
2697:
2645:
2530:
2473:
2463:
2414:
2365:
2308:
2142:
2042:
563:These variants are typically aggregates,
199:and his team pioneered the techniques to
2285:"Reshaping human antibodies for therapy"
1020:
957:by their ability to bind to and inhibit
829:
8477:
4368:"Monoclonal antibodies to treat cancer"
3988:
3986:
3960:
3958:
3956:
3954:
3952:
3950:
3948:
3946:
3944:
3942:
3922:Takimoto CH, Calvo E. (1 January 2005)
2252:
2250:
2248:
1928:
1789:Several monoclonal antibodies, such as
815:or other active conjugate or to design
360:or antigen microarray assay) or immuno-
336:The selective culture medium is called
2389:Nadler LM, Roberts WC (October 2007).
513:Transferrin can instead be removed by
348:. This medium is selective for fused (
143:Nobel Prize for Physiology or Medicine
4415:
4413:
4344:. U.S. Food and Drug Administration.
2843:The Journal of Clinical Investigation
2667:
2665:
2117:Tansey EM, Catterall PP (July 1994).
1286:targets myeloid cell surface antigen
305:(HGPRT), an enzyme necessary for the
268:Bathing prepared slides in a solution
190:Nobel Prize in Physiology or Medicine
7:
4098:"Emergency Use Authorization letter"
3738:The American Journal of Managed Care
3035:Current Pharmaceutical Biotechnology
2941:The Journal of Experimental Medicine
2596:"Recommendation 1: Executive Summary
110:in the treatment of e.g. cancer and
3768:"Therapeutic monoclonal antibodies"
3703:International Reviews of Immunology
1001:For a more comprehensive list, see
378:The medium must be enriched during
67:affinity, binding only to the same
8346:Small modular immunopharmaceutical
3460:Transfusion Clinique et Biologique
1976:Trends in Pharmacological Sciences
1800:Some common side effects include:
1672:inhibits an RSV fusion (F) protein
14:
4348:from the original on 8 April 2024
4140:Bernstein L (14 September 2021).
4025:(Press release). 21 November 2020
4000:. Springer Nature Switzerland AG.
3558:Zhang YF, Ho M (September 2016).
2211:Journal of the History of Biology
846:complement-dependent cytotoxicity
835:Monoclonal antibodies for cancer.
8492:
8480:
8281:
4121:
4078:
4068:(Press release). 9 February 2021
4035:
3994:"Bavituximab - Avid Bioservices"
3668:Current Opinion in Biotechnology
393:Novel mAb development technology
85:Bispecific monoclonal antibodies
2678:International Journal of Cancer
1858:Arterial and venous blood clots
977:and thereby help prevent acute
929:Monoclonal antibodies used for
160:. These abnormal antibodies or
4915:Immunoglobulin class switching
4525:Antibody Purification Handbook
4342:"EUA 122 Invivyd Pemgarda LOA"
4283:10.1002/14651858.cd014945.pub2
4231:10.1002/14651858.cd013825.pub2
3813:Australian Prescriber (2006).
2407:10.1080/08998280.2007.11928327
2015:Annals of Medicine and Surgery
1:
3784:10.1016/S0140-6736(00)01034-5
3680:10.1016/S0958-1669(00)00198-1
3472:10.1016/S1246-7820(01)00210-5
3133:10.1080/19420862.2018.1553476
1988:10.1016/S0165-6147(99)01382-6
1917:List of monoclonal antibodies
1885:Decrease in white blood cells
1003:List of monoclonal antibodies
678:" or "humanised" DNA through
539:support. If the antigen is a
515:size exclusion chromatography
499:anion exchange chromatography
4558:Resources in other libraries
4340:Cavazzoni P (3 April 2024).
4314:MacMillan C (5 April 2024).
4107:Food and Drug Administration
4064:Food and Drug Administration
4021:Food and Drug Administration
3832:10.18773/austprescr.2006.079
3337:10.1016/j.chroma.2011.05.061
2748:10.1371/journal.pone.0086184
2340:Altmann DM (November 2018).
2009:Liu JK (11 September 2014).
1899:Gastrointestinal perforation
1734:Food and Drug Administration
1730:emergency use authorizations
1242:moderate-to-severe allergic
592:and then with cation beads.
3325:Journal of Chromatography A
1643:, targets spike protein of
1608:, targets spike protein of
1573:, targets spike protein of
791:One possible treatment for
775:Monoclonal antibody therapy
710:and single B cell cloning.
668:human anti-mouse antibodies
596:Displacement chromatography
483:ion exchange chromatography
192:in 1984 for the discovery.
8561:
4744:Polyclonal B cell response
4473:10.1038/d41586-019-02840-w
4184:10.1038/d41586-021-03829-0
3233:Nature Reviews. Immunology
3047:10.2174/138920108786786402
2027:10.1016/j.amsu.2014.09.001
1511:Anti-cancer and anti-viral
1007:
1000:
772:
757:Analytic and chemical uses
655:
276:
8540:Reagents for biochemistry
8279:
8168:
7750:Mirvetuximab soravtansine
7171:
6573:
6049:
5021:
4553:Resources in your library
3846:Rosenn (September 2023).
3715:10.3109/08830189509061738
2135:10.1017/s0025727300036632
1653:
1543:
1510:
1267:
1040:
941:, which are effective in
819:that can bind with their
8304:fragment / Fab' fragment
4513:Medical Subject Headings
2564:10.1385/1-59745-005-7:59
1779:pre-exposure prophylaxis
1434:squamous cell carcinomas
1404:squamous cell carcinomas
721:University of Pittsburgh
493:is used at a low enough
321:analogue which inhibits
311:de novo purine synthesis
8146:Depatuxizumab mafodotin
8087:Tucotuzumab celmoleukin
8038:Rovalpituzumab tesirine
7953:Lorvotuzumab mertansine
7843:Clivatuzumab tetraxetan
5012:Monoclonal antibodies (
4530:5 December 2008 at the
4494:Kimball's Biology Pages
4490:"Monoclonal Antibodies"
4372:American Cancer Society
2807:10.1126/science.1086907
2465:10.1073/pnas.92.20.9348
2263:WhatisBiotechnology.org
2088:10.1126/science.2047874
1722:bamlanivimab/etesevimab
1585:bamlanivimab/etesevimab
1352:targets phosphoprotein
981:of kidney transplants.
529:affinity chromatography
323:dihydrofolate reductase
256:to form a hybrid cell (
129:proposed the idea of a
106:and infections and are
8545:Therapeutic antibodies
8341:Single-domain antibody
8271:Trifunctional antibody
8107:Vorsetuzumab mafodotin
8063:Tacatuzumab tetraxetan
7868:Denintuzumab mafodotin
7813:Bivatuzumab mertansine
7740:Loncastuximab tesirine
7730:Indatuximab ravtansine
7607:Naptumomab estafenatox
7089:Angiogenesis inhibitor
6189:+maftivimab/odesivimab
4858:Tolerance in pregnancy
4600:adaptive immune system
3933:4 October 2013 at the
3882:Nature Reviews. Cancer
3537:10.1053/plac.1999.0511
3531:(Suppl A): S106–S112.
3494:"Dr. George Pieczenik"
3427:10.1126/sciadv.aaw1822
3292:10.4161/mabs.2.6.13333
3074:Biotechnology Progress
3002:10.1038/nprot.2013.117
1862:Autoimmune thyroiditis
1760:tixagevimab/cilgavimab
1699:inhibits the receptor
1667:infections in children
1343:non-Hodgkin's lymphoma
1280:acute myeloid leukemia
1109:ankylosing spondylitis
1069:ankylosing spondylitis
955:ankylosing spondylitis
849:
695:
556:Antibody heterogeneity
269:
261:
237:
229:
26:
8515:Monoclonal antibodies
8450:Kunitz domain peptide
8316:Chemically linked Fab
8246:monoclonal antibodies
8092:Vandortuzumab vedotin
8043:Sacituzumab govitecan
7933:Inotuzumab ozogamicin
7918:Gemtuzumab ozogamicin
7848:Cofetuzumab pelidotin
7828:Cantuzumab ravtansine
7823:Cantuzumab mertansine
7642:Nofetumomab merpentan
7602:Moxetumomab pasudotox
7356:Glembatumumab vedotin
7242:Monoclonal antibodies
6120:Monoclonal antibodies
5133:Camidanlumab tesirine
5055:Monoclonal antibodies
4893:Somatic hypermutation
4727:Polyclonal antibodies
4722:Monoclonal antibodies
4509:Monoclonal+antibodies
3865:10.3390/immuno3030021
3819:Australian Prescriber
3191:10.3390/antib11040073
2626:Antibody Therapeutics
2556:Nuclear Reprogramming
1750:As of December 2021,
1726:casirivimab/imdevimab
1549:casirivimab/imdevimab
1215:of kidney transplants
1184:of kidney transplants
878:Gemtuzumab ozogamicin
833:
817:bispecific antibodies
693:
454:) may be preferable.
277:Further information:
273:Hybridoma development
267:
243:
235:
227:
156:– a cancer affecting
77:polyclonal antibodies
37:, more rarely called
24:
8139:Chimeric + humanized
7943:Lifastuzumab vedotin
7803:Belantamab mafodotin
7572:Ibritumomab tiuxetan
7537:Anatumomab mafenatox
5808:Chimeric + humanized
5337:Nivolumab/relatlimab
4910:Junctional diversity
4678:Antigen presentation
3360:Nature Biotechnology
2953:10.1084/jem.20080611
2904:10.1038/nprot.2009.3
1693:coronary angioplasty
1464:colorectal carcinoma
1438:colorectal carcinoma
1347:rheumatoid arthritis
1094:rheumatoid arthritis
1054:rheumatoid arthritis
943:rheumatoid arthritis
747:immunohistochemistry
434:, nucleic acids and
340:because it contains
279:Hybridoma technology
122:In the early 1900s,
108:used therapeutically
63:antibodies can have
8058:Sofituzumab vedotin
8028:Polatuzumab vedotin
8023:Pinatuzumab vedotin
8008:Oportuzumab monatox
7838:Citatuzumab bogatox
7700:Derlotuximab biotin
7685:Brentuximab vedotin
7637:Taplitumomab paptox
7627:Satumomab pendetide
7597:Nacolomab tafenatox
7532:Altumomab pentetate
4905:V(D)J recombination
4888:Affinity maturation
4640:Antigenic variation
4544:Monoclonal antibody
4465:2019Natur.575...47R
4146:The Washington Post
3629:1984Natur.312..643B
3576:2016NatSR...633878Z
3504:on 23 December 2012
3419:2019SciA....5.1822V
2798:2003Sci...301.1374W
2792:(5638): 1374–1377.
2739:2014PLoSO...986184S
2515:2015NatSR...5E9928Z
2456:1995PNAS...92.9348S
2301:1988Natur.332..323R
2080:1991Sci...252.1657W
2074:(5013): 1657–1662.
1942:home.ccr.cancer.gov
1374:breast cancer with
1316:targets an antigen
1248:chronic ideopathic
1149:psoriatic arthritis
931:autoimmune diseases
925:Autoimmune diseases
763:immunoprecipitation
658:Chimeric antibodies
652:Chimeric antibodies
422:. In contrast, the
298:Polyethylene glycol
31:monoclonal antibody
8195:Never to phase III
7557:Capromab pendetide
7331:Enfortumab vedotin
7198:Never to phase III
6967:Anti-amyloid drugs
6600:Never to phase III
6124:infectious disease
6076:Never to phase III
5522:Certolizumab pegol
5489:Immunosuppressive:
4111:. 16 December 2021
3564:Scientific Reports
3372:10.1038/nbt0109-26
2638:10.1093/abt/tby001
2620:Ho M (June 2018).
2503:Scientific Reports
2223:10.1007/BF00162840
1948:on 15 October 2004
1765:Over 2021–22, two
1535:phosphatidylserine
1139:ulcerative colitis
1104:ulcerative colitis
1064:ulcerative colitis
951:ulcerative colitis
850:
751:immunofluorescence
696:
615:The production of
586:ion chromatography
344:, aminopterin and
270:
262:
238:
230:
141:received the 1908
27:
8525:Cancer treatments
8468:
8467:
8410:Affibody molecule
8402:Antibody mimetics
8328:Variable fragment
8250:antibody mimetics
8211:
8210:
8205:
8204:
8164:
8163:
7486:Tisotumab vedotin
7208:
7207:
7167:
7166:
7112:
7111:
7083:
7082:
6946:
6945:
6731:
6730:
6702:
6701:
6610:
6609:
6569:
6568:
6498:
6497:
6409:
6408:
6159:
6158:
6086:
6085:
6045:
6044:
6000:
5999:
5834:
5833:
5789:Immune activation
5281:Immune activation
5083:Immunosuppression
5004:
5003:
4932:
4931:
4682:professional APCs
4539:Library resources
3928:Cancer Management
3778:(9205): 735–740.
3766:(February 2000).
3623:(5995): 643–646.
3584:10.1038/srep33878
3331:(31): 5079–5086.
3086:10.1002/btpr.2327
2996:(10): 1907–1915.
2690:10.1002/ijc.25557
2523:10.1038/srep09928
2450:(20): 9348–9352.
2358:10.1111/imm.13008
2295:(6162): 323–327.
2174:(5416): 444–447.
1713:
1712:
1634:COVID-19 pandemic
1599:COVID-19 pandemic
1564:COVID-19 pandemic
605:Quality by Design
503:isoelectric point
369:peritoneal cavity
307:salvage synthesis
288:these cells with
96:molecular biology
8552:
8497:
8496:
8485:
8484:
8483:
8476:
8285:
8238:
8231:
8224:
8215:
8117:Rat/mouse hybrid
7256:
7235:
7228:
7221:
7212:
7123:
7094:
6957:
6874:Alacizumab pegol
6742:
6713:
6658:
6637:
6630:
6623:
6614:
6509:
6420:
6170:
6141:
6113:
6106:
6099:
6090:
6012:
5845:
5403:Zolimomab aritox
5393:Telimomab aritox
5072:
5048:
5041:
5034:
5025:
5008:
4898:Clonal selection
4870:Immune privilege
4865:Immunodeficiency
4820:Cross-reactivity
4810:Hypersensitivity
4615:
4590:
4583:
4576:
4567:
4497:
4484:
4437:
4436:
4434:
4432:
4417:
4408:
4407:
4405:
4403:
4389:
4383:
4382:
4380:
4378:
4364:
4358:
4357:
4355:
4353:
4337:
4331:
4330:
4328:
4326:
4311:
4305:
4304:
4294:
4262:
4253:
4252:
4242:
4210:
4204:
4203:
4171:
4165:
4164:
4162:
4160:
4137:
4131:
4125:
4124:
4120:
4118:
4116:
4102:
4094:
4088:
4082:
4081:
4077:
4075:
4073:
4054:
4045:
4039:
4038:
4034:
4032:
4030:
4011:
4002:
4001:
3990:
3981:
3980:
3965:Rang HP (2003).
3962:
3937:
3920:
3914:
3913:
3894:10.1038/35101072
3876:
3870:
3869:
3867:
3843:
3837:
3836:
3834:
3810:
3804:
3803:
3760:
3754:
3753:
3733:
3727:
3726:
3698:
3692:
3691:
3663:
3657:
3656:
3637:10.1038/312643a0
3612:
3606:
3605:
3595:
3555:
3549:
3548:
3520:
3514:
3513:
3511:
3509:
3490:
3484:
3483:
3455:
3449:
3448:
3438:
3407:Science Advances
3398:
3392:
3391:
3355:
3349:
3348:
3320:
3314:
3313:
3303:
3271:
3265:
3264:
3228:
3222:
3221:
3211:
3193:
3169:
3163:
3162:
3152:
3112:
3106:
3105:
3080:(5): 1103–1112.
3065:
3059:
3058:
3030:
3024:
3023:
3013:
2990:Nature Protocols
2981:
2975:
2974:
2964:
2932:
2926:
2925:
2915:
2892:Nature Protocols
2883:
2877:
2876:
2866:
2855:10.1172/JCI27628
2849:(6): 1558–1565.
2834:
2828:
2827:
2809:
2777:
2771:
2770:
2760:
2750:
2718:
2712:
2711:
2701:
2684:(9): 2020–2030.
2669:
2660:
2659:
2649:
2617:
2606:
2592:
2586:
2585:
2551:
2545:
2544:
2534:
2494:
2488:
2487:
2477:
2467:
2435:
2429:
2428:
2418:
2386:
2380:
2379:
2369:
2337:
2331:
2330:
2312:
2310:10.1038/332323a0
2280:
2274:
2273:
2271:
2269:
2254:
2243:
2242:
2206:
2200:
2199:
2180:10.1038/244444a0
2163:
2157:
2156:
2146:
2114:
2108:
2107:
2063:
2057:
2056:
2046:
2006:
2000:
1999:
1964:
1958:
1957:
1955:
1953:
1944:. Archived from
1933:
1834:Loss of appetite
1831:General weakness
1767:Cochrane reviews
1746:
1743:
1387:(erbB2) receptor
1258:immunoglobulin E
1144:plaque psoriasis
1033:Mechanism/Target
1021:
987:immunoglobulin E
787:Cancer treatment
769:Therapeutic uses
733:Diagnostic tests
686:Human antibodies
463:membrane fouling
294:rabbit hybridoma
206:James P. Allison
170:Jerrold Schwaber
154:multiple myeloma
139:Élie Metchnikoff
71:(the part of an
55:white blood cell
45:produced from a
8560:
8559:
8555:
8554:
8553:
8551:
8550:
8549:
8505:
8504:
8503:
8491:
8481:
8479:
8471:
8469:
8464:
8396:
8380:
8364:
8322:
8303:
8286:
8277:
8252:
8242:
8212:
8207:
8206:
8201:
8200:
8185:Clinical trials
8160:
8134:
8111:
7774:
7661:
7515:
7245:
7239:
7209:
7204:
7203:
7188:Clinical trials
7163:
7142:
7108:
7079:
7013:
6942:
6860:
6837:
6816:
6727:
6708:Musculoskeletal
6698:
6677:
6647:
6641:
6611:
6606:
6605:
6590:Clinical trials
6565:
6549:
6533:
6494:
6478:
6460:
6444:
6405:
6367:
6334:
6155:
6130:
6117:
6087:
6082:
6081:
6066:Clinical trials
6041:
5996:
5980:
5909:
5830:
5821:Rozanolixizumab
5809:
5803:
5795:
5784:
5597:Lulizumab pegol
5475:
5407:
5341:
5061:
5052:
5017:
5005:
5000:
4974:
4928:
4874:
4853:Clonal deletion
4781:
4775:
4705:
4606:
4594:
4564:
4563:
4562:
4547:
4546:
4542:
4532:Wayback Machine
4505:
4500:
4487:
4459:(7781): 47–49.
4450:
4446:
4444:Further reading
4441:
4440:
4430:
4428:
4419:
4418:
4411:
4401:
4399:
4391:
4390:
4386:
4376:
4374:
4366:
4365:
4361:
4351:
4349:
4339:
4338:
4334:
4324:
4322:
4313:
4312:
4308:
4277:(6): CD014945.
4264:
4263:
4256:
4225:(9): CD013825.
4212:
4211:
4207:
4173:
4172:
4168:
4158:
4156:
4139:
4138:
4134:
4122:
4114:
4112:
4100:
4096:
4095:
4091:
4079:
4071:
4069:
4056:
4055:
4048:
4036:
4028:
4026:
4013:
4012:
4005:
3992:
3991:
3984:
3977:
3964:
3963:
3940:
3935:Wayback Machine
3921:
3917:
3879:
3877:
3873:
3845:
3844:
3840:
3812:
3811:
3807:
3762:
3761:
3757:
3735:
3734:
3730:
3700:
3699:
3695:
3665:
3664:
3660:
3614:
3613:
3609:
3557:
3556:
3552:
3522:
3521:
3517:
3507:
3505:
3492:
3491:
3487:
3457:
3456:
3452:
3413:(8): eaaw1822.
3400:
3399:
3395:
3357:
3356:
3352:
3322:
3321:
3317:
3273:
3272:
3268:
3245:10.1038/nri2747
3230:
3229:
3225:
3171:
3170:
3166:
3114:
3113:
3109:
3067:
3066:
3062:
3032:
3031:
3027:
2983:
2982:
2978:
2934:
2933:
2929:
2885:
2884:
2880:
2836:
2835:
2831:
2779:
2778:
2774:
2720:
2719:
2715:
2671:
2670:
2663:
2619:
2618:
2609:
2593:
2589:
2574:
2553:
2552:
2548:
2496:
2495:
2491:
2437:
2436:
2432:
2388:
2387:
2383:
2339:
2338:
2334:
2282:
2281:
2277:
2267:
2265:
2256:
2255:
2246:
2208:
2207:
2203:
2165:
2164:
2160:
2123:Medical History
2116:
2115:
2111:
2065:
2064:
2060:
2008:
2007:
2003:
1982:(11): 432–438.
1966:
1965:
1961:
1951:
1949:
1935:
1934:
1930:
1925:
1913:
1908:
1843:
1787:
1773:In March 2024,
1744:
1741:
1718:
1493:Anti-angiogenic
1310:B cell leukemia
1256:inhibits human
1134:Crohn's disease
1099:Crohn's disease
1059:Crohn's disease
1042:
1012:
1006:
999:
985:inhibits human
947:Crohn's disease
927:
922:
801:immune response
789:
777:
771:
759:
735:
730:
716:
704:transgenic mice
688:
660:
654:
613:
600:pharmacokinetic
574:bioavailability
558:
468:ultrafiltration
411:
395:
281:
275:
222:
186:Niels Kaj Jerne
120:
17:
12:
11:
5:
8558:
8556:
8548:
8547:
8542:
8537:
8532:
8527:
8522:
8517:
8507:
8506:
8502:
8501:
8489:
8466:
8465:
8463:
8462:
8457:
8452:
8447:
8442:
8437:
8432:
8427:
8422:
8417:
8412:
8406:
8404:
8398:
8397:
8395:
8394:
8388:
8386:
8382:
8381:
8379:
8378:
8372:
8370:
8366:
8365:
8363:
8362:
8361:
8360:
8358:T-cell engager
8348:
8343:
8338:
8332:
8330:
8324:
8323:
8321:
8320:
8319:
8318:
8306:
8301:
8296:
8294:
8288:
8287:
8280:
8278:
8276:
8275:
8274:
8273:
8260:
8258:
8257:Whole antibody
8254:
8253:
8243:
8241:
8240:
8233:
8226:
8218:
8209:
8208:
8203:
8202:
8199:
8198:
8197:
8196:
8193:
8182:
8176:
8170:
8169:
8166:
8165:
8162:
8161:
8159:
8158:
8153:
8151:Duvortuxizumab
8148:
8142:
8140:
8136:
8135:
8133:
8132:
8127:
8121:
8119:
8113:
8112:
8110:
8109:
8104:
8099:
8094:
8089:
8084:
8070:
8065:
8060:
8055:
8050:
8045:
8040:
8035:
8030:
8025:
8020:
8015:
8010:
8005:
8000:
7995:
7990:
7985:
7980:
7975:
7970:
7965:
7960:
7955:
7950:
7945:
7940:
7935:
7930:
7925:
7920:
7915:
7910:
7905:
7900:
7895:
7890:
7885:
7880:
7875:
7870:
7865:
7860:
7855:
7850:
7845:
7840:
7835:
7830:
7825:
7820:
7818:Brontictuzumab
7815:
7810:
7805:
7800:
7795:
7790:
7784:
7782:
7776:
7775:
7773:
7772:
7767:
7762:
7757:
7752:
7747:
7742:
7737:
7732:
7727:
7722:
7717:
7712:
7707:
7702:
7697:
7692:
7687:
7682:
7677:
7671:
7669:
7663:
7662:
7660:
7659:
7654:
7649:
7644:
7639:
7634:
7629:
7624:
7619:
7614:
7609:
7604:
7599:
7594:
7589:
7584:
7579:
7574:
7569:
7564:
7559:
7554:
7549:
7544:
7539:
7534:
7529:
7523:
7521:
7517:
7516:
7514:
7513:
7508:
7503:
7498:
7493:
7488:
7483:
7478:
7473:
7468:
7463:
7458:
7453:
7448:
7443:
7438:
7433:
7428:
7423:
7418:
7413:
7408:
7403:
7398:
7393:
7388:
7383:
7378:
7373:
7368:
7363:
7358:
7353:
7348:
7343:
7338:
7333:
7328:
7323:
7318:
7313:
7308:
7303:
7298:
7293:
7288:
7285:+hyaluronidase
7278:
7273:
7268:
7262:
7260:
7253:
7247:
7246:
7240:
7238:
7237:
7230:
7223:
7215:
7206:
7205:
7202:
7201:
7200:
7199:
7196:
7185:
7179:
7173:
7172:
7169:
7168:
7165:
7164:
7162:
7161:
7156:
7150:
7148:
7144:
7143:
7141:
7140:
7135:
7129:
7127:
7120:
7114:
7113:
7110:
7109:
7107:
7106:
7100:
7098:
7091:
7085:
7084:
7081:
7080:
7078:
7077:
7072:
7067:
7062:
7057:
7052:
7047:
7042:
7037:
7032:
7027:
7021:
7019:
7015:
7014:
7012:
7011:
7006:
7001:
6996:
6991:
6990:
6989:
6984:
6979:
6974:
6963:
6961:
6954:
6948:
6947:
6944:
6943:
6941:
6940:
6935:
6930:
6925:
6920:
6915:
6910:
6905:
6900:
6895:
6890:
6885:
6876:
6870:
6868:
6862:
6861:
6859:
6858:
6853:
6847:
6845:
6839:
6838:
6836:
6835:
6830:
6824:
6822:
6818:
6817:
6815:
6814:
6809:
6804:
6799:
6794:
6789:
6784:
6779:
6774:
6769:
6764:
6759:
6754:
6748:
6746:
6739:
6733:
6732:
6729:
6728:
6726:
6725:
6719:
6717:
6710:
6704:
6703:
6700:
6699:
6697:
6696:
6691:
6685:
6683:
6679:
6678:
6676:
6675:
6670:
6664:
6662:
6655:
6649:
6648:
6642:
6640:
6639:
6632:
6625:
6617:
6608:
6607:
6604:
6603:
6602:
6601:
6598:
6587:
6581:
6575:
6574:
6571:
6570:
6567:
6566:
6564:
6563:
6557:
6555:
6551:
6550:
6548:
6547:
6541:
6539:
6535:
6534:
6532:
6531:
6526:
6521:
6515:
6513:
6506:
6500:
6499:
6496:
6495:
6493:
6492:
6486:
6484:
6480:
6479:
6477:
6476:
6470:
6468:
6462:
6461:
6459:
6458:
6452:
6450:
6446:
6445:
6443:
6442:
6437:
6432:
6426:
6424:
6417:
6411:
6410:
6407:
6406:
6404:
6403:
6398:
6393:
6388:
6383:
6377:
6375:
6369:
6368:
6366:
6365:
6360:
6355:
6350:
6344:
6342:
6336:
6335:
6333:
6332:
6327:
6317:
6312:
6307:
6302:
6297:
6292:
6287:
6282:
6277:
6272:
6267:
6262:
6252:
6247:
6242:
6237:
6232:
6222:
6212:
6207:
6197:
6192:
6182:
6176:
6174:
6167:
6161:
6160:
6157:
6156:
6154:
6153:
6147:
6145:
6138:
6132:
6131:
6118:
6116:
6115:
6108:
6101:
6093:
6084:
6083:
6080:
6079:
6078:
6077:
6074:
6063:
6057:
6051:
6050:
6047:
6046:
6043:
6042:
6040:
6039:
6034:
6029:
6024:
6018:
6016:
6009:
6002:
6001:
5998:
5997:
5995:
5994:
5988:
5986:
5982:
5981:
5979:
5978:
5973:
5968:
5963:
5958:
5953:
5948:
5943:
5938:
5933:
5928:
5923:
5917:
5915:
5911:
5910:
5908:
5907:
5902:
5897:
5892:
5887:
5882:
5877:
5872:
5867:
5862:
5857:
5851:
5849:
5842:
5836:
5835:
5832:
5831:
5829:
5828:
5823:
5818:
5812:
5810:
5805:
5804:
5786:
5785:
5779:
5774:
5769:
5764:
5759:
5754:
5749:
5744:
5739:
5734:
5729:
5724:
5719:
5714:
5709:
5704:
5699:
5694:
5689:
5684:
5679:
5674:
5669:
5664:
5659:
5654:
5649:
5644:
5639:
5634:
5629:
5624:
5621:+hyaluronidase
5614:
5609:
5604:
5599:
5594:
5589:
5584:
5579:
5574:
5569:
5564:
5559:
5554:
5549:
5544:
5539:
5534:
5529:
5524:
5519:
5514:
5509:
5504:
5499:
5494:
5485:
5483:
5477:
5476:
5474:
5473:
5468:
5463:
5458:
5453:
5448:
5443:
5438:
5433:
5428:
5423:
5417:
5415:
5409:
5408:
5406:
5405:
5400:
5395:
5390:
5385:
5380:
5375:
5370:
5365:
5360:
5355:
5349:
5347:
5343:
5342:
5340:
5339:
5329:
5328:
5323:
5318:
5308:
5307:
5302:
5297:
5292:
5287:
5277:
5276:
5271:
5266:
5261:
5256:
5251:
5246:
5241:
5236:
5231:
5225:
5220:
5215:
5210:
5205:
5200:
5195:
5190:
5185:
5180:
5175:
5170:
5165:
5160:
5155:
5150:
5145:
5140:
5135:
5130:
5125:
5120:
5115:
5110:
5105:
5100:
5095:
5090:
5078:
5076:
5069:
5063:
5062:
5053:
5051:
5050:
5043:
5036:
5028:
5022:
5019:
5018:
5011:
5002:
5001:
4999:
4998:
4993:
4988:
4982:
4980:
4976:
4975:
4973:
4972:
4967:
4966:
4965:
4955:
4954:
4953:
4942:
4940:
4934:
4933:
4930:
4929:
4927:
4926:
4917:
4912:
4907:
4902:
4901:
4900:
4895:
4884:
4882:
4880:Immunogenetics
4876:
4875:
4873:
4872:
4867:
4862:
4861:
4860:
4855:
4850:
4845:
4840:
4828:
4827:
4825:Co-stimulation
4822:
4817:
4812:
4807:
4802:
4797:
4792:
4785:
4783:
4777:
4776:
4774:
4773:
4768:
4766:Immune complex
4762:
4761:
4756:
4751:
4746:
4741:
4740:
4739:
4734:
4729:
4724:
4713:
4711:
4707:
4706:
4704:
4703:
4698:
4693:
4688:
4686:Dendritic cell
4674:
4673:
4668:
4667:
4666:
4664:Conformational
4661:
4650:
4649:
4644:
4643:
4642:
4637:
4632:
4621:
4619:
4612:
4608:
4607:
4595:
4593:
4592:
4585:
4578:
4570:
4561:
4560:
4555:
4549:
4548:
4537:
4536:
4535:
4534:
4522:
4516:
4504:
4503:External links
4501:
4499:
4498:
4485:
4447:
4445:
4442:
4439:
4438:
4409:
4384:
4359:
4332:
4306:
4254:
4205:
4166:
4132:
4089:
4046:
4003:
3982:
3976:978-0443071454
3975:
3938:
3915:
3888:(2): 118–129.
3878:Modified from
3871:
3858:(3): 346-357.
3838:
3825:(5): 130–133.
3805:
3755:
3744:(2): 109–112.
3728:
3693:
3674:(2): 188–194.
3658:
3607:
3550:
3515:
3485:
3450:
3393:
3350:
3315:
3286:(6): 613–624.
3266:
3239:(5): 345–352.
3223:
3164:
3127:(2): 239–264.
3107:
3060:
3041:(6): 468–481.
3025:
2976:
2947:(1): 139–151.
2927:
2898:(3): 372–384.
2878:
2829:
2772:
2713:
2661:
2607:
2604:978-0309075114
2587:
2572:
2546:
2489:
2430:
2381:
2352:(3): 283–284.
2332:
2275:
2244:
2217:(2): 175–230.
2201:
2158:
2129:(3): 322–327.
2109:
2058:
2021:(4): 113–116.
2001:
1959:
1927:
1926:
1924:
1921:
1920:
1919:
1912:
1909:
1907:
1906:
1901:
1896:
1891:
1886:
1883:
1878:
1875:
1872:
1869:
1867:Hypothyroidism
1864:
1859:
1856:
1853:
1847:
1842:
1841:
1838:
1835:
1832:
1829:
1826:
1823:
1820:
1817:
1812:
1809:
1806:
1802:
1786:
1783:
1717:
1714:
1711:
1710:
1707:
1697:
1696:
1695:
1683:
1677:
1676:
1673:
1670:
1669:
1668:
1660:
1655:
1651:
1650:
1647:
1638:
1637:
1636:
1622:
1616:
1615:
1612:
1603:
1602:
1601:
1587:
1581:
1580:
1577:
1568:
1567:
1566:
1552:
1545:
1541:
1540:
1537:
1528:
1527:
1526:
1517:
1512:
1508:
1507:
1504:
1498:
1497:
1496:
1495:cancer therapy
1488:
1478:
1477:
1474:
1468:
1467:
1466:
1458:
1452:
1451:
1448:
1442:
1441:
1440:
1428:
1422:
1421:
1418:
1412:
1411:
1410:
1407:
1398:
1392:
1391:
1388:
1381:
1380:
1379:
1378:overexpression
1370:
1364:
1363:
1360:
1350:
1349:
1348:
1345:
1338:
1332:
1331:
1328:
1314:
1313:
1312:
1305:
1299:
1298:
1295:
1284:
1283:
1282:
1274:
1269:
1265:
1264:
1261:
1254:
1253:
1252:
1246:
1238:
1232:
1231:
1228:
1218:
1217:
1216:
1207:
1201:
1200:
1197:
1187:
1186:
1185:
1176:
1170:
1169:
1166:
1153:
1152:
1151:
1146:
1141:
1136:
1129:
1123:
1122:
1119:
1113:
1112:
1111:
1106:
1101:
1096:
1089:
1083:
1082:
1079:
1073:
1072:
1071:
1066:
1061:
1056:
1049:
1044:
1038:
1037:
1034:
1031:
1028:
1025:
998:
995:
926:
923:
921:
920:
915:
910:
905:
900:
895:
890:
885:
880:
875:
870:
865:
860:
854:
799:and induce an
788:
785:
773:Main article:
770:
767:
758:
755:
734:
731:
729:
726:
715:
712:
687:
684:
656:Main article:
653:
650:
612:
609:
578:immunogenicity
557:
554:
491:chromatography
410:
407:
394:
391:
274:
271:
221:
218:
197:Gregory Winter
182:César Milstein
178:Georges Köhler
148:By the 1970s,
119:
116:
15:
13:
10:
9:
6:
4:
3:
2:
8557:
8546:
8543:
8541:
8538:
8536:
8533:
8531:
8530:Immune system
8528:
8526:
8523:
8521:
8520:Biotechnology
8518:
8516:
8513:
8512:
8510:
8500:
8495:
8490:
8488:
8478:
8474:
8461:
8458:
8456:
8453:
8451:
8448:
8446:
8443:
8441:
8438:
8436:
8433:
8431:
8428:
8426:
8423:
8421:
8418:
8416:
8413:
8411:
8408:
8407:
8405:
8403:
8399:
8393:
8390:
8389:
8387:
8385:Intracellular
8383:
8377:
8376:Microantibody
8374:
8373:
8371:
8369:Smaller units
8367:
8359:
8356:
8355:
8354:
8353:
8349:
8347:
8344:
8342:
8339:
8337:
8334:
8333:
8331:
8329:
8325:
8317:
8314:
8313:
8312:
8311:
8307:
8305:
8298:
8297:
8295:
8293:
8289:
8284:
8272:
8269:
8268:
8267:
8266:
8262:
8261:
8259:
8255:
8251:
8247:
8239:
8234:
8232:
8227:
8225:
8220:
8219:
8216:
8194:
8192:
8189:
8188:
8186:
8183:
8180:
8177:
8175:
8172:
8171:
8167:
8157:
8154:
8152:
8149:
8147:
8144:
8143:
8141:
8137:
8131:
8128:
8126:
8123:
8122:
8120:
8118:
8114:
8108:
8105:
8103:
8100:
8098:
8095:
8093:
8090:
8088:
8085:
8082:
8078:
8074:
8071:
8069:
8066:
8064:
8061:
8059:
8056:
8054:
8051:
8049:
8046:
8044:
8041:
8039:
8036:
8034:
8033:Rosmantuzumab
8031:
8029:
8026:
8024:
8021:
8019:
8016:
8014:
8011:
8009:
8006:
8004:
8001:
7999:
7996:
7994:
7991:
7989:
7986:
7984:
7981:
7979:
7976:
7974:
7971:
7969:
7966:
7964:
7961:
7959:
7956:
7954:
7951:
7949:
7946:
7944:
7941:
7939:
7936:
7934:
7931:
7929:
7926:
7924:
7921:
7919:
7916:
7914:
7911:
7909:
7906:
7904:
7901:
7899:
7896:
7894:
7891:
7889:
7888:Enoblituzumab
7886:
7884:
7881:
7879:
7876:
7874:
7871:
7869:
7866:
7864:
7861:
7859:
7856:
7854:
7851:
7849:
7846:
7844:
7841:
7839:
7836:
7834:
7831:
7829:
7826:
7824:
7821:
7819:
7816:
7814:
7811:
7809:
7806:
7804:
7801:
7799:
7796:
7794:
7791:
7789:
7786:
7785:
7783:
7781:
7777:
7771:
7768:
7766:
7763:
7761:
7758:
7756:
7753:
7751:
7748:
7746:
7743:
7741:
7738:
7736:
7733:
7731:
7728:
7726:
7723:
7721:
7718:
7716:
7713:
7711:
7708:
7706:
7703:
7701:
7698:
7696:
7693:
7691:
7688:
7686:
7683:
7681:
7678:
7676:
7673:
7672:
7670:
7668:
7664:
7658:
7655:
7653:
7650:
7648:
7645:
7643:
7640:
7638:
7635:
7633:
7630:
7628:
7625:
7623:
7620:
7618:
7615:
7613:
7610:
7608:
7605:
7603:
7600:
7598:
7595:
7593:
7590:
7588:
7585:
7583:
7580:
7578:
7575:
7573:
7570:
7568:
7565:
7563:
7560:
7558:
7555:
7553:
7550:
7548:
7545:
7543:
7540:
7538:
7535:
7533:
7530:
7528:
7525:
7524:
7522:
7518:
7512:
7509:
7507:
7504:
7502:
7499:
7497:
7494:
7492:
7489:
7487:
7484:
7482:
7479:
7477:
7474:
7472:
7469:
7467:
7464:
7462:
7459:
7457:
7454:
7452:
7449:
7447:
7444:
7442:
7441:Pembrolizumab
7439:
7437:
7434:
7432:
7429:
7427:
7424:
7422:
7419:
7417:
7414:
7412:
7409:
7407:
7404:
7402:
7399:
7397:
7394:
7392:
7389:
7387:
7384:
7382:
7379:
7377:
7374:
7372:
7369:
7367:
7364:
7362:
7359:
7357:
7354:
7352:
7349:
7347:
7344:
7342:
7339:
7337:
7334:
7332:
7329:
7327:
7324:
7322:
7319:
7317:
7314:
7312:
7309:
7307:
7304:
7302:
7299:
7297:
7294:
7292:
7289:
7286:
7282:
7279:
7277:
7276:Ascrinvacumab
7274:
7272:
7269:
7267:
7264:
7263:
7261:
7257:
7254:
7252:
7248:
7243:
7236:
7231:
7229:
7224:
7222:
7217:
7216:
7213:
7197:
7195:
7192:
7191:
7189:
7186:
7183:
7180:
7178:
7175:
7174:
7170:
7160:
7159:Landogrozumab
7157:
7155:
7152:
7151:
7149:
7145:
7139:
7136:
7134:
7131:
7130:
7128:
7124:
7121:
7119:
7118:Growth factor
7115:
7105:
7102:
7101:
7099:
7095:
7092:
7090:
7086:
7076:
7073:
7071:
7068:
7066:
7063:
7061:
7058:
7056:
7053:
7051:
7048:
7046:
7043:
7041:
7038:
7036:
7033:
7031:
7028:
7026:
7023:
7022:
7020:
7016:
7010:
7007:
7005:
7002:
7000:
6997:
6995:
6992:
6988:
6985:
6983:
6980:
6978:
6975:
6973:
6970:
6969:
6968:
6965:
6964:
6962:
6958:
6955:
6953:
6949:
6939:
6936:
6934:
6931:
6929:
6928:Ralpancizumab
6926:
6924:
6921:
6919:
6916:
6914:
6911:
6909:
6906:
6904:
6901:
6899:
6896:
6894:
6891:
6889:
6886:
6884:
6880:
6877:
6875:
6872:
6871:
6869:
6867:
6863:
6857:
6854:
6852:
6849:
6848:
6846:
6844:
6840:
6834:
6831:
6829:
6826:
6825:
6823:
6819:
6813:
6810:
6808:
6805:
6803:
6800:
6798:
6795:
6793:
6790:
6788:
6785:
6783:
6780:
6778:
6775:
6773:
6770:
6768:
6765:
6763:
6762:Ascrinvacumab
6760:
6758:
6755:
6753:
6750:
6749:
6747:
6743:
6740:
6738:
6734:
6724:
6721:
6720:
6718:
6714:
6711:
6709:
6705:
6695:
6692:
6690:
6687:
6686:
6684:
6680:
6674:
6671:
6669:
6666:
6665:
6663:
6659:
6656:
6654:
6650:
6645:
6638:
6633:
6631:
6626:
6624:
6619:
6618:
6615:
6599:
6597:
6594:
6593:
6591:
6588:
6585:
6582:
6580:
6577:
6576:
6572:
6562:
6559:
6558:
6556:
6552:
6546:
6545:Obiltoxaximab
6543:
6542:
6540:
6536:
6530:
6527:
6525:
6522:
6520:
6517:
6516:
6514:
6510:
6507:
6505:
6501:
6491:
6488:
6487:
6485:
6481:
6475:
6472:
6471:
6469:
6467:
6463:
6457:
6454:
6453:
6451:
6447:
6441:
6438:
6436:
6433:
6431:
6428:
6427:
6425:
6421:
6418:
6416:
6412:
6402:
6399:
6397:
6394:
6392:
6389:
6387:
6384:
6382:
6379:
6378:
6376:
6374:
6370:
6364:
6361:
6359:
6356:
6354:
6351:
6349:
6348:Cosfroviximab
6346:
6345:
6343:
6341:
6337:
6331:
6328:
6325:
6321:
6318:
6316:
6313:
6311:
6308:
6306:
6303:
6301:
6298:
6296:
6293:
6291:
6288:
6286:
6283:
6281:
6278:
6276:
6273:
6271:
6268:
6266:
6263:
6260:
6256:
6253:
6251:
6248:
6246:
6243:
6241:
6238:
6236:
6233:
6230:
6226:
6223:
6220:
6216:
6213:
6211:
6208:
6205:
6201:
6198:
6196:
6193:
6190:
6186:
6183:
6181:
6178:
6177:
6175:
6171:
6168:
6166:
6162:
6152:
6149:
6148:
6146:
6142:
6139:
6137:
6133:
6129:
6125:
6121:
6114:
6109:
6107:
6102:
6100:
6095:
6094:
6091:
6075:
6073:
6070:
6069:
6067:
6064:
6061:
6058:
6056:
6053:
6052:
6048:
6038:
6035:
6033:
6030:
6028:
6025:
6023:
6020:
6019:
6017:
6013:
6010:
6008:
6005:Inflammatory
6003:
5993:
5990:
5989:
5987:
5983:
5977:
5976:Tildrakizumab
5974:
5972:
5969:
5967:
5964:
5962:
5959:
5957:
5954:
5952:
5949:
5947:
5944:
5942:
5939:
5937:
5934:
5932:
5929:
5927:
5924:
5922:
5919:
5918:
5916:
5912:
5906:
5903:
5901:
5898:
5896:
5893:
5891:
5888:
5886:
5883:
5881:
5878:
5876:
5873:
5871:
5868:
5866:
5863:
5861:
5858:
5856:
5853:
5852:
5850:
5846:
5843:
5841:
5837:
5827:
5824:
5822:
5819:
5817:
5814:
5813:
5811:
5806:
5802:
5801:
5798:
5794:
5790:
5783:
5780:
5778:
5777:Vobarilizumab
5775:
5773:
5770:
5768:
5765:
5763:
5760:
5758:
5755:
5753:
5750:
5748:
5745:
5743:
5740:
5738:
5735:
5733:
5730:
5728:
5725:
5723:
5722:Spartalizumab
5720:
5718:
5715:
5713:
5710:
5708:
5705:
5703:
5700:
5698:
5695:
5693:
5690:
5688:
5685:
5683:
5680:
5678:
5675:
5673:
5670:
5668:
5665:
5663:
5660:
5658:
5655:
5653:
5650:
5648:
5647:Pembrolizumab
5645:
5643:
5640:
5638:
5635:
5633:
5630:
5628:
5625:
5622:
5618:
5615:
5613:
5610:
5608:
5607:Mogamulizumab
5605:
5603:
5600:
5598:
5595:
5593:
5590:
5588:
5585:
5583:
5580:
5578:
5575:
5573:
5570:
5568:
5565:
5563:
5560:
5558:
5555:
5553:
5550:
5548:
5545:
5543:
5540:
5538:
5535:
5533:
5530:
5528:
5527:Crizanlizumab
5525:
5523:
5520:
5518:
5515:
5513:
5510:
5508:
5505:
5503:
5500:
5498:
5495:
5493:
5490:
5487:
5486:
5484:
5482:
5478:
5472:
5469:
5467:
5464:
5462:
5459:
5457:
5454:
5452:
5449:
5447:
5444:
5442:
5439:
5437:
5434:
5432:
5429:
5427:
5424:
5422:
5421:Andecaliximab
5419:
5418:
5416:
5414:
5410:
5404:
5401:
5399:
5396:
5394:
5391:
5389:
5386:
5384:
5381:
5379:
5376:
5374:
5371:
5369:
5366:
5364:
5361:
5359:
5356:
5354:
5351:
5350:
5348:
5344:
5338:
5334:
5331:
5330:
5327:
5324:
5322:
5319:
5317:
5313:
5310:
5309:
5306:
5303:
5301:
5298:
5296:
5293:
5291:
5288:
5286:
5282:
5279:
5278:
5275:
5272:
5270:
5267:
5265:
5262:
5260:
5257:
5255:
5252:
5250:
5247:
5245:
5242:
5240:
5237:
5235:
5232:
5229:
5226:
5224:
5221:
5219:
5216:
5214:
5211:
5209:
5206:
5204:
5201:
5199:
5196:
5194:
5191:
5189:
5186:
5184:
5181:
5179:
5176:
5174:
5171:
5169:
5166:
5164:
5161:
5159:
5156:
5154:
5151:
5149:
5146:
5144:
5141:
5139:
5136:
5134:
5131:
5129:
5126:
5124:
5121:
5119:
5116:
5114:
5111:
5109:
5106:
5104:
5101:
5099:
5096:
5094:
5091:
5089:
5085:
5084:
5080:
5079:
5077:
5073:
5070:
5068:
5067:Immune system
5064:
5060:
5059:immune system
5056:
5049:
5044:
5042:
5037:
5035:
5030:
5029:
5026:
5020:
5015:
5009:
4997:
4994:
4992:
4989:
4987:
4984:
4983:
4981:
4977:
4971:
4968:
4964:
4961:
4960:
4959:
4956:
4952:
4949:
4948:
4947:
4944:
4943:
4941:
4939:
4935:
4925:
4921:
4918:
4916:
4913:
4911:
4908:
4906:
4903:
4899:
4896:
4894:
4891:
4890:
4889:
4886:
4885:
4883:
4881:
4877:
4871:
4868:
4866:
4863:
4859:
4856:
4854:
4851:
4849:
4848:Clonal anergy
4846:
4844:
4841:
4839:
4836:
4835:
4834:
4830:
4829:
4826:
4823:
4821:
4818:
4816:
4813:
4811:
4808:
4806:
4803:
4801:
4798:
4796:
4793:
4791:
4787:
4786:
4784:
4778:
4772:
4769:
4767:
4764:
4763:
4760:
4757:
4755:
4752:
4750:
4747:
4745:
4742:
4738:
4737:Microantibody
4735:
4733:
4730:
4728:
4725:
4723:
4720:
4719:
4718:
4715:
4714:
4712:
4708:
4702:
4699:
4697:
4694:
4692:
4689:
4687:
4683:
4679:
4676:
4675:
4672:
4669:
4665:
4662:
4660:
4657:
4656:
4655:
4652:
4651:
4648:
4645:
4641:
4638:
4636:
4633:
4631:
4628:
4627:
4626:
4623:
4622:
4620:
4616:
4613:
4609:
4605:
4601:
4598:
4591:
4586:
4584:
4579:
4577:
4572:
4571:
4568:
4559:
4556:
4554:
4551:
4550:
4545:
4540:
4533:
4529:
4526:
4523:
4520:
4519:Antibodypedia
4517:
4514:
4510:
4507:
4506:
4502:
4495:
4491:
4486:
4482:
4478:
4474:
4470:
4466:
4462:
4458:
4454:
4449:
4448:
4443:
4427:
4423:
4416:
4414:
4410:
4398:
4394:
4388:
4385:
4373:
4369:
4363:
4360:
4347:
4343:
4336:
4333:
4321:
4320:Yale Medicine
4317:
4310:
4307:
4302:
4298:
4293:
4288:
4284:
4280:
4276:
4272:
4268:
4261:
4259:
4255:
4250:
4246:
4241:
4236:
4232:
4228:
4224:
4220:
4216:
4209:
4206:
4201:
4197:
4193:
4189:
4185:
4181:
4177:
4170:
4167:
4155:
4151:
4147:
4143:
4136:
4133:
4129:
4128:public domain
4110:
4108:
4099:
4093:
4090:
4086:
4085:public domain
4067:
4065:
4059:
4053:
4051:
4047:
4043:
4042:public domain
4024:
4022:
4016:
4010:
4008:
4004:
3999:
3995:
3989:
3987:
3983:
3978:
3972:
3968:
3961:
3959:
3957:
3955:
3953:
3951:
3949:
3947:
3945:
3943:
3939:
3936:
3932:
3929:
3925:
3919:
3916:
3911:
3907:
3903:
3899:
3895:
3891:
3887:
3883:
3875:
3872:
3866:
3861:
3857:
3853:
3849:
3842:
3839:
3833:
3828:
3824:
3820:
3816:
3809:
3806:
3801:
3797:
3793:
3789:
3785:
3781:
3777:
3773:
3769:
3765:
3759:
3756:
3751:
3747:
3743:
3739:
3732:
3729:
3724:
3720:
3716:
3712:
3708:
3704:
3697:
3694:
3689:
3685:
3681:
3677:
3673:
3669:
3662:
3659:
3654:
3650:
3646:
3642:
3638:
3634:
3630:
3626:
3622:
3618:
3611:
3608:
3603:
3599:
3594:
3589:
3585:
3581:
3577:
3573:
3569:
3565:
3561:
3554:
3551:
3546:
3542:
3538:
3534:
3530:
3526:
3519:
3516:
3503:
3499:
3495:
3489:
3486:
3481:
3477:
3473:
3469:
3465:
3461:
3454:
3451:
3446:
3442:
3437:
3432:
3428:
3424:
3420:
3416:
3412:
3408:
3404:
3397:
3394:
3389:
3385:
3381:
3377:
3373:
3369:
3365:
3361:
3354:
3351:
3346:
3342:
3338:
3334:
3330:
3326:
3319:
3316:
3311:
3307:
3302:
3297:
3293:
3289:
3285:
3281:
3277:
3270:
3267:
3262:
3258:
3254:
3250:
3246:
3242:
3238:
3234:
3227:
3224:
3219:
3215:
3210:
3205:
3201:
3197:
3192:
3187:
3183:
3179:
3175:
3168:
3165:
3160:
3156:
3151:
3146:
3142:
3138:
3134:
3130:
3126:
3122:
3118:
3111:
3108:
3103:
3099:
3095:
3091:
3087:
3083:
3079:
3075:
3071:
3064:
3061:
3056:
3052:
3048:
3044:
3040:
3036:
3029:
3026:
3021:
3017:
3012:
3007:
3003:
2999:
2995:
2991:
2987:
2980:
2977:
2972:
2968:
2963:
2958:
2954:
2950:
2946:
2942:
2938:
2931:
2928:
2923:
2919:
2914:
2909:
2905:
2901:
2897:
2893:
2889:
2882:
2879:
2874:
2870:
2865:
2860:
2856:
2852:
2848:
2844:
2840:
2833:
2830:
2825:
2821:
2817:
2813:
2808:
2803:
2799:
2795:
2791:
2787:
2783:
2776:
2773:
2768:
2764:
2759:
2754:
2749:
2744:
2740:
2736:
2733:(2): e86184.
2732:
2728:
2724:
2717:
2714:
2709:
2705:
2700:
2695:
2691:
2687:
2683:
2679:
2675:
2668:
2666:
2662:
2657:
2653:
2648:
2643:
2639:
2635:
2631:
2627:
2623:
2616:
2614:
2612:
2608:
2605:
2601:
2597:
2591:
2588:
2583:
2579:
2575:
2573:1-59745-005-7
2569:
2565:
2561:
2557:
2550:
2547:
2542:
2538:
2533:
2528:
2524:
2520:
2516:
2512:
2508:
2504:
2500:
2493:
2490:
2485:
2481:
2476:
2471:
2466:
2461:
2457:
2453:
2449:
2445:
2441:
2434:
2431:
2426:
2422:
2417:
2412:
2408:
2404:
2400:
2396:
2392:
2385:
2382:
2377:
2373:
2368:
2363:
2359:
2355:
2351:
2347:
2343:
2336:
2333:
2328:
2324:
2320:
2316:
2311:
2306:
2302:
2298:
2294:
2290:
2286:
2279:
2276:
2264:
2260:
2253:
2251:
2249:
2245:
2240:
2236:
2232:
2228:
2224:
2220:
2216:
2212:
2205:
2202:
2197:
2193:
2189:
2185:
2181:
2177:
2173:
2169:
2162:
2159:
2154:
2150:
2145:
2140:
2136:
2132:
2128:
2124:
2120:
2113:
2110:
2105:
2101:
2097:
2093:
2089:
2085:
2081:
2077:
2073:
2069:
2062:
2059:
2054:
2050:
2045:
2040:
2036:
2032:
2028:
2024:
2020:
2016:
2012:
2005:
2002:
1997:
1993:
1989:
1985:
1981:
1977:
1973:
1969:
1963:
1960:
1947:
1943:
1939:
1932:
1929:
1922:
1918:
1915:
1914:
1910:
1905:
1902:
1900:
1897:
1895:
1894:Enterocolitis
1892:
1890:
1887:
1884:
1882:
1879:
1876:
1874:Heart failure
1873:
1870:
1868:
1865:
1863:
1860:
1857:
1854:
1852:
1849:
1848:
1846:
1839:
1836:
1833:
1830:
1827:
1824:
1821:
1818:
1816:
1813:
1810:
1807:
1804:
1803:
1801:
1798:
1796:
1792:
1784:
1782:
1780:
1776:
1771:
1768:
1763:
1761:
1757:
1753:
1748:
1739:
1735:
1731:
1727:
1723:
1715:
1708:
1706:
1702:
1698:
1694:
1690:
1686:
1685:
1684:
1682:
1679:
1678:
1674:
1671:
1666:
1663:
1662:
1661:
1659:
1656:
1652:
1648:
1646:
1642:
1641:immunotherapy
1639:
1635:
1631:
1628:
1625:
1624:
1623:
1621:
1618:
1617:
1613:
1611:
1607:
1606:immunotherapy
1604:
1600:
1596:
1593:
1590:
1589:
1588:
1586:
1583:
1582:
1578:
1576:
1572:
1571:immunotherapy
1569:
1565:
1561:
1558:
1555:
1554:
1553:
1551:
1550:
1546:
1542:
1538:
1536:
1532:
1531:immunotherapy
1529:
1524:
1520:
1519:
1518:
1516:
1513:
1509:
1505:
1503:
1499:
1494:
1491:
1490:
1489:
1487:
1483:
1480:
1479:
1475:
1472:
1469:
1465:
1461:
1460:
1459:
1457:
1454:
1453:
1449:
1446:
1443:
1439:
1435:
1431:
1430:
1429:
1427:
1424:
1423:
1419:
1416:
1413:
1408:
1405:
1401:
1400:
1399:
1397:
1394:
1393:
1389:
1386:
1382:
1377:
1373:
1372:
1371:
1369:
1366:
1365:
1361:
1359:
1358:B lymphocytes
1355:
1351:
1346:
1344:
1341:
1340:
1339:
1337:
1334:
1333:
1329:
1327:
1326:B-lymphocytes
1323:
1319:
1315:
1311:
1308:
1307:
1306:
1304:
1301:
1300:
1296:
1293:
1289:
1285:
1281:
1277:
1276:
1275:
1273:
1270:
1266:
1262:
1259:
1255:
1251:
1247:
1245:
1241:
1240:
1239:
1237:
1234:
1233:
1229:
1227:
1224:on activated
1223:
1219:
1214:
1210:
1209:
1208:
1206:
1203:
1202:
1198:
1196:
1193:on activated
1192:
1188:
1183:
1179:
1178:
1177:
1175:
1172:
1171:
1167:
1165:
1161:
1158:
1154:
1150:
1147:
1145:
1142:
1140:
1137:
1135:
1132:
1131:
1130:
1128:
1125:
1124:
1120:
1118:
1114:
1110:
1107:
1105:
1102:
1100:
1097:
1095:
1092:
1091:
1090:
1088:
1085:
1084:
1080:
1078:
1074:
1070:
1067:
1065:
1062:
1060:
1057:
1055:
1052:
1051:
1050:
1048:
1045:
1039:
1035:
1032:
1029:
1026:
1024:Main category
1023:
1022:
1019:
1017:
1011:
1004:
996:
994:
992:
988:
984:
980:
976:
973:on activated
972:
968:
964:
960:
956:
952:
948:
944:
940:
936:
932:
924:
919:
916:
914:
911:
909:
906:
904:
903:Pembrolizumab
901:
899:
896:
894:
891:
889:
886:
884:
881:
879:
876:
874:
871:
869:
866:
864:
861:
859:
856:
855:
853:
847:
843:
839:
836:
832:
828:
826:
822:
818:
814:
810:
806:
802:
798:
794:
786:
784:
782:
776:
768:
766:
764:
756:
754:
752:
748:
744:
740:
732:
727:
725:
722:
713:
711:
709:
708:phage display
705:
701:
692:
685:
683:
681:
677:
671:
669:
665:
659:
651:
649:
646:
642:
638:
634:
633:yeast display
630:
629:phage display
626:
622:
618:
610:
608:
606:
601:
597:
593:
591:
588:, first with
587:
583:
579:
575:
570:
569:glycosylation
566:
561:
555:
553:
550:
546:
542:
538:
532:
530:
527:
522:
520:
516:
511:
508:
504:
500:
496:
492:
488:
484:
480:
475:
473:
469:
464:
460:
455:
453:
449:
445:
441:
437:
433:
429:
425:
421:
417:
408:
406:
404:
400:
399:phage display
392:
390:
388:
383:
382:
376:
374:
370:
365:
363:
359:
353:
351:
347:
343:
339:
334:
332:
331:nucleic acids
328:
324:
320:
316:
312:
308:
304:
299:
295:
291:
287:
280:
272:
266:
259:
255:
252:
248:
242:
234:
226:
219:
217:
213:
211:
207:
202:
198:
193:
191:
187:
183:
179:
175:
171:
167:
163:
159:
155:
151:
146:
144:
140:
136:
132:
128:
125:
117:
115:
113:
109:
105:
101:
97:
93:
88:
86:
82:
78:
74:
70:
66:
62:
58:
56:
52:
48:
44:
40:
36:
32:
23:
19:
8350:
8308:
8292:Fab fragment
8263:
8245:
8048:Sibrotuzumab
8013:Parsatuzumab
7998:Otlertuzumab
7993:Odronextamab
7988:Ocaratuzumab
7983:Obinutuzumab
7958:Lumretuzumab
7913:Flotetuzumab
7908:Ficlatuzumab
7903:Farletuzumab
7898:Etaracizumab
7883:Emibetuzumab
7770:Zolbetuximab
7745:Margetuximab
7725:Girentuximab
7587:Minretumomab
7552:Blinatumomab
7491:Teprotumumab
7471:Seribantumab
7296:Botensilimab
7281:Atezolizumab
7266:Adecatumumab
7241:
7154:Domagrozumab
7045:Galcanezumab
7040:Fremanezumab
7025:Bapineuzumab
6982:Gantenerumab
6923:Idarucizumab
6913:Etaracizumab
6898:Caplacizumab
6893:Brolucizumab
6529:Suvratoxumab
6524:Bezlotoxumab
6358:Porgaviximab
6353:Larcaviximab
6259:+casirivimab
6229:+tixagevimab
6210:Bebtelovimab
6200:Bamlanivimab
5966:Risankizumab
5951:Lebrikizumab
5931:Clazakizumab
5921:Anrukinzumab
5900:Tralokinumab
5816:Otelixizumab
5796:
5788:
5787:
5757:Tregalizumab
5742:Tislelizumab
5712:Satralizumab
5692:Rontalizumab
5687:Retifanlimab
5662:Plozalizumab
5642:Pateclizumab
5637:Pascolizumab
5632:Ozoralizumab
5582:Lampalizumab
5572:Inebilizumab
5562:Fontolizumab
5512:Camrelizumab
5507:Benralizumab
5502:Atezolizumab
5488:
5431:Clenoliximab
5332:
5316:Bertilimumab
5311:
5300:Tremelimumab
5280:
5203:Mavrilimumab
5193:Lirentelimab
5188:Lerdelimumab
5163:Fresolimumab
5103:Atorolimumab
5081:
5013:
4815:Inflammation
4800:Alloimmunity
4795:Autoimmunity
4780:Immunity vs.
4732:Autoantibody
4721:
4630:Superantigen
4543:
4493:
4488:Kimball JA.
4456:
4452:
4429:. Retrieved
4425:
4400:. Retrieved
4396:
4387:
4375:. Retrieved
4371:
4362:
4350:. Retrieved
4335:
4323:. Retrieved
4319:
4309:
4274:
4270:
4222:
4218:
4208:
4175:
4169:
4157:. Retrieved
4145:
4135:
4113:. Retrieved
4104:
4092:
4070:. Retrieved
4061:
4027:. Retrieved
4018:
3997:
3967:Pharmacology
3966:
3918:
3885:
3881:
3874:
3855:
3851:
3841:
3822:
3818:
3808:
3775:
3771:
3764:Breedveld FC
3758:
3741:
3737:
3731:
3709:(1): 65–93.
3706:
3702:
3696:
3671:
3667:
3661:
3620:
3616:
3610:
3567:
3563:
3553:
3528:
3524:
3518:
3506:. Retrieved
3502:the original
3497:
3488:
3466:(1): 15–22.
3463:
3459:
3453:
3410:
3406:
3396:
3366:(1): 26–34.
3363:
3359:
3353:
3328:
3324:
3318:
3283:
3279:
3269:
3236:
3232:
3226:
3181:
3177:
3167:
3124:
3120:
3110:
3077:
3073:
3063:
3038:
3034:
3028:
2993:
2989:
2979:
2944:
2940:
2930:
2895:
2891:
2881:
2846:
2842:
2832:
2789:
2785:
2775:
2730:
2726:
2716:
2681:
2677:
2629:
2625:
2590:
2555:
2549:
2506:
2502:
2492:
2447:
2443:
2433:
2398:
2394:
2384:
2349:
2345:
2335:
2292:
2288:
2278:
2268:23 September
2266:. Retrieved
2262:
2214:
2210:
2204:
2171:
2167:
2161:
2126:
2122:
2112:
2071:
2067:
2061:
2018:
2014:
2004:
1979:
1975:
1962:
1950:. Retrieved
1946:the original
1941:
1936:Gelboin HV.
1931:
1844:
1840:Constipation
1799:
1788:
1785:Side effects
1772:
1764:
1751:
1749:
1719:
1632:causing the
1597:causing the
1562:causing the
1547:
1462:approved in
1432:approved in
1402:approved in
1383:targets the
1268:Anti-cancer
1043:inflammatory
1013:
928:
851:
834:
809:radioisotope
790:
778:
760:
739:Western blot
736:
728:Applications
717:
699:
697:
680:cell culture
672:
661:
614:
594:
562:
559:
533:
523:
512:
476:
456:
451:
447:
423:
420:transferrins
412:
409:Purification
396:
379:
377:
366:
354:
342:hypoxanthine
335:
282:
214:
210:Tasuku Honjo
194:
162:paraproteins
147:
135:magic bullet
130:
127:Paul Ehrlich
124:immunologist
121:
112:inflammatory
92:biochemistry
89:
59:
47:cell lineage
38:
34:
30:
28:
18:
8244:Engineered
8181:from market
8156:Ontuxizumab
8130:Ertumaxomab
8125:Catumaxomab
8097:Vanucizumab
8077:+deruxtecan
8073:Trastuzumab
8068:Tigatuzumab
8003:Onartuzumab
7978:Nimotuzumab
7968:Milatuzumab
7938:Labetuzumab
7928:Imgatuzumab
7893:Epcoritamab
7878:Emactuzumab
7863:Dalotuzumab
7853:Dacetuzumab
7833:Cirmtuzumab
7808:Bevacizumab
7793:Alemtuzumab
7765:Ublituximab
7715:Ensituximab
7710:Ecromeximab
7705:Dinutuximab
7690:Carotuximab
7680:Bavituximab
7657:Tositumomab
7652:Tenatumomab
7622:Racotumomab
7567:Edrecolomab
7542:Arcitumomab
7511:Zalutumumab
7501:Vantictumab
7476:Sugemalimab
7466:Robatumumab
7461:Rilotumumab
7456:Ramucirumab
7431:Panitumumab
7406:Necitumumab
7396:Mapatumumab
7391:Lucatumumab
7386:Lexatumumab
7376:Istiratumab
7361:Intetumumab
7346:Flanvotumab
7341:Figitumumab
7326:Dusigitumab
7321:Duligotumab
7311:Daratumumab
7306:Conatumumab
7301:Cixutumumab
7291:Balstilimab
7271:Amivantamab
7184:from market
7138:Trevogrumab
7104:Ranibizumab
7070:Solanezumab
7065:Semorinemab
7060:Refanezumab
7035:Eptinezumab
6938:Vanucizumab
6933:Tadocizumab
6888:Bococizumab
6883:Ranibizumab
6879:Bevacizumab
6856:Volociximab
6807:Ramucirumab
6767:Bentracimab
6737:Circulatory
6694:Romosozumab
6644:Monoclonals
6586:from market
6561:Urtoxazumab
6490:Tefibazumab
6474:Pagibaximab
6440:Raxibacumab
6435:Panobacumab
6396:Palivizumab
6391:Motavizumab
6386:Lenvervimab
6363:Vilobelimab
6324:+cilgavimab
6320:Tixagevimab
6300:Regdanvimab
6295:Regavirumab
6290:Rafivirumab
6265:Libivirumab
6250:Foravirumab
6245:Exbivirumab
6235:Diridavumab
6215:Casirivimab
6204:+etesevimab
6195:Avdoralimab
6185:Atoltivimab
6062:from market
6032:Lemalesomab
6027:Fanolesomab
6022:Besilesomab
5961:Perakizumab
5946:Mirikizumab
5936:Gevokizumab
5926:Bimekizumab
5905:Ustekinumab
5890:Secukinumab
5875:Fezakinumab
5870:Canakinumab
5865:Briakinumab
5840:Interleukin
5793:Dostarlimab
5772:Visilizumab
5767:Vedolizumab
5762:Vatelizumab
5752:Toralizumab
5747:Tocilizumab
5732:Teclistamab
5707:Samalizumab
5697:Rovelizumab
5677:Ravulizumab
5657:Pidilizumab
5652:Pexelizumab
5617:Ocrelizumab
5612:Natalizumab
5602:Mepolizumab
5592:Ligelizumab
5587:Letolizumab
5557:Etrolizumab
5547:Epratuzumab
5517:Cedelizumab
5471:Vapaliximab
5466:Teneliximab
5456:Lumiliximab
5441:Gomiliximab
5426:Basiliximab
5398:Vepalimomab
5383:Nerelimomab
5368:Gavilimomab
5363:Faralimomab
5358:Elsilimomab
5333:Combination
5326:Zanolimumab
5321:Ontamalimab
5269:Ulocuplumab
5264:Tezepelumab
5254:Sifalimumab
5234:Pamrevlumab
5213:Morolimumab
5208:Metelimumab
5178:Lanadelumab
5113:Avdoralimab
5098:Anifrolumab
4938:Lymphocytes
4597:Lymphocytic
4426:MedicineNet
4397:Mayo Clinic
4159:21 December
4072:10 February
4029:21 November
3998:AdisInsight
3508:17 November
2395:Proceedings
1851:Anaphylaxis
1791:bevacizumab
1728:were given
1689:coagulation
1658:palivizumab
1630:coronavirus
1595:coronavirus
1560:coronavirus
1523:hepatitis C
1515:bavituximab
1486:ranibizumab
1482:bevacizumab
1456:panitumumab
1396:nimotuzumab
1368:trastuzumab
1303:alemtuzumab
1174:basiliximab
1157:interleukin
1127:ustekinumab
1030:Application
963:Basiliximab
918:Trastuzumab
908:Ranibizumab
898:Panitumumab
873:Dostarlimab
863:Bevacizumab
858:Alemtuzumab
821:Fab regions
741:and immuno
625:CRISPR/Cas9
617:recombinant
611:Recombinant
590:anion beads
565:deamidation
526:protein A/G
403:recombinant
327:auxotrophic
315:aminopterin
249:cell and a
188:shared the
176:. In 1975,
168:. In 1973,
150:lymphocytes
131:Zauberkugel
81:plasma cell
8535:Immunology
8509:Categories
8352:bispecific
8310:bispecific
8265:bispecific
8102:Veltuzumab
8081:+emtansine
8053:Simtuzumab
8018:Pertuzumab
7948:Lintuzumab
7923:Glofitamab
7873:Elotuzumab
7858:Demcizumab
7798:Axatilimab
7788:Abituzumab
7760:Siltuximab
7735:Isatuximab
7675:Amatuximab
7647:Pintumomab
7617:Pemtumomab
7612:Oregovomab
7547:Bectumomab
7527:Abagovomab
7481:Tarextumab
7451:Radretumab
7446:Pritumumab
7436:Patritumab
7426:Olaratumab
7421:Ofatumumab
7411:Nesvacumab
7401:Narnatumab
7371:Iratumumab
7366:Ipilimumab
7336:Enoticumab
7316:Drozitumab
7244:for tumors
7133:Bimagrumab
7050:Ozanezumab
7030:Crenezumab
7009:Opicinumab
7004:Fulranumab
6972:Aducanumab
6952:Neurologic
6908:Emicizumab
6903:Demcizumab
6797:Nesvacumab
6792:Inclacumab
6782:Evolocumab
6777:Evinacumab
6772:Enoticumab
6757:Alirocumab
6752:Abelacimab
6723:Stamulumab
6689:Blosozumab
6456:Edobacomab
6381:Felvizumab
6315:Suptavumab
6310:Sotrovimab
6285:Pemivibart
6280:Odesivimab
6275:Nirsevimab
6270:Maftivimab
6240:Etesevimab
6225:Cilgavimab
6219:+imdevimab
5992:Lokivetmab
5985:Veterinary
5971:Spesolimab
5956:Olokizumab
5941:Ixekizumab
5885:Guselkumab
5880:Fletikumab
5860:Brazikumab
5855:Bermekimab
5826:Sutimlimab
5800:Ibalizumab
5737:Teplizumab
5717:Siplizumab
5702:Ruplizumab
5682:Reslizumab
5672:Quilizumab
5627:Omalizumab
5577:Itolizumab
5567:Frexalimab
5542:Efalizumab
5537:Eculizumab
5532:Daclizumab
5497:Aselizumab
5492:Apolizumab
5461:Priliximab
5446:Infliximab
5388:Odulimomab
5378:Maslimomab
5373:Inolimomab
5353:Afelimomab
5290:Durvalumab
5285:Ipilimumab
5274:Varlilumab
5244:Relatlimab
5239:Placulumab
5183:Lenzilumab
5158:Emapalumab
5143:Cemiplimab
5128:Brodalumab
5123:Bleselumab
5093:Adalimumab
4979:Substances
4843:Peripheral
4831:Inaction:
4710:Antibodies
4691:Macrophage
4604:complement
3498:LMB Alumni
3178:Antibodies
2632:(1): 1–5.
2346:Immunology
2257:Marks LV.
1968:Gelboin HV
1923:References
1889:Stomatitis
1775:pemivibart
1756:sotrovimab
1732:by the US
1701:GpIIb/IIIa
1675:humanized
1645:SARS-CoV-2
1627:SARS-CoV-2
1620:Sotrovimab
1610:SARS-CoV-2
1592:SARS-CoV-2
1575:SARS-CoV-2
1557:SARS-CoV-2
1544:Anti-viral
1533:, targets
1506:humanized
1420:humanized
1390:humanized
1330:humanized
1297:humanized
1272:gemtuzumab
1263:humanized
1236:omalizumab
1230:humanized
1205:daclizumab
1087:adalimumab
1047:infliximab
1008:See also:
983:Omalizumab
967:daclizumab
939:adalimumab
935:infliximab
893:Ofatumumab
883:Ipilimumab
745:tests. In
645:amino acid
567:products,
459:filtration
444:endotoxins
338:HAT medium
319:folic acid
254:lymphocyte
220:Production
174:hybridomas
114:diseases.
83:lineages.
65:monovalent
61:Monoclonal
8460:nanoCLAMP
8435:Anticalin
8430:Alphabody
8392:Intrabody
8191:Phase III
8179:Withdrawn
7973:Naxitamab
7963:Matuzumab
7780:Humanized
7755:Rituximab
7720:Futuximab
7695:Cetuximab
7632:Solitomab
7592:Mitumomab
7582:Lilotomab
7562:Detumomab
7506:Votumumab
7496:Tovetumab
7416:Nivolumab
7381:Icrucumab
7351:Ganitumab
7194:Phase III
7182:Withdrawn
7147:Humanized
7097:Humanized
7075:Tanezumab
7055:Ponezumab
7018:Humanized
6999:Fasinumab
6987:Lecanemab
6977:Donanemab
6918:Faricimab
6866:Humanized
6851:Abciximab
6833:Imciromab
6828:Biciromab
6812:Rinucumab
6802:Orticumab
6787:Icrucumab
6682:Humanized
6673:Denosumab
6668:Burosumab
6596:Phase III
6584:Withdrawn
6554:Humanized
6519:Actoxumab
6483:Humanized
6430:Nebacumab
6415:Bacterial
6401:Suvizumab
6373:Humanized
6330:Tuvirumab
6305:Sevirumab
6255:Imdevimab
6180:Ansuvimab
6151:Efungumab
6072:Phase III
6060:Withdrawn
6037:Sulesomab
5914:Humanized
5895:Sirukumab
5727:Talizumab
5552:Erlizumab
5481:Humanized
5451:Keliximab
5436:Galiximab
5295:Nivolumab
5259:Tabalumab
5249:Sarilumab
5223:Oleclumab
5218:Namilumab
5198:Lirilumab
5173:Ianalumab
5168:Golimumab
5153:Eldelumab
5148:Dupilumab
5118:Belimumab
5088:Abrilumab
4996:Cytolysin
4986:Cytokines
4833:Tolerance
4782:tolerance
4701:Immunogen
4200:245442677
4154:0190-8286
4115:6 January
3570:: 33878.
3200:2073-4468
3184:(4): 73.
3141:1942-0862
3094:1520-6033
2035:2049-0801
1904:Mucositis
1871:Hepatitis
1828:Back pain
1811:Allergies
1808:Headaches
1805:Dizziness
1795:cetuximab
1709:chimeric
1705:platelets
1681:abciximab
1539:chimeric
1525:infection
1500:inhibits
1473:inhibitor
1450:chimeric
1447:inhibitor
1426:cetuximab
1417:inhibitor
1362:chimeric
1336:rituximab
1278:relapsed
1250:urticaria
1220:inhibits
1213:rejection
1199:chimeric
1189:inhibits
1182:rejection
1115:inhibits
1081:chimeric
1075:inhibits
979:rejection
913:Rituximab
888:Nivolumab
868:Cetuximab
825:Fc region
781:apoptosis
582:protein A
489:exchange
485:. Either
440:cytokines
432:nucleases
428:proteases
387:unethical
350:hybridoma
346:thymidine
258:hybridoma
195:In 1988,
53:a unique
8499:COVID-19
8487:Medicine
8455:Monobody
7667:Chimeric
7577:Igovomab
6994:Erenumab
6843:Chimeric
6538:Chimeric
6466:Chimeric
6340:Chimeric
5413:Chimeric
5305:Urelumab
5228:Oxelumab
5138:Carlumab
5108:Avelumab
5057:for the
4946:Cellular
4790:Immunity
4788:Action:
4771:Paratope
4759:Idiotype
4749:Allotype
4717:Antibody
4671:Mimotope
4635:Allergen
4618:Antigens
4611:Lymphoid
4528:Archived
4481:31686050
4431:19 April
4402:19 April
4377:19 April
4346:Archived
4301:35713300
4249:34473343
4192:34937889
3931:Archived
3910:10169378
3902:11905803
3800:43781004
3792:10703815
3750:29461857
3688:11287236
3602:27667400
3545:10831134
3525:Placenta
3480:11889896
3445:31489367
3380:19131992
3345:21700290
3310:20818176
3261:29689097
3253:20414207
3218:36412839
3159:30543482
3102:27452958
3055:19075686
3020:24030440
2971:19103878
2922:19247287
2873:17510706
2824:43459065
2816:12920303
2767:24503933
2727:PLOS ONE
2708:20635390
2656:30101214
2582:16761719
2541:25996440
2509:: 9928.
2425:17948113
2376:30320408
2239:45615711
2231:11623041
2104:19615695
2053:25568796
1996:10542439
1911:See also
1855:Bleeding
1837:Insomnia
1815:Diarrhea
1752:in vitro
1738:COVID-19
1716:COVID-19
1687:prevent
1521:cancer,
1406:, Glioma
1385:HER2/neu
1376:HER2/neu
1292:leukemia
969:inhibit
933:include
813:cytokine
797:antigens
743:dot blot
676:chimeric
670:(HAMA).
545:cysteine
472:dialysis
452:in vitro
416:hormones
381:in vitro
362:dot blot
201:humanize
100:medicine
49:made by
43:antibody
41:) is an
8473:Portals
8425:Affitin
8420:Affimer
8415:Affilin
6007:lesions
5782:TGN1412
5667:PRO 140
4991:Opsonin
4970:NK cell
4958:Humoral
4838:Central
4805:Allergy
4754:Isotype
4654:Epitope
4625:Antigen
4461:Bibcode
4352:8 April
4325:8 April
4292:9205158
4240:8411904
3723:7494109
3653:4311503
3645:6095115
3625:Bibcode
3593:5036187
3572:Bibcode
3436:6713500
3415:Bibcode
3388:5523554
3301:3011216
3209:9703962
3150:6380400
3011:4844175
2962:2626668
2913:2750034
2864:1866247
2794:Bibcode
2786:Science
2758:3913575
2735:Bibcode
2699:2978266
2647:6086361
2532:4440525
2511:Bibcode
2484:7568130
2452:Bibcode
2416:2014809
2367:6187215
2327:4335569
2319:3127726
2297:Bibcode
2196:4171375
2188:4200460
2153:7934322
2144:1036884
2096:2047874
2076:Bibcode
2068:Science
2044:4284445
1952:2 April
1825:Itching
1742:US$ 2.9
1226:T cells
1195:T cells
1155:blocks
975:T cells
637:viruses
621:cloning
541:peptide
537:agarose
521:times.
519:elution
507:albumin
448:in vivo
436:viruses
424:in vivo
375:fluid.
373:ascites
290:myeloma
247:myeloma
166:antigen
158:B-cells
118:History
73:antigen
69:epitope
51:cloning
8445:DARPin
8440:Avimer
8300:F(ab')
8174:WHO-EM
7177:WHO-EM
6579:WHO-EM
6136:Fungal
6128:toxins
6055:WHO-EM
5797:Other:
5014:"-mab"
4963:B cell
4951:T cell
4696:B cell
4659:Linear
4647:Hapten
4541:about
4515:(MeSH)
4479:
4453:Nature
4299:
4289:
4247:
4237:
4198:
4190:
4176:Nature
4152:
3973:
3908:
3900:
3852:Immuno
3798:
3790:
3772:Lancet
3748:
3721:
3686:
3651:
3643:
3617:Nature
3600:
3590:
3543:
3478:
3443:
3433:
3386:
3378:
3343:
3308:
3298:
3259:
3251:
3216:
3206:
3198:
3157:
3147:
3139:
3100:
3092:
3053:
3018:
3008:
2969:
2959:
2920:
2910:
2871:
2861:
2822:
2814:
2765:
2755:
2706:
2696:
2654:
2644:
2602:
2580:
2570:
2539:
2529:
2482:
2472:
2423:
2413:
2374:
2364:
2325:
2317:
2289:Nature
2237:
2229:
2194:
2186:
2168:Nature
2151:
2141:
2102:
2094:
2051:
2041:
2033:
1994:
1881:Anemia
1877:Cancer
1745:
1649:human
1614:human
1579:human
1484:&
1476:human
1294:cells
1260:(IgE)
1244:asthma
1211:acute
1180:acute
1168:human
1121:human
1016:CiteAb
991:asthma
793:cancer
664:murine
487:cation
479:anions
286:fusing
104:cancer
98:, and
7520:Mouse
7259:Human
7251:Tumor
7126:Human
6960:Human
6821:Mouse
6745:Human
6716:Human
6661:Human
6512:Human
6504:Toxin
6449:Mouse
6423:Human
6173:Human
6165:Viral
6144:Human
6015:Mouse
5848:Human
5346:Mouse
5312:Other
5075:Human
4196:S2CID
4109:(FDA)
4105:U.S.
4101:(PDF)
4066:(FDA)
4062:U.S.
4023:(FDA)
4019:U.S.
3906:S2CID
3796:S2CID
3649:S2CID
3384:S2CID
3257:S2CID
2820:S2CID
2475:40982
2323:S2CID
2235:S2CID
2192:S2CID
2100:S2CID
1822:Fever
1819:Cough
1654:Other
1164:IL-23
1160:IL-12
1117:TNF-α
1077:TNF-α
1041:Anti-
1036:Mode
959:TNF-α
838:ADEPT
805:toxin
700:fully
641:yeast
627:, or
358:ELISA
251:mouse
8248:and
6653:Bone
6126:and
6122:for
4602:and
4477:PMID
4433:2018
4404:2018
4379:2018
4354:2024
4327:2024
4297:PMID
4275:2022
4245:PMID
4223:2021
4188:PMID
4161:2021
4150:ISSN
4117:2022
4074:2021
4031:2020
3971:ISBN
3898:PMID
3788:PMID
3746:PMID
3719:PMID
3684:PMID
3641:PMID
3598:PMID
3541:PMID
3510:2012
3476:PMID
3441:PMID
3376:PMID
3341:PMID
3329:1218
3306:PMID
3280:mAbs
3249:PMID
3214:PMID
3196:ISSN
3155:PMID
3137:ISSN
3121:mAbs
3098:PMID
3090:ISSN
3051:PMID
3016:PMID
2967:PMID
2918:PMID
2869:PMID
2812:PMID
2763:PMID
2704:PMID
2652:PMID
2600:ISBN
2578:PMID
2568:ISBN
2537:PMID
2480:PMID
2421:PMID
2372:PMID
2315:PMID
2270:2020
2227:PMID
2184:PMID
2149:PMID
2092:PMID
2049:PMID
2031:ISSN
1992:PMID
1954:2018
1793:and
1758:and
1724:and
1502:VEGF
1471:EGFR
1445:EGFR
1415:EGFR
1354:CD20
1324:and
1318:CD52
1288:CD33
1222:IL-2
1191:IL-2
1162:and
1027:Type
971:IL-2
965:and
953:and
937:and
842:ADCC
714:Cost
576:and
418:and
329:for
208:and
180:and
39:moAb
4924:HLA
4920:MHC
4469:doi
4457:575
4287:PMC
4279:doi
4235:PMC
4227:doi
4180:doi
3890:doi
3860:doi
3827:doi
3780:doi
3776:355
3711:doi
3676:doi
3633:doi
3621:312
3588:PMC
3580:doi
3533:doi
3468:doi
3431:PMC
3423:doi
3368:doi
3333:doi
3296:PMC
3288:doi
3241:doi
3204:PMC
3186:doi
3145:PMC
3129:doi
3082:doi
3043:doi
3006:PMC
2998:doi
2957:PMC
2949:doi
2945:206
2908:PMC
2900:doi
2859:PMC
2851:doi
2847:117
2802:doi
2790:301
2753:PMC
2743:doi
2694:PMC
2686:doi
2682:128
2642:PMC
2634:doi
2560:doi
2527:PMC
2519:doi
2470:PMC
2460:doi
2411:PMC
2403:doi
2362:PMC
2354:doi
2350:155
2305:doi
2293:332
2219:doi
2176:doi
2172:244
2139:PMC
2131:doi
2084:doi
2072:252
2039:PMC
2023:doi
1984:doi
1703:on
1691:in
1665:RSV
1356:on
1320:on
1290:on
639:or
549:KLH
470:or
450:or
317:(a
133:– "
35:mAb
8511::
8187::
8079:/
7190::
6592::
6068::
5791::
5335::
5314::
5283::
5086::
4684::
4492:.
4475:.
4467:.
4455:.
4424:.
4412:^
4395:.
4370:.
4318:.
4295:.
4285:.
4273:.
4269:.
4257:^
4243:.
4233:.
4221:.
4217:.
4194:.
4186:.
4178:.
4148:.
4144:.
4103:.
4060:.
4049:^
4017:.
4006:^
3996:.
3985:^
3941:^
3904:.
3896:.
3884:.
3854:.
3850:.
3823:29
3821:.
3817:.
3794:.
3786:.
3774:.
3770:.
3742:24
3740:.
3717:.
3707:13
3705:.
3682:.
3672:12
3670:.
3647:.
3639:.
3631:.
3619:.
3596:.
3586:.
3578:.
3566:.
3562:.
3539:.
3529:21
3527:.
3496:.
3474:.
3462:.
3439:.
3429:.
3421:.
3409:.
3405:.
3382:.
3374:.
3364:27
3362:.
3339:.
3327:.
3304:.
3294:.
3282:.
3278:.
3255:.
3247:.
3237:10
3235:.
3212:.
3202:.
3194:.
3182:11
3180:.
3176:.
3153:.
3143:.
3135:.
3125:11
3123:.
3119:.
3096:.
3088:.
3078:32
3076:.
3072:.
3049:.
3037:.
3014:.
3004:.
2992:.
2988:.
2965:.
2955:.
2943:.
2939:.
2916:.
2906:.
2894:.
2890:.
2867:.
2857:.
2845:.
2841:.
2818:.
2810:.
2800:.
2788:.
2784:.
2761:.
2751:.
2741:.
2729:.
2725:.
2702:.
2692:.
2680:.
2676:.
2664:^
2650:.
2640:.
2628:.
2624:.
2610:^
2576:.
2566:.
2535:.
2525:.
2517:.
2505:.
2501:.
2478:.
2468:.
2458:.
2448:92
2446:.
2442:.
2419:.
2409:.
2399:20
2397:.
2393:.
2370:.
2360:.
2348:.
2344:.
2321:.
2313:.
2303:.
2291:.
2287:.
2261:.
2247:^
2233:.
2225:.
2215:25
2213:.
2190:.
2182:.
2170:.
2147:.
2137:.
2127:38
2125:.
2121:.
2098:.
2090:.
2082:.
2070:.
2047:.
2037:.
2029:.
2017:.
2013:.
1990:.
1980:20
1978:.
1974:.
1940:.
1436:,
1322:T-
993:.
961:.
949:,
945:,
827:.
811:,
807:,
765:.
706:,
623:,
495:pH
474:.
430:,
389:.
296:.
260:).
94:,
29:A
8475::
8302:2
8237:e
8230:t
8223:v
8083:)
8075:(
7287:)
7283:(
7234:e
7227:t
7220:v
6881:/
6636:e
6629:t
6622:v
6326:)
6322:(
6261:)
6257:(
6231:)
6227:(
6221:)
6217:(
6206:)
6202:(
6191:)
6187:(
6112:e
6105:t
6098:v
5623:)
5619:(
5230:§
5047:e
5040:t
5033:v
5016:)
4922:/
4680:/
4589:e
4582:t
4575:v
4496:.
4483:.
4471::
4463::
4435:.
4406:.
4381:.
4356:.
4329:.
4303:.
4281::
4251:.
4229::
4202:.
4182::
4163:.
4130:.
4119:.
4087:.
4076:.
4044:.
4033:.
3979:.
3912:.
3892::
3886:1
3868:.
3862::
3856:3
3835:.
3829::
3802:.
3782::
3752:.
3725:.
3713::
3690:.
3678::
3655:.
3635::
3627::
3604:.
3582::
3574::
3568:6
3547:.
3535::
3512:.
3482:.
3470::
3464:9
3447:.
3425::
3417::
3411:5
3390:.
3370::
3347:.
3335::
3312:.
3290::
3284:2
3263:.
3243::
3220:.
3188::
3161:.
3131::
3104:.
3084::
3057:.
3045::
3039:9
3022:.
3000::
2994:8
2973:.
2951::
2924:.
2902::
2896:4
2875:.
2853::
2826:.
2804::
2796::
2769:.
2745::
2737::
2731:9
2710:.
2688::
2658:.
2636::
2630:1
2584:.
2562::
2543:.
2521::
2513::
2507:5
2486:.
2462::
2454::
2427:.
2405::
2378:.
2356::
2329:.
2307::
2299::
2272:.
2241:.
2221::
2198:.
2178::
2155:.
2133::
2106:.
2086::
2078::
2055:.
2025::
2019:3
1998:.
1986::
1956:.
1005:.
674:"
631:/
33:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.