1134:) may be reported following treatment. In clinical trials, lymphocyte levels above Grade 0 (≥1000 cells/mm) and Grade 1 (<1000–800 cells/mm) were maintained in most patients, with levels continuing to improve after the 2-year dosing period. Less than 1% of patients developed Grade 4 lymphopenia (<200 cells/mm). It is important that patients with lymphocyte counts below 500 cells/mm should be actively monitored for signs suggestive of infection and that anti-infective treatments are given to at-risk patients.
645:
622:
919:
cells. This helps to explain which B cells are more vulnerable to cladribine-mediated apoptosis. DCK is the rate-limiting enzyme for conversion of the cladribine prodrug into its active triphosphate form, leading to the selective depletion of dividing and non-dividing T and B lymphocytes. In contrast, the DCK:5'-NT ratio is relatively low in other cell types, thus sparing numerous non-hematologic cells.
42:
1097:
cladribine tablets (3.5 mg/kg) in the CLARITY study had a 58% reduction in annualized relapse rate and 47% of patients showed no evidence of disease activity at 2 years. Clinical improvements can be observed at Week 24 of treatment, and benefits may be sustained up to 4 years, beyond the 2-year dosing period and recovery of total lymphocytes.
7301:
1018:
multiple sclerosis due to his sister having the disease, initiated a very productive collaboration. Ortho-Clinical, a subsidiary of
Johnson & Johnson, filed a new drug application for cladribine for multiple sclerosis in 1997 but withdrew it in the late 1990s after discussion with the FDA proved that more clinical data would be needed.
1144:
has been reported in patients with hairy cell leukemia treated with parenteral cladribine. However, in up to 10 years of follow-up of patients receiving cladribine tablets for multiple sclerosis, no cases of progressive multifocal leukoencephalopathy have been observed; baseline MRI must be performed
946:
and recognised that because the lack of adenosine deaminase led to the destruction of B cell lymphocytes, a drug designed to inhibit adenosine deaminase might be useful in lymphomas. Carson then synthesised cladribine, and through clinical research at
Scripps starting in the 1980s, Beutler tested it
1129:
with minimal impact on innate immune cells. Although the exact mechanism by which cladribine exerts its therapeutic effect is not fully elucidated, it is proposed to have a transient effect on B and T lymphocyte depletion, interrupting the cascade of immune events central to multiple sclerosis. As a
1072:
Cladribine tablets are administered as 2 courses separated by 1 year (a maximum of 20 days of treatment). The recommended cumulative dose is 3.5 mg/kg weight over 2 years, administered as 1 treatment course of 1.75 mg/kg per year. Each treatment course consists of 2 treatment weeks, one at
1137:
Despite the initial reduction in lymphocyte counts following treatment, studies showed the overall risk of infection in patients receiving cladribine tablets was comparable to those who received placebo, except for herpes zoster infection. Due to this increased risk, it is recommended that patients
1047:
Based on the supporting data from the completed clinical trials that confirmed no increased risk of cancer, Merck announced it would again seek regulatory approval. In 2016, the EMA accepted its application for review. On 22 June 2017, the EMA's
Committee for Medicinal Products for Human Use (CHMP)
918:
Accumulation of cladribine into cells is dependent on the ratio of DCK and 5'-NT. This ratio differs between cell types, with high levels in T and B lymphocytes, making them particularly susceptible to cell death. The cells with the highest ratios are B cells, especially germinal centre and naïve B
1104:
Further analyses of a subgroup of patients in the CLARITY study who had very active multiple sclerosis showed a 67% reduction in relapse rates and an 82% reduction in disability progression in those treated with cladribine tablets. Similarly, clinical improvements were seen in lesion burden on MRI
1080:
with cladribine tablets, patients who are antibody-negative for varicella zoster virus are recommended to be vaccinated before starting treatment. Treatment should not be initiated within 4 to 6 weeks of receiving a live or attenuated live vaccine because of a risk of active infection. Vaccination
922:
In multiple sclerosis, cladribine's effectiveness may be due to depletion of B cells, in particular memory B cells. In the pivotal phase 3 clinical trial of oral cladribine in multiple sclerosis, CLARITY, cladribine selectively depleted 80% of peripheral B cells, compared to only 40–45% of CD4+ T
874:
Following EMA approval of cladribine tablets for the treatment of adult patients with highly active relapsing-remitting multiple sclerosis in 2017, as of July 2020, cladribine tablets have gained marketing authorisation in over 75 countries. In 2019, cladribine tablets were approved by the FDA for
3073:
926:
Although cladribine is selective for B cells, the long-term suppression of memory B cells, which may contribute to its effect in multiple sclerosis, is not explained by gene or protein expression. Instead, cladribine appears to deplete the entire B cell department, but while naïve B cells rapidly
894:
As a purine analogue, cladribine is taken up into rapidly proliferating cells, including B and T lymphocytes, to be incorporated into DNA synthesis. Chemically, it mimics nucleoside adenosine; however, unlike adenosine, cladribine has a chlorine molecule at position 2, which renders it partially
858:
Cladribine is used as a first- and second-line treatment for symptomatic hairy cell leukemia and for B-cell chronic lymphocytic leukaemia, and is administered by intravenous or subcutaneous infusion. Some investigators have used the parenteral formulation orally to treat patients with hairy cell
1060:
As per the EU label, cladribine tablets are indicated for the treatment of adult patients with highly active relapsing multiple sclerosis as defined by clinical or imaging features: (i) patients with a relapse in the previous year and at least one T1 Gd+ lesion or 9 or more T2 lesions, while on
1043:
The ratio of benefit to harm was not clear to regulators, and further studies were requested to address concerns related to severe lymphopenia and cancer cases observed during pivotal trials. Clinical studies of multiple sclerosis were still ongoing at the time of the rejections, and Merck KGaA
1017:
In the mid-1990s, Beutler, in collaboration with Jack Sipe, a neurologist at
Scripps Institute, ran several clinical trials exploring the utility of cladribine in multiple sclerosis, based on the drug's immunosuppressive effects. Sipe's insight into multiple sclerosis, and Beutler's interest in
843:(5’-NT), which breaks down and inactivates the compound. This ratio differs between cell types, with high levels in T and B lymphocytes, resulting in selective targeting of these cells. In contrast, DCK:5'NT is relatively low in other cell types, thus sparing numerous non-haematological cells.
1096:
Clinical trial results have shown that cladribine tablets can be an effective treatment for highly active, relapsing forms of multiple sclerosis, with significant clinical benefits in relapse rate, disability progression, and radiological measures. Compared with placebo, patients who received
914:
Another family of enzymes, the 5'-nucleotidase (5'-NT) family, is also capable of dephosphorylating cladribine, making it inactive. The most important subtypes of this group appear to be cytosolic 5'-NT, c-5NCT1A and c-NT1B, which are cytosolically active and specific for purine analogues.
1148:
In clinical trials, malignancies were observed more frequently in patients treated with cladribine tablets compared with patients who received placebo. Compared with a matched reference population from the Global Cancer
Observatory database, cladribine tablets had no increased risk of
898:
Cladribine is taken up by specific nucleoside transporter proteins. Once inside a cell, cladribine undergoes phosphorylation by the enzyme deoxycytidine kinase (DCK) to produce mononucleotide 2-chlorodeoxyadenosine 5’monophosphate (2-CdAMP), which is subsequently phosphorylated to the
2860:
Giovannoni G, Cook S, Rammohan K, Rieckmann P, Sørensen PS, Vermersch P, et al. (April 2011). "Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis".
1112:
Furthermore, treatment with cladribine tablets has been shown to significantly reduce the rate of brain atrophy in patients with highly active relapsing-remitting multiple sclerosis. This reduction correlated with a reduced risk in disability progression in a retrospective analysis.
1068:
and alternatively, immune reconstitution therapy. Classified as the latter, cladribine tablets are administered intermittently as a short treatment course without continuous immunosuppression. In contrast to maintenance therapies, clinical efficacy extends beyond the dosing period.
835:(DCK) to produce mononucleotide 2-chlorodeoxyadenosine 5’monophosphate (2-CdAMP), which is subsequently phosphorylated to the triphosphorylated active compound 2-chlorodeoxyadenosine 5’triphosphate (2-CdATP). Activated cladribine is incorporated into cellular DNA, which triggers
1108:
Studies evaluating the treatment effects of cladribine tablets across a spectrum of baseline demographics and disease characteristics showed that the relative risk of relapse was significantly reduced compared with placebo, irrespective of previous treatment experience.
1073:
the beginning of the first month and one at the beginning of the second month of the respective treatment year. Each treatment week consists of 4 or 5 days on which a patient receives 10 mg or 20 mg (1 or 2 tablets) as a single daily dose based on body weight.
951:, so Beutler's lab synthesised and packaged it and supplied it to the hospital pharmacy; the laboratory also developed a test to monitor blood levels. This was the first treatment that led to prolonged remission of hairy cell leukemia, which was previously untreatable.
3081:
1051:
Cladribine tablets were later approved in Europe, in August 2017, for highly active relapsing-remitting multiple sclerosis, and has since been approved by the FDA for the treatment of relapsing-remitting and secondary progressive multiple sclerosis in the US.
1044:
committed to completing them. A meta-analysis of data from clinical trials comparing the risk of cancer and other disease-modifying therapies showed that cladribine tablets did not increase the risk of cancer at the doses used in the initial clinical trials.
1116:
In clinical trials, higher cumulative doses of cladribine tablets did not result in further improvement in efficacy nor did additional courses after the 2-year treatment period, but was associated with a higher incidence of Grade 3 and Grade 4 lymphopenia.
3180:
Rammohan K, Giovannoni G, Comi G, Cook S, Rieckmann P, Soelberg Sørensen P, et al. (January 2012). "Cladribine tablets for relapsing-remitting multiple sclerosis: Efficacy across patient subgroups from the phase III CLARITY study".
1138:
are screened for varicella zoster virus and antibody-negative patients are vaccinated prior to receiving treatment. In an analysis of post-approval data, as of 2020, no new infection safety signals were observed in over 18,000 patients.
831:, which causes it to accumulate in targeted cells and interfere with the cell's ability to process DNA. Cladribine is taken up by cells via transporter proteins. Once inside a cell, cladribine undergoes phosphorylation by the enzyme
902:
Activated cladribine is incorporated into the DNA synthesis pathway, where it disrupts DNA repair and synthesis, resulting in an accumulation of DNA strand breaks This is followed by the activation of transcription factor
1087:
The use of cladribine tablets is contraindicated in pregnant women, and women of childbearing potential must use effective contraception to prevent pregnancy during treatment and 6 months after receiving the last dose.
2531:
2229:
1442:
3800:
3056:
Giovannoni G (2017). "Effect of cladribine tablets on relapse rates and the proportions qualified relapse-free in patients with multiple sclerosis: analysis of the CLARITY and CLARITY extension studies".
375:
Approximately 10 hours after both intravenous infusion and subcutaneous bolus injection ranging from 5.6 to 7.6 hours and 18.4 to 19.7 hours after oral administration, indicative of different elimination
2550:
2393:
251:
927:
move from lymphoid organs, the memory B cell pool repopulates slowly from the bone marrow. Both hairy cell leukemia and B-cell chronic lymphocytic leukaemia are types of B cell blood cancers.
3074:"An exploratory analysis of the efficacy of cladribine tablets 3.5mg/kg in patients with relapsing multiple sclerosis stratified according to age above and below 45 years in the CLARITY study"
3891:
947:
as intravenous infusion and found it was especially useful to treat hairy cell leukemia. No pharmaceutical companies were interested in selling the drug because hairy cell leukemia was an
2193:
3457:
7351:
2918:
Comi G, Cook SD, Giovannoni G, Rammohan K, Rieckmann P, Sørensen PS, et al. (April 2013). "MRI outcomes with cladribine tablets for multiple sclerosis in the CLARITY study".
3601:
911:
from mitochondria and eventual programmed cell death (apoptosis). This process occurs over approximately 2 months, with a peak level of cell depletion 4–8 weeks after treatment.
183:
1930:
Beutler E, Piro LD, Saven A, Kay AC, McMillan R, Longmire R, et al. (1991). "2-Chlorodeoxyadenosine (2-CdA): A Potent
Chemotherapeutic and Immunosuppressive Nucleoside".
1241:
923:
cells and 15‒30% CD8+ T cells. More recently, cladribine has been shown to induce long term, selective suppression of certain subtypes of B cells, especially memory B cells.
717:
4137:
3790:
1021:
Ivax acquired the rights for oral administration of cladribine to treat multiple sclerosis from
Scripps in 2000, and partnered with Serono in 2002. Ivax was acquired by
2168:
1081:
with live or attenuated live vaccines should also be avoided during and after treatment, but can be considered when lymphocyte counts have recovered to ≥1000 cells/mm.
2240:
1009:. At the dosage used to treat hairy cell leukemia in two clinical trials, 16% of people had rashes and 22% had nausea, the nausea generally did not lead to vomiting.
7341:
2741:"Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study"
1450:
3886:
3232:"Reduced brain atrophy rates are associated with lower risk of disability progression in patients with relapsing multiple sclerosis treated with cladribine tablets"
2513:
879:
disease, in adult patients who have had an inadequate response to, or are unable to tolerate, an alternate drug indicated for the treatment of multiple sclerosis.
72:
5869:
4837:
731:
3450:
3119:"Efficacy of Cladribine Tablets in high disease activity subgroups of patients with relapsing multiple sclerosis: A post hoc analysis of the CLARITY study"
1835:"Cladribine Treatment Improved Homocysteine Metabolism and Increased Total Serum Antioxidant Activity in Secondary Progressive Multiple Sclerosis Patients"
859:
leukemia. About 37–51% of oral cladribine is bioavailable orally. It is used, often in combination with other cytotoxic agents, to treat various kinds of
1141:
2404:
3838:
2339:
6785:
3942:
3475:
2975:
Schippling S (2018). "CLARITY: An analysis of severity and frequency of relapses in patients with RRMS treated with cladribine tablets or placebo".
1061:
another disease-modifying therapies or (ii) patients with two or more relapses in the previous year, whether on disease-modifying treatment or not.
895:
resistant to breakdown by adenosine deaminase. This causes it to accumulate in cells and interfere with the targeted cell's ability to process DNA.
1040:
in 2009, which was rejected in 2010, and an appeal was denied in 2011. Likewise Merck KGaA's new drug application with the FDA rejected in 2011.
2204:
3990:
3947:
3823:
3770:
3443:
3425:
3347:
3279:
3216:
3166:
3103:
3042:
2961:
2904:
2843:
2788:
2499:
2033:
1965:
1048:
adopted a positive opinion, recommending the granting of a marketing authorisation for the treatment of relapsing forms of multiple sclerosis.
806:
1769:
The combination of cytarabine and cladribine is the current standard for second-line therapy of refractory cases with vital organ dysfunction.
5813:
4130:
3957:
3762:
1659:
The selection and use of essential medicines 2023: web annex A: World Health
Organization model list of essential medicines: 23rd list (2023)
1076:
Before initiating treatment with cladribine tablets, blood tests, MRI and infection screening must be performed. Due to an increased risk of
847:
2321:
3922:
3848:
3808:
5420:
798:
1530:
4572:
4528:
4406:
969:
The subcutaneous formulation was developed in
Switzerland in the early 1990s and it was commercialised by Lipomed GmbH in the 2000s.
7346:
6728:
6098:
4331:
751:
3881:
1715:
1345:
5862:
4123:
3952:
3828:
3818:
3775:
3629:
3596:
3576:
1101:
analyses of clinical trial data showed that 89% of patients remained free from disability progression two years after treatment.
3917:
1689:
7137:
5053:
4847:
3549:
399:
236:
104:
2995:"Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis"
2642:
Giovannoni G (June 2018). "Disease-modifying treatments for early and advanced multiple sclerosis: a new treatment paradigm".
2532:"Merck Receives European Medicines Agency Acceptance for Review of Marketing Authorization Application for Cladribine Tablets"
5466:
4468:
4358:
3833:
3745:
2176:
1166:
1036:
was developed by Ivax and Serono, and then Merck KGaA conducted clinical trials. Merck KGaA submitted an application to the
1022:
943:
759:
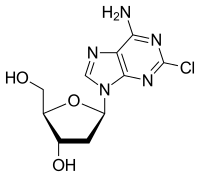
InChI=1S/C10H12ClN5O3/c11-10-14-8(12)7-9(15-10)16(3-13-7)6-1-4(18)5(2-17)19-6/h3-6,17-18H,1-2H2,(H2,12,14,15)/t4-,5+,6+/m0/s1
2146:
1475:
5388:
5371:
5246:
5010:
4388:
868:
7291:
5934:
5120:
4716:
4043:
3912:
3876:
3571:
521:
864:
7326:
6043:
5855:
5830:
5322:
4115:
601:
123:
5913:
4502:
3983:
6591:
5688:
5568:
5558:
4038:
3813:
3698:
3488:
2287:
1654:
1037:
1562:
Leist TP, Weissert R (2011). "Cladribine: mode of action and implications for treatment of multiple sclerosis".
7336:
6971:
5740:
5673:
5643:
5261:
4511:
1084:
Following completion of the two treatment courses, no further treatment or additional monitoring is required.
470:
6966:
5818:
5725:
5543:
5258:
4744:
4592:
4519:
3533:
986:
883:
160:
3300:"Safety of cladribine tablets in the treatment of patients with multiple sclerosis: An integrated analysis"
958:
to bring intravenous cladribine to market, and by
December of that year, Johnson & Johnson had filed a
640:
6771:
6197:
5878:
5623:
5613:
5346:
5188:
3467:
2551:"Cladribine Tablets Receives Positive CHMP Opinion for Treatment of Relapsing Forms of Multiple Sclerosis"
2347:
1126:
336:
171:
2802:
Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Soelberg Sørensen P, et al. (February 2010).
1213:"FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)"
7321:
6958:
4895:
4712:
4682:
4542:
3976:
3419:
3341:
3273:
3210:
3160:
3097:
3036:
2955:
2898:
2837:
2782:
2739:
Giovannoni G, Soelberg Sorensen P, Cook S, Rammohan K, Rieckmann P, Comi G, et al. (October 2018).
2493:
2027:
1959:
1064:
Two main approaches to multiple sclerosis treatment maintenance therapy are used – immunomodulation and
461:
3435:
3230:
De Stefano N, Giorgio A, Battaglini M, De Leucio A, Hicking C, Dangond F, et al. (February 2018).
1786:"The Development of Cladribine Tablets for the Treatment of Multiple Sclerosis: A Comprehensive Review"
590:
362:
After oral administration, 25% (±21%) of dose is excreted unchanged in urine and 3.8% as a metabolite.
5847:
5293:
5170:
4691:
4607:
3856:
3780:
3513:
3117:
Giovannoni G, Soelberg Sorensen P, Cook S, Rammohan KW, Rieckmann P, Comi G, et al. (May 2019).
1887:
Johnston JB (June 2011). "Mechanism of action of pentostatin and cladribine in hairy cell leukemia".
1212:
1162:
978:
959:
955:
832:
367:
6179:
5578:
5478:
5457:
5178:
5000:
4832:
4563:
4459:
4058:
3684:
3606:
989:); data from hairy cell leukemia studies showed that about 70% of people taking the drug developed
882:
Cladribine may cause foetal harm when administered to a pregnant woman and is listed by the FDA as
828:
794:
617:
416:
3072:
Giovannoni G, Rammohan K, Cook S, Soelberg-Sørensen P, Vermersch P, Keller B, et al. (2018).
7128:
6229:
4636:
4048:
3785:
3669:
3664:
3659:
3649:
3329:
3024:
2943:
2886:
2770:
2667:
1912:
1587:
1320:
876:
817:
220:
134:
7168:
7026:
5785:
2436:
1784:
Rammohan K, Coyle PK, Sylvester E, Galazka A, Dangond F, Grosso M, et al. (December 2020).
840:
7178:
6864:
6305:
4666:
4646:
4631:
2993:
Comi G, Cook S, Giovannoni G, Rieckmann P, Sørensen PS, Vermersch P, et al. (April 2019).
2575:
570:
7133:
7123:
5924:
5882:
5760:
5334:
4168:
3861:
3644:
3554:
3407:
3321:
3261:
3198:
3148:
3016:
2935:
2878:
2825:
2762:
2716:
2659:
2624:
2481:
2127:
2078:
2049:"Memory B Cells are Major Targets for Effective Immunotherapy in Relapsing Multiple Sclerosis"
2015:
1947:
1904:
1866:
1815:
1760:
1636:
1579:
1424:
1312:
1269:
1065:
275:
263:
54:
2452:"No evidence for higher risk of cancer in patients with multiple sclerosis taking cladribine"
2450:
Pakpoor J, Disanto G, Altmann DR, Pavitt S, Turner BP, Marta M, et al. (December 2015).
1739:"Langerhans cell histiocytosis in children: from the bench to bedside for an updated therapy"
7331:
6929:
6832:
6495:
6391:
5765:
5522:
5237:
4871:
4856:
4577:
3586:
3397:
3389:
3376:
Hasanali ZS, Saroya BS, Stuart A, Shimko S, Evans J, Vinod Shah M, et al. (June 2015).
3311:
3251:
3243:
3190:
3138:
3130:
3006:
2927:
2870:
2815:
2752:
2706:
2698:
2651:
2614:
2606:
2471:
2463:
2370:
2117:
2109:
2068:
2060:
2005:
1997:
1939:
1896:
1856:
1846:
1805:
1797:
1750:
1662:
1626:
1618:
1571:
1414:
1406:
1304:
939:
657:
310:
1986:"Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells"
530:
510:
7305:
7228:
7113:
5204:
5166:
4957:
4379:
4294:
3896:
3866:
2514:"Four years after a transatlantic slapdown, Merck KGaA will once again seek cladribine OK"
2269:
1131:
998:
425:
346:
318:
3298:
Cook S, Leist T, Comi G, Montalban X, Giovannoni G, Nolting A, et al. (April 2019).
1984:
Ceronie B, Jacobs BM, Baker D, Dubuisson N, Mao Z, Ammoscato F, et al. (May 2018).
827:. However, unlike deoxyadenosine, it is relatively resistant to breakdown by the enzyme
644:
621:
7118:
6986:
6634:
6468:
6447:
6443:
6439:
6067:
5904:
5824:
5358:
4949:
4443:
4307:
4194:
4164:
4151:
4083:
3871:
3561:
3523:
3402:
3377:
3256:
3231:
3143:
3118:
2711:
2686:
2619:
2594:
2476:
2451:
2122:
2097:
2073:
2048:
2010:
1985:
1861:
1834:
1810:
1785:
1723:
1631:
1606:
1419:
1394:
948:
935:
824:
2874:
2685:
Baker D, Herrod SS, Alvarez-Gonzalez C, Zalewski L, Albor C, Schmierer K (July 2017).
2096:
Baker D, Herrod SS, Alvarez-Gonzalez C, Zalewski L, Albor C, Schmierer K (July 2017).
1186:
7315:
7268:
7198:
7108:
7015:
6801:
6501:
6423:
6375:
6355:
6213:
6129:
5980:
5946:
5698:
5658:
5028:
4901:
4782:
4725:
4483:
4432:
4053:
3719:
3378:"Epigenetic therapy overcomes treatment resistance in T cell prolymphocytic leukemia"
2302:
1916:
1308:
1246:
860:
633:
352:
288:
96:
3333:
3028:
2890:
1591:
1324:
899:
triphosphorylated active compound, 2-chlorodeoxyadenosine 5’triphosphate (2-CdATP).
7278:
7238:
7223:
7218:
6934:
6884:
6879:
6859:
6739:
6734:
6597:
6582:
6530:
6506:
6453:
6330:
6320:
6139:
6056:
6051:
6009:
5962:
5919:
5886:
5745:
5709:
5593:
5583:
5537:
5533:
5495:
5403:
5193:
5132:
5128:
5109:
5104:
5083:
5072:
4886:
4880:
4866:
4861:
4792:
4787:
4740:
4626:
4493:
4473:
4402:
4351:
4341:
4155:
4147:
4068:
3999:
3679:
2947:
2774:
2671:
1033:
994:
908:
211:
206:
196:
191:
1833:
Jamroz-Wiśniewska A, Bełtowski J, Wójcicka G, Bartosik-Psujek H, Rejdak K (2020).
450:
82:
3393:
2702:
2655:
2467:
2113:
1900:
1575:
7273:
7213:
7203:
7193:
7093:
7083:
7073:
7068:
7033:
6944:
6939:
6914:
6904:
6894:
6869:
6854:
6849:
6807:
6777:
6763:
6758:
6744:
6687:
6645:
6640:
6626:
6621:
6607:
6587:
6540:
6516:
6474:
6458:
6429:
6413:
6381:
6365:
6360:
6335:
6285:
6275:
6270:
6265:
6254:
6244:
6149:
6061:
6019:
6004:
5998:
5994:
5941:
5790:
5770:
5755:
5750:
5735:
5693:
5663:
5603:
5573:
5517:
5490:
5363:
5304:
5276:
5266:
5219:
5183:
5099:
5061:
5043:
5038:
5018:
4995:
4966:
4944:
4924:
4914:
4765:
4750:
4730:
4616:
4547:
4533:
4488:
4421:
4416:
4393:
4368:
4336:
4279:
4242:
4219:
4204:
4199:
4184:
4099:
3694:
3689:
3634:
3316:
3299:
3194:
3011:
2994:
2804:"A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis"
2064:
1483:
990:
982:
963:
813:
324:
167:
90:
1801:
7263:
7258:
7243:
7183:
7173:
7158:
7153:
7148:
7098:
7088:
7078:
7043:
7021:
7007:
6919:
6909:
6899:
6889:
6874:
6817:
6812:
6791:
6706:
6701:
6659:
6653:
6554:
6511:
6407:
6397:
6350:
6340:
6315:
6310:
6300:
6295:
6290:
6280:
6239:
6224:
6219:
6203:
6144:
6124:
6073:
6033:
6014:
5956:
5780:
5683:
5678:
5653:
5648:
5638:
5633:
5628:
5598:
5588:
5553:
5548:
5528:
5483:
5471:
5446:
5441:
5431:
5413:
5408:
5398:
5393:
5351:
5339:
5327:
5299:
5286:
5281:
5251:
5214:
5209:
5147:
5142:
5088:
5033:
4985:
4971:
4919:
4906:
4875:
4808:
4797:
4770:
4760:
4755:
4701:
4656:
4651:
4552:
4516:
4448:
4438:
4427:
4363:
4346:
4322:
4274:
4262:
4214:
4033:
4018:
3738:
3674:
3654:
3613:
3498:
2931:
2001:
1943:
1622:
1410:
1150:
1026:
839:. Accumulation of cladribine into cells is dependent on the ratio of DCK and
821:
693:
501:
201:
3247:
3134:
2757:
2740:
2610:
977:
Injectable cladribine suppresses the body's ability to make new lymphocytes,
7253:
7248:
7233:
7208:
7163:
7143:
7103:
7038:
7002:
6924:
6720:
6673:
6602:
6568:
6535:
6345:
6325:
6234:
6134:
5974:
5795:
5775:
5730:
5720:
5714:
5618:
5563:
5512:
5436:
5426:
5381:
5368:
5309:
5271:
5199:
5137:
5078:
5067:
5023:
4980:
4939:
4933:
4929:
4891:
4818:
4813:
4803:
4775:
4735:
4696:
4671:
4661:
4621:
4267:
4257:
4252:
4247:
4209:
4063:
4023:
3714:
3709:
3518:
3503:
1295:
Liliemark J (February 1997). "The clinical pharmacokinetics of cladribine".
1006:
836:
381:
360:
Intravenous and subcutaneous bolus injection: 15-18% is excreted unchanged
76:
3411:
3325:
3265:
3202:
3152:
3020:
2939:
2882:
2829:
2766:
2720:
2663:
2628:
2485:
2131:
2082:
2019:
1951:
1908:
1870:
1851:
1819:
1764:
1640:
1583:
1428:
962:; cladribine was approved by the FDA in 1993 for hairy cell leukemia as an
33:
2820:
2803:
1316:
7188:
7063:
6752:
6260:
6170:
6106:
6028:
5704:
5668:
5608:
5376:
5314:
5094:
4990:
4962:
4641:
4478:
4078:
4013:
3704:
3493:
1077:
481:
175:
118:
1667:
812:
Cladribine is a purine analogue that selectively targets and suppresses
490:
6681:
4597:
4498:
4312:
4173:
3508:
1476:"Leustat Injection. - Summary of Product Characteristics (SPC) - (eMC)"
436:
41:
2047:
Baker D, Marta M, Pryce G, Giovannoni G, Schmierer K (February 2017).
1755:
1738:
6996:
6827:
4237:
4104:
4073:
3483:
1607:"Update on the biology and treatment options for hairy cell leukemia"
1002:
581:
355:
2687:"Both cladribine and alemtuzumab may effect MS via B-cell depletion"
2098:"Both cladribine and alemtuzumab may effect MS via B-cell depletion"
17:
993:
and about 30% developed infections and some of those progressed to
875:
the treatment of relapsing forms of multiple sclerosis, to include
550:
6695:
6548:
805:, is used for the treatment of adults with highly active forms of
716:
707:
561:
328:
216:
6667:
6615:
6576:
6562:
6116:
5990:
3528:
1355:. St Leonards, Australia: Orphan Australia Pty. Ltd. 10 May 2010
541:
297:
269:
5851:
4119:
3972:
3968:
3439:
282:
113:
6524:
1388:
1386:
1384:
1382:
1380:
1378:
1376:
1374:
1372:
1370:
1220:
904:
2734:
2732:
2730:
2595:"Pulsed immune reconstitution therapy in multiple sclerosis"
886:; safety and efficacy in children has not been established.
877:
relapsing-remitting disease and active secondary progressive
606:
407:
5-(6-Amino-2-chloro-purin-9-yl)-2-(hydroxymethyl)oxolan-3-ol
1395:"Cladribine to Treat Relapsing Forms of Multiple Sclerosis"
1346:"PRODUCT INFORMATION LITAK© 2 mg/mL solution for injection"
245:
145:
3892:
Megalencephalic leukoencephalopathy with subcortical cysts
1121:
Safety profile of cladribine tablets in multiple sclerosis
2855:
2853:
67:
2-Chlorodeoxyadenosine; 2-Chloro-2'-deoxyadenosine; 2-CdA
258:
848:
World Health Organization's List of Essential Medicines
776:
2322:"Teva to Acquire Ivax, Another Maker of Generic Drugs"
2270:"A potential new MS treatment's long and winding road"
7289:
3602:
Lesional demyelinations of the central nervous system
1557:
1555:
1553:
1551:
1549:
1547:
954:
In February 1991, Scripps began a collaboration with
1092:
Efficacy of cladribine tablets in multiple sclerosis
1029:
acquired control of Serono's drug business in 2006.
7056:
6984:
6957:
6842:
6485:
6187:
6178:
6169:
6162:
6115:
6097:
6088:
6042:
5973:
5903:
5894:
5505:
5456:
5236:
5229:
5165:
5119:
5052:
5009:
4846:
4831:
4711:
4681:
4606:
4591:
4562:
4458:
4378:
4321:
4304:
4293:
4229:
4182:
4163:
4092:
4006:
3935:
3905:
3847:
3799:
3761:
3754:
3728:
3622:
3542:
3474:
2388:
2386:
2384:
2288:"Ivax to Develop Cladribine for Multiple Sclerosis"
2169:"Ortho Biotech's Leustatin For Hairy Cell Leukemia"
1161:Cladribine has been studied as part of a multidrug
973:
Safety profile of cladribine in hairy cell leukemia
705:
692:
656:
651:
632:
600:
580:
560:
540:
520:
500:
480:
469:
460:
435:
415:
390:
380:
366:
345:
335:
317:
309:
235:
230:
182:
159:
133:
103:
89:
71:
63:
53:
48:
2691:Neurology: Neuroimmunology & Neuroinflammation
3791:Chronic inflammatory demyelinating polyneuropathy
2437:"Merck KGaA Throws in Towel on Cladribine for MS"
2303:"Serono Purchases Rights To Experimental MS Drug"
2230:"Litak: Background Information on the Procedure"
1882:
1880:
1779:
1777:
997:; about 40% of people taking the drug had fewer
820:and B-cell leukaemia. Chemically, it mimics the
449:
3887:Leukoencephalopathy with vanishing white matter
2147:"Biographical Memoir: Ernest Beutler 1928–2008"
424:
2599:Therapeutic Advances in Neurological Disorders
2307:Dow Jones Newswires in the Wall Street Journal
2188:
2186:
966:, and was approved in Europe later that year.
793:, among others, is a medication used to treat
7352:World Health Organization essential medicines
5863:
4131:
3984:
3451:
2274:News & Views - Scripps Research Institute
1979:
1977:
1975:
816:implicated in the underlying pathogenesis of
8:
3293:
3291:
3289:
2988:
2986:
1605:Jain P, Pemmaraju N, Ravandi F (June 2014).
1525:
1523:
1521:
122:
32:
2394:"Withdrawal Assessment Report for Movectro"
2263:
2261:
1519:
1517:
1515:
1513:
1511:
1509:
1507:
1505:
1503:
1501:
1125:Cladribine tablets target the cells of the
991:dangerously low levels of white blood cells
6184:
6175:
6166:
6094:
5900:
5870:
5856:
5848:
5233:
4843:
4603:
4318:
4301:
4179:
4138:
4124:
4116:
3991:
3977:
3969:
3758:
3458:
3444:
3436:
2430:
2428:
2194:"Litak EMA package: Scientific Discussion"
1142:Progressive multifocal leukoencephalopathy
643:
620:
509:
3839:Experimental autoimmune encephalomyelitis
3401:
3315:
3255:
3142:
3010:
2819:
2756:
2710:
2618:
2475:
2121:
2072:
2009:
1860:
1850:
1839:Oxidative Medicine and Cellular Longevity
1809:
1754:
1666:
1630:
1418:
1130:result, a reduction in lymphocyte count (
529:
7342:Drugs developed by Johnson & Johnson
3943:List of multiple sclerosis organizations
3304:Multiple Sclerosis and Related Disorders
3183:Multiple Sclerosis and Related Disorders
2999:Multiple Sclerosis and Related Disorders
1684:
1682:
1680:
1678:
801:. Cladribine, sold under the brand name
7296:
1178:
1153:in long-term real-world evidence data.
756:
736:
616:
489:
404:
95:
3948:List of people with multiple sclerosis
3824:Neuromyelitis optica spectrum disorder
3771:Neuromyelitis optica spectrum disorder
3417:
3339:
3271:
3208:
3158:
3095:
3034:
2953:
2896:
2835:
2780:
2491:
2025:
1957:
1005:; and about 10% of people had too few
807:relapsing-remitting multiple sclerosis
634:
341:25% (range 5-50%); up to 20% (orally)
31:
1661:. Geneva: World Health Organization.
1611:Current Treatment Options in Oncology
1470:
1468:
1443:"European Medicines Agency - - Litak"
1340:
1338:
1336:
1334:
1032:An oral formulation of the drug with
797:(leukemic reticuloendotheliosis) and
589:
569:
81:
7:
3923:Mitochondrial DNA depletion syndrome
3809:Acute disseminated encephalomyelitis
2340:"Teva Completes Acquisition of Ivax"
210:
195:
4407:ribonucleotide reductase inhibitors
2808:The New England Journal of Medicine
1449:. 17 September 2018. Archived from
799:B-cell chronic lymphocytic leukemia
549:
440:
4573:Ribonucleotide reductase inhibitor
4529:Ribonucleotide reductase inhibitor
3424:: CS1 maint: overridden setting (
3346:: CS1 maint: overridden setting (
3278:: CS1 maint: overridden setting (
3215:: CS1 maint: overridden setting (
3165:: CS1 maint: overridden setting (
3102:: CS1 maint: overridden setting (
3041:: CS1 maint: overridden setting (
2960:: CS1 maint: overridden setting (
2903:: CS1 maint: overridden setting (
2842:: CS1 maint: overridden setting (
2787:: CS1 maint: overridden setting (
2593:Sorensen PS, Sellebjerg F (2019).
2498:: CS1 maint: overridden setting (
2032:: CS1 maint: overridden setting (
1964:: CS1 maint: overridden setting (
1690:"Cladribine- cladribine injection"
25:
4332:Dihydrofolate reductase inhibitor
358:; 15-18% is excreted unchanged.
7299:
3953:Multiple sclerosis drug pipeline
3829:Diffuse myelinoclastic sclerosis
3819:Marburg acute multiple sclerosis
3776:Diffuse myelinoclastic sclerosis
3630:Management of multiple sclerosis
3597:Radiologically isolated syndrome
3577:Expanded Disability Status Scale
1309:10.2165/00003088-199732020-00003
677:
674:
668:
40:
3550:Diagnosis of multiple sclerosis
2576:"Cladribine approved in Europe"
1274:European Medicines Agency (EMA)
1167:T-cell prolymphocytic leukaemia
1145:prior to initiating treatment.
764:Key:PTOAARAWEBMLNO-KVQBGUIXSA-N
739:Clc1nc(c2ncn(c2n1)3O((O)C3)CO)N
4469:Thymidylate synthase inhibitor
4359:Thymidylate synthase inhibitor
3834:Tumefactive multiple sclerosis
3746:Research in multiple sclerosis
3466:Demyelinating diseases of the
3382:Science Translational Medicine
2371:"Merck KGaA to acquire Serono"
1743:British Journal of Haematology
944:adenosine deaminase deficiency
931:History in hairy cell leukemia
683:
662:
1:
4389:Adenosine deaminase inhibitor
4230:Block microtubule disassembly
3361:Giovannoni G (2020). "A965".
2875:10.1016/S1474-4422(11)70023-0
2420:Procedure No. EMEA/H/C/001197
2301:Sargent C (31 October 2002).
1393:Giovannoni G (October 2017).
1013:History in multiple sclerosis
869:Langerhans cell histiocytosis
5935:dihydroorotate dehydrogenase
4044:Human chorionic gonadotropin
3913:Central pontine myelinolysis
3882:Pelizaeus–Merzbacher disease
3877:Metachromatic leukodystrophy
3572:Clinically isolated syndrome
3543:Investigations and diagnosis
3394:10.1126/scitranslmed.aaa5079
2703:10.1212/NXI.0000000000000360
2656:10.1097/WCO.0000000000000561
2644:Current Opinion in Neurology
2468:10.1212/nxi.0000000000000158
2154:National Academy of Sciences
2114:10.1212/NXI.0000000000000360
1901:10.3109/10428194.2011.570394
1576:10.1097/WNF.0b013e318204cd90
789:, sold under the brand name
59:Leustatin, Mavenclad, others
5914:purine synthesis inhibitors
3918:Marchiafava–Bignami disease
3317:10.1016/j.msard.2018.11.021
3195:10.1016/j.msard.2011.08.006
3012:10.1016/j.msard.2019.01.038
2369:Staff (21 September 2006).
2290:. Reuters. 4 December 2000.
2065:10.1016/j.ebiom.2017.01.042
7368:
2401:Europeans Medicines Agency
2237:Europeans Medicines Agency
2201:Europeans Medicines Agency
1802:10.1007/s40265-020-01422-9
1671:. WHO/MHP/HPS/EML/2023.02.
1564:Clinical Neuropharmacology
1105:scans in this population.
652:Chemical and physical data
6099:IL-1 receptor antagonists
5808:
5689:Omacetaxine mepesuccinate
5569:Ciltacabtagene autoleucel
5559:Brexucabtagene autoleucel
4039:Gonadotropin preparations
3814:Balo concentric sclerosis
2932:10.1007/s00415-012-6775-0
2002:10.1007/s00415-018-8830-y
1944:10.3109/10428199109068099
1720:Histiocytosis Association
1716:"Erdheim-Chester Disease"
1655:World Health Organization
1623:10.1007/s11864-014-0285-5
1538:European Medicines Agency
1411:10.1007/s13311-017-0573-4
1297:Clinical Pharmacokinetics
1056:Use in multiple sclerosis
1038:European Medicines Agency
772:
747:
727:
395:
39:
7347:Drugs developed by Merck
6972:Anti-lymphocyte globulin
5741:Talimogene laherparepvec
5674:Nadofaragene firadenovec
5644:Lisocabtagene maraleucel
4593:Topoisomerase inhibitors
4512:DNA polymerase inhibitor
3248:10.1177/1352458517690269
3135:10.1177/1352458518771875
2758:10.1177/1352458517727603
2611:10.1177/1756286419836913
2435:Gever J (22 June 2011).
2320:Bayot J (26 July 2005).
1242:"Neurological therapies"
6967:Anti-thymocyte globulin
5879:Immunosuppressive drugs
5544:Axicabtagene ciloleucel
4148:chemotherapeutic agents
1932:Leukemia & Lymphoma
1889:Leukemia & Lymphoma
865:Erdheim–Chester disease
6772:Interleukin-6 receptor
6198:Complement component 5
5726:Sitimagene ceradenovec
5624:Idecabtagene vicleucel
5189:Methyl aminolevulinate
3755:Demyelinating diseases
3468:central nervous system
3080:: 1204. Archived from
2403:. 2011. Archived from
2346:. 2006. Archived from
2239:. 2004. Archived from
2203:. 2004. Archived from
2167:Staff (8 March 1993).
1353:TGA eBusiness Services
1127:adaptive immune system
5200:Porphyrin derivatives
4896:Melphalan flufenamide
4543:Hypomethylating agent
4152:antineoplastic agents
2863:The Lancet. Neurology
2821:10.1056/NEJMoa0902533
1737:Aricò M (June 2016).
956:Johnson & Johnson
5529:Asparagine depleters
5458:Receptor antagonists
5372:+abiraterone acetate
3857:Adrenoleukodystrophy
3781:MOG antibody disease
3583:Serological and CSF
3534:Uhthoff's phenomenon
2920:Journal of Neurology
2605:: 1756286419836913.
2555:www.prnewswire.co.uk
2210:on 24 September 2015
2145:Lichtman MA (2012).
1990:Journal of Neurology
1852:10.1155/2020/1654754
1480:www.medicines.org.uk
1163:chemotherapy regimen
1001:and became severely
979:natural killer cells
960:new drug application
884:pregnancy category D
833:deoxycytidine kinase
5579:Denileukin diftitox
5479:Retinoid X receptor
5179:Aminolevulinic acid
5001:Triethylenemelamine
4833:Crosslinking of DNA
4564:Deoxyribonucleotide
4503:+gimeracil/oteracil
4059:Luteinizing hormone
3685:Monomethyl fumarate
2580:Merck Press Release
2350:on 18 December 2019
1531:"Mavenclad EU SmPC"
1165:for drug-resistant
890:Mechanism of action
829:adenosine deaminase
795:hairy cell leukemia
278:(Prescription only)
254:(Prescription only)
36:
27:Pharmaceutical drug
7327:Purine antagonists
7129:Diroximel fumarate
6802:IL-2 receptor/CD25
6230:Certolizumab pegol
5883:Immunosuppressants
5835:Never to phase III
4637:Etirinotecan pegol
4049:Interferon beta-1a
3786:Multiple sclerosis
3670:Interferon beta-1b
3665:Interferon beta-1a
3660:Glatiramer acetate
3650:Diroximel fumarate
3623:Approved treatment
3476:Signs and symptoms
3236:Multiple Sclerosis
3123:Multiple Sclerosis
2745:Multiple Sclerosis
2344:Teva Press Release
2179:on 3 October 2017.
1895:(Suppl 2): 43–45.
1696:. 31 December 2019
1193:. 28 February 2020
818:multiple sclerosis
221:multiple sclerosis
7287:
7286:
7134:Efgartigimod alfa
7124:Dimethyl fumarate
7052:
7051:
6980:
6979:
6953:
6952:
6158:
6157:
6084:
6083:
5925:Mycophenolic acid
5845:
5844:
5804:
5803:
5761:Tigilanol tiglate
5238:Enzyme inhibitors
5161:
5160:
5157:
5156:
4857:Nitrogen mustards
4827:
4826:
4587:
4586:
4289:
4288:
4113:
4112:
3966:
3965:
3931:
3930:
3862:Alexander disease
3645:Dimethyl fumarate
3587:Oligoclonal bands
3555:McDonald criteria
3388:(293): 293ra102.
2751:(12): 1594–1604.
2582:. 25 August 2017.
2410:on 21 August 2016
2375:First Word Pharma
2268:Sauter E, Ono M.
2246:on 21 August 2016
1796:(18): 1901–1928.
1756:10.1111/bjh.13955
1486:on 3 October 2017
1447:www.ema.europa.eu
1399:Neurotherapeutics
1066:immunosuppression
907:, the release of
784:
783:
718:Interactive image
602:CompTox Dashboard
301:
286:
273:
261:
249:
149:
116:
16:(Redirected from
7359:
7304:
7303:
7302:
7295:
6930:Telimomab aritox
6833:Zolimomab aritox
6654:CD62L/L-selectin
6392:Immunoglobulin E
6185:
6176:
6167:
6095:
5901:
5872:
5865:
5858:
5849:
5766:Tisagenlecleucel
5523:Arsenic trioxide
5234:
5167:Photosensitizers
4958:Alkyl sulfonates
4872:Cyclophosphamide
4844:
4783:Anthracenediones
4604:
4578:Hydroxycarbamide
4319:
4302:
4180:
4140:
4133:
4126:
4117:
3993:
3986:
3979:
3970:
3759:
3729:Other treatments
3607:Dawson's fingers
3460:
3453:
3446:
3437:
3430:
3429:
3423:
3415:
3405:
3373:
3367:
3366:
3358:
3352:
3351:
3345:
3337:
3319:
3295:
3284:
3283:
3277:
3269:
3259:
3227:
3221:
3220:
3214:
3206:
3177:
3171:
3170:
3164:
3156:
3146:
3114:
3108:
3107:
3101:
3093:
3091:
3089:
3069:
3063:
3062:
3053:
3047:
3046:
3040:
3032:
3014:
2990:
2981:
2980:
2972:
2966:
2965:
2959:
2951:
2926:(4): 1136–1146.
2915:
2909:
2908:
2902:
2894:
2857:
2848:
2847:
2841:
2833:
2823:
2799:
2793:
2792:
2786:
2778:
2760:
2736:
2725:
2724:
2714:
2682:
2676:
2675:
2639:
2633:
2632:
2622:
2590:
2584:
2583:
2572:
2566:
2565:
2563:
2561:
2546:
2540:
2539:
2528:
2522:
2521:
2510:
2504:
2503:
2497:
2489:
2479:
2447:
2441:
2440:
2432:
2423:
2422:
2417:
2415:
2409:
2398:
2390:
2379:
2378:
2366:
2360:
2359:
2357:
2355:
2336:
2330:
2329:
2317:
2311:
2310:
2298:
2292:
2291:
2284:
2278:
2277:
2265:
2256:
2255:
2253:
2251:
2245:
2234:
2226:
2220:
2219:
2217:
2215:
2209:
2198:
2190:
2181:
2180:
2175:. Archived from
2164:
2158:
2157:
2151:
2142:
2136:
2135:
2125:
2093:
2087:
2086:
2076:
2044:
2038:
2037:
2031:
2023:
2013:
1996:(5): 1199–1209.
1981:
1970:
1969:
1963:
1955:
1927:
1921:
1920:
1884:
1875:
1874:
1864:
1854:
1830:
1824:
1823:
1813:
1781:
1772:
1771:
1758:
1734:
1728:
1727:
1722:. Archived from
1712:
1706:
1705:
1703:
1701:
1686:
1673:
1672:
1670:
1651:
1645:
1644:
1634:
1602:
1596:
1595:
1559:
1542:
1541:
1540:. February 2021.
1535:
1527:
1496:
1495:
1493:
1491:
1482:. Archived from
1472:
1463:
1462:
1460:
1458:
1453:on 3 August 2018
1439:
1433:
1432:
1422:
1390:
1365:
1364:
1362:
1360:
1350:
1342:
1329:
1328:
1292:
1286:
1285:
1283:
1281:
1276:. 22 August 2017
1270:"Mavenclad EPAR"
1266:
1260:
1259:
1257:
1255:
1238:
1232:
1231:
1229:
1227:
1217:nctr-crs.fda.gov
1209:
1203:
1202:
1200:
1198:
1183:
987:myelosuppression
940:Dennis A. Carson
780:
779:
720:
700:
685:
679:
676:
670:
664:
647:
636:
625:
624:
610:
608:
593:
573:
553:
533:
513:
493:
473:
453:
443:
442:
428:
371:
299:
296:
291:
284:
281:
271:
268:
260:
257:
247:
244:
214:
199:
147:
144:
126:
115:
112:
99:
85:
44:
37:
35:
21:
7367:
7366:
7362:
7361:
7360:
7358:
7357:
7356:
7337:Organochlorides
7312:
7311:
7310:
7300:
7298:
7290:
7288:
7283:
7229:Rozanolixizumab
7114:Deucravacitinib
7048:
6976:
6949:
6838:
6487:
6481:
6189:
6154:
6111:
6090:
6080:
6038:
5978:
5969:
5905:Antimetabolites
5896:
5890:
5876:
5846:
5841:
5840:
5825:Clinical trials
5800:
5506:Other/ungrouped
5501:
5452:
5225:
5205:Porfimer sodium
5153:
5115:
5048:
5005:
4835:
4823:
4707:
4677:
4595:
4583:
4558:
4454:
4374:
4310:
4308:antimetabolites
4306:
4305:DNA precursors/
4297:
4295:DNA replication
4285:
4225:
4195:Vinca alkaloids
4171:
4159:
4144:
4114:
4109:
4088:
4002:
3997:
3967:
3962:
3958:Pathophysiology
3927:
3901:
3897:CAMFAK syndrome
3867:Canavan disease
3843:
3795:
3750:
3724:
3618:
3538:
3470:
3464:
3434:
3433:
3416:
3375:
3374:
3370:
3363:Actrims-Ectrims
3360:
3359:
3355:
3338:
3297:
3296:
3287:
3270:
3229:
3228:
3224:
3207:
3179:
3178:
3174:
3157:
3116:
3115:
3111:
3094:
3087:
3085:
3084:on 17 July 2021
3071:
3070:
3066:
3055:
3054:
3050:
3033:
2992:
2991:
2984:
2974:
2973:
2969:
2952:
2917:
2916:
2912:
2895:
2859:
2858:
2851:
2834:
2801:
2800:
2796:
2779:
2738:
2737:
2728:
2684:
2683:
2679:
2641:
2640:
2636:
2592:
2591:
2587:
2574:
2573:
2569:
2559:
2557:
2548:
2547:
2543:
2538:. 18 July 2016.
2530:
2529:
2525:
2512:
2511:
2507:
2490:
2449:
2448:
2444:
2434:
2433:
2426:
2413:
2411:
2407:
2396:
2392:
2391:
2382:
2368:
2367:
2363:
2353:
2351:
2338:
2337:
2333:
2319:
2318:
2314:
2300:
2299:
2295:
2286:
2285:
2281:
2267:
2266:
2259:
2249:
2247:
2243:
2232:
2228:
2227:
2223:
2213:
2211:
2207:
2196:
2192:
2191:
2184:
2166:
2165:
2161:
2149:
2144:
2143:
2139:
2095:
2094:
2090:
2046:
2045:
2041:
2024:
1983:
1982:
1973:
1956:
1929:
1928:
1924:
1886:
1885:
1878:
1832:
1831:
1827:
1783:
1782:
1775:
1736:
1735:
1731:
1726:on 6 June 2019.
1714:
1713:
1709:
1699:
1697:
1688:
1687:
1676:
1653:
1652:
1648:
1604:
1603:
1599:
1561:
1560:
1545:
1533:
1529:
1528:
1499:
1489:
1487:
1474:
1473:
1466:
1456:
1454:
1441:
1440:
1436:
1392:
1391:
1368:
1358:
1356:
1348:
1344:
1343:
1332:
1294:
1293:
1289:
1279:
1277:
1268:
1267:
1263:
1253:
1251:
1240:
1239:
1235:
1225:
1223:
1211:
1210:
1206:
1196:
1194:
1185:
1184:
1180:
1175:
1159:
1123:
1094:
1058:
1015:
999:red blood cells
975:
933:
892:
856:
841:5'-nucleotidase
775:
773:
768:
765:
760:
755:
754:
743:
740:
735:
734:
723:
698:
688:
682:
673:
667:
628:
604:
596:
576:
556:
536:
516:
496:
476:
456:
439:
431:
411:
408:
403:
402:
369:
337:Protein binding
319:Bioavailability
311:Pharmacokinetic
305:
289:
226:
205:
162:
155:
136:
129:
28:
23:
22:
15:
12:
11:
5:
7365:
7363:
7355:
7354:
7349:
7344:
7339:
7334:
7329:
7324:
7314:
7313:
7309:
7308:
7285:
7284:
7282:
7281:
7276:
7271:
7266:
7261:
7256:
7251:
7246:
7241:
7236:
7231:
7226:
7221:
7216:
7211:
7206:
7201:
7196:
7191:
7186:
7181:
7176:
7171:
7166:
7161:
7156:
7151:
7146:
7141:
7138:+hyaluronidase
7131:
7126:
7121:
7119:Deuruxolitinib
7116:
7111:
7106:
7101:
7096:
7091:
7086:
7081:
7076:
7071:
7066:
7060:
7058:
7054:
7053:
7050:
7049:
7047:
7046:
7041:
7036:
7031:
7030:
7029:
7024:
7012:
7011:
7010:
7005:
6992:
6990:
6982:
6981:
6978:
6977:
6975:
6974:
6969:
6963:
6961:
6955:
6954:
6951:
6950:
6948:
6947:
6942:
6937:
6932:
6927:
6922:
6917:
6912:
6907:
6902:
6897:
6892:
6887:
6882:
6877:
6872:
6867:
6862:
6857:
6852:
6846:
6844:
6840:
6839:
6837:
6836:
6823:
6822:
6821:
6820:
6815:
6810:
6797:
6796:
6795:
6794:
6782:
6781:
6780:
6768:
6767:
6766:
6761:
6749:
6748:
6747:
6742:
6737:
6725:
6724:
6723:
6712:
6711:
6710:
6709:
6704:
6692:
6691:
6690:
6678:
6677:
6676:
6664:
6663:
6662:
6650:
6649:
6648:
6643:
6631:
6630:
6629:
6624:
6612:
6611:
6610:
6605:
6600:
6595:
6592:+hyaluronidase
6585:
6573:
6572:
6571:
6559:
6558:
6557:
6545:
6544:
6543:
6538:
6533:
6521:
6520:
6519:
6514:
6509:
6504:
6491:
6489:
6483:
6482:
6480:
6479:
6478:
6477:
6464:
6463:
6462:
6461:
6456:
6435:
6434:
6433:
6432:
6419:
6418:
6417:
6416:
6403:
6402:
6401:
6400:
6387:
6386:
6385:
6384:
6371:
6370:
6369:
6368:
6363:
6358:
6353:
6348:
6343:
6338:
6333:
6328:
6323:
6318:
6313:
6308:
6303:
6298:
6293:
6288:
6283:
6278:
6273:
6268:
6263:
6250:
6249:
6248:
6247:
6242:
6237:
6232:
6227:
6222:
6209:
6208:
6207:
6206:
6193:
6191:
6182:
6173:
6164:
6160:
6159:
6156:
6155:
6153:
6152:
6147:
6142:
6137:
6132:
6127:
6121:
6119:
6113:
6112:
6110:
6109:
6103:
6101:
6092:
6086:
6085:
6082:
6081:
6079:
6078:
6077:
6076:
6068:PDE4 inhibitor
6064:
6059:
6054:
6048:
6046:
6040:
6039:
6037:
6036:
6031:
6025:
6024:
6023:
6022:
6017:
6012:
6007:
5986:
5984:
5971:
5970:
5968:
5967:
5966:
5965:
5952:
5951:
5950:
5949:
5944:
5930:
5929:
5928:
5927:
5922:
5909:
5907:
5898:
5892:
5891:
5877:
5875:
5874:
5867:
5860:
5852:
5843:
5842:
5839:
5838:
5837:
5836:
5833:
5822:
5816:
5810:
5809:
5806:
5805:
5802:
5801:
5799:
5798:
5793:
5788:
5783:
5778:
5773:
5768:
5763:
5758:
5753:
5748:
5743:
5738:
5733:
5728:
5723:
5718:
5712:
5701:
5696:
5691:
5686:
5681:
5676:
5671:
5666:
5661:
5656:
5651:
5646:
5641:
5636:
5631:
5626:
5621:
5616:
5611:
5606:
5601:
5596:
5591:
5586:
5581:
5576:
5571:
5566:
5561:
5556:
5551:
5546:
5541:
5525:
5520:
5515:
5509:
5507:
5503:
5502:
5500:
5499:
5487:
5475:
5462:
5460:
5454:
5453:
5451:
5450:
5444:
5439:
5434:
5429:
5417:
5411:
5406:
5401:
5396:
5385:
5379:
5374:
5366:
5359:PARP inhibitor
5355:
5343:
5331:
5319:
5318:
5317:
5312:
5307:
5302:
5290:
5284:
5279:
5274:
5269:
5255:
5242:
5240:
5231:
5227:
5226:
5224:
5223:
5217:
5212:
5207:
5196:
5191:
5186:
5181:
5175:
5173:
5163:
5162:
5159:
5158:
5155:
5154:
5152:
5151:
5145:
5140:
5135:
5125:
5123:
5117:
5116:
5114:
5113:
5107:
5102:
5091:
5086:
5081:
5076:
5064:
5058:
5056:
5050:
5049:
5047:
5046:
5041:
5036:
5031:
5026:
5021:
5015:
5013:
5011:Platinum-based
5007:
5006:
5004:
5003:
4998:
4993:
4988:
4976:
4975:
4969:
4953:
4952:
4947:
4942:
4937:
4927:
4922:
4910:
4909:
4904:
4899:
4889:
4884:
4878:
4869:
4864:
4852:
4850:
4841:
4829:
4828:
4825:
4824:
4822:
4821:
4816:
4811:
4806:
4801:
4795:
4790:
4779:
4773:
4768:
4763:
4758:
4753:
4748:
4738:
4733:
4726:Anthracyclines
4721:
4719:
4709:
4708:
4706:
4705:
4699:
4687:
4685:
4679:
4678:
4676:
4675:
4669:
4664:
4659:
4654:
4649:
4644:
4639:
4634:
4629:
4624:
4612:
4610:
4601:
4589:
4588:
4585:
4584:
4582:
4581:
4568:
4566:
4560:
4559:
4557:
4556:
4550:
4538:
4537:
4524:
4523:
4507:
4506:
4496:
4491:
4486:
4481:
4476:
4464:
4462:
4456:
4455:
4453:
4452:
4446:
4444:Mercaptopurine
4435:
4430:
4425:
4419:
4414:
4398:
4397:
4384:
4382:
4376:
4375:
4373:
4372:
4366:
4355:
4349:
4344:
4339:
4327:
4325:
4316:
4299:
4291:
4290:
4287:
4286:
4284:
4283:
4271:
4265:
4260:
4255:
4250:
4245:
4233:
4231:
4227:
4226:
4224:
4223:
4217:
4212:
4207:
4202:
4190:
4188:
4177:
4161:
4160:
4146:Intracellular
4145:
4143:
4142:
4135:
4128:
4120:
4111:
4110:
4108:
4107:
4102:
4096:
4094:
4090:
4089:
4087:
4086:
4084:Tegafur/uracil
4081:
4076:
4071:
4066:
4061:
4056:
4051:
4046:
4041:
4036:
4031:
4026:
4021:
4016:
4010:
4008:
4004:
4003:
3998:
3996:
3995:
3988:
3981:
3973:
3964:
3963:
3961:
3960:
3955:
3950:
3945:
3939:
3937:
3933:
3932:
3929:
3928:
3926:
3925:
3920:
3915:
3909:
3907:
3903:
3902:
3900:
3899:
3894:
3889:
3884:
3879:
3874:
3872:Krabbe disease
3869:
3864:
3859:
3853:
3851:
3845:
3844:
3842:
3841:
3836:
3831:
3826:
3821:
3816:
3811:
3805:
3803:
3797:
3796:
3794:
3793:
3788:
3783:
3778:
3773:
3767:
3765:
3756:
3752:
3751:
3749:
3748:
3743:
3742:
3741:
3732:
3730:
3726:
3725:
3723:
3722:
3717:
3712:
3707:
3702:
3699:+hyaluronidase
3692:
3687:
3682:
3677:
3672:
3667:
3662:
3657:
3652:
3647:
3642:
3637:
3632:
3626:
3624:
3620:
3619:
3617:
3616:
3611:
3610:
3609:
3604:
3599:
3591:
3590:
3589:
3581:
3580:
3579:
3574:
3565:
3564:
3562:Poser criteria
3559:
3558:
3557:
3546:
3544:
3540:
3539:
3537:
3536:
3531:
3526:
3524:Optic neuritis
3521:
3516:
3511:
3506:
3501:
3496:
3491:
3486:
3480:
3478:
3472:
3471:
3465:
3463:
3462:
3455:
3448:
3440:
3432:
3431:
3368:
3353:
3285:
3242:(2): 222–226.
3222:
3172:
3129:(6): 819–827.
3109:
3064:
3048:
2982:
2967:
2910:
2869:(4): 329–337.
2849:
2814:(5): 416–426.
2794:
2726:
2677:
2650:(3): 233–243.
2634:
2585:
2567:
2541:
2523:
2518:Fierce Biotech
2505:
2442:
2424:
2380:
2361:
2331:
2326:New York Times
2312:
2293:
2279:
2257:
2221:
2182:
2173:The Pink Sheet
2159:
2137:
2088:
2039:
1971:
1922:
1876:
1825:
1773:
1749:(5): 663–670.
1729:
1707:
1674:
1646:
1617:(2): 187–209.
1597:
1543:
1497:
1464:
1434:
1405:(4): 874–887.
1366:
1330:
1303:(2): 120–131.
1287:
1261:
1233:
1204:
1177:
1176:
1174:
1171:
1158:
1155:
1122:
1119:
1093:
1090:
1057:
1054:
1014:
1011:
974:
971:
949:orphan disease
936:Ernest Beutler
932:
929:
891:
888:
855:
852:
825:deoxyadenosine
782:
781:
770:
769:
767:
766:
763:
761:
758:
750:
749:
748:
745:
744:
742:
741:
738:
730:
729:
728:
725:
724:
722:
721:
713:
711:
703:
702:
696:
690:
689:
686:
680:
671:
665:
660:
654:
653:
649:
648:
638:
630:
629:
627:
626:
613:
611:
598:
597:
595:
594:
586:
584:
578:
577:
575:
574:
566:
564:
558:
557:
555:
554:
546:
544:
538:
537:
535:
534:
526:
524:
518:
517:
515:
514:
506:
504:
498:
497:
495:
494:
486:
484:
478:
477:
475:
474:
466:
464:
458:
457:
455:
454:
446:
444:
433:
432:
430:
429:
421:
419:
413:
412:
410:
409:
406:
398:
397:
396:
393:
392:
388:
387:
384:
378:
377:
373:
364:
363:
349:
343:
342:
339:
333:
332:
327:); 37 to 51% (
321:
315:
314:
307:
306:
304:
303:
294:
279:
266:
255:
241:
239:
233:
232:
228:
227:
225:
224:
188:
186:
180:
179:
165:
163:administration
157:
156:
154:
153:
151:
141:
139:
131:
130:
128:
127:
109:
107:
101:
100:
93:
87:
86:
79:
69:
68:
65:
61:
60:
57:
51:
50:
46:
45:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
7364:
7353:
7350:
7348:
7345:
7343:
7340:
7338:
7335:
7333:
7330:
7328:
7325:
7323:
7320:
7319:
7317:
7307:
7297:
7293:
7280:
7277:
7275:
7272:
7270:
7269:Tildrakizumab
7267:
7265:
7262:
7260:
7257:
7255:
7252:
7250:
7247:
7245:
7242:
7240:
7237:
7235:
7232:
7230:
7227:
7225:
7222:
7220:
7217:
7215:
7212:
7210:
7207:
7205:
7202:
7200:
7199:Pegcetacoplan
7197:
7195:
7192:
7190:
7187:
7185:
7182:
7180:
7177:
7175:
7172:
7170:
7167:
7165:
7162:
7160:
7157:
7155:
7152:
7150:
7147:
7145:
7142:
7139:
7135:
7132:
7130:
7127:
7125:
7122:
7120:
7117:
7115:
7112:
7110:
7109:Darvadstrocel
7107:
7105:
7102:
7100:
7097:
7095:
7092:
7090:
7087:
7085:
7082:
7080:
7077:
7075:
7072:
7070:
7067:
7065:
7062:
7061:
7059:
7055:
7045:
7042:
7040:
7037:
7035:
7032:
7028:
7025:
7023:
7020:
7019:
7018:
7017:
7016:TNF inhibitor
7013:
7009:
7006:
7004:
7001:
7000:
6999:
6998:
6994:
6993:
6991:
6988:
6983:
6973:
6970:
6968:
6965:
6964:
6962:
6960:
6956:
6946:
6943:
6941:
6938:
6936:
6933:
6931:
6928:
6926:
6923:
6921:
6918:
6916:
6913:
6911:
6908:
6906:
6903:
6901:
6898:
6896:
6893:
6891:
6888:
6886:
6883:
6881:
6878:
6876:
6873:
6871:
6868:
6866:
6863:
6861:
6858:
6856:
6853:
6851:
6848:
6847:
6845:
6841:
6834:
6830:
6829:
6825:
6824:
6819:
6816:
6814:
6811:
6809:
6806:
6805:
6804:
6803:
6799:
6798:
6793:
6790:
6789:
6788:
6787:
6783:
6779:
6776:
6775:
6774:
6773:
6769:
6765:
6762:
6760:
6757:
6756:
6755:
6754:
6750:
6746:
6743:
6741:
6738:
6736:
6733:
6732:
6731:
6730:
6726:
6722:
6719:
6718:
6717:
6714:
6713:
6708:
6705:
6703:
6700:
6699:
6698:
6697:
6693:
6689:
6686:
6685:
6684:
6683:
6682:CD147/Basigin
6679:
6675:
6672:
6671:
6670:
6669:
6665:
6661:
6658:
6657:
6656:
6655:
6651:
6647:
6644:
6642:
6639:
6638:
6637:
6636:
6632:
6628:
6625:
6623:
6620:
6619:
6618:
6617:
6613:
6609:
6606:
6604:
6601:
6599:
6596:
6593:
6589:
6586:
6584:
6581:
6580:
6579:
6578:
6574:
6570:
6567:
6566:
6565:
6564:
6560:
6556:
6553:
6552:
6551:
6550:
6546:
6542:
6539:
6537:
6534:
6532:
6529:
6528:
6527:
6526:
6522:
6518:
6515:
6513:
6510:
6508:
6505:
6503:
6502:Muromonab-CD3
6500:
6499:
6498:
6497:
6493:
6492:
6490:
6484:
6476:
6473:
6472:
6471:
6470:
6466:
6465:
6460:
6457:
6455:
6452:
6451:
6450:
6449:
6445:
6441:
6437:
6436:
6431:
6428:
6427:
6426:
6425:
6421:
6420:
6415:
6412:
6411:
6410:
6409:
6405:
6404:
6399:
6396:
6395:
6394:
6393:
6389:
6388:
6383:
6380:
6379:
6378:
6377:
6376:Interleukin 5
6373:
6372:
6367:
6364:
6362:
6359:
6357:
6356:Tildrakizumab
6354:
6352:
6349:
6347:
6344:
6342:
6339:
6337:
6334:
6332:
6329:
6327:
6324:
6322:
6319:
6317:
6314:
6312:
6309:
6307:
6304:
6302:
6299:
6297:
6294:
6292:
6289:
6287:
6284:
6282:
6279:
6277:
6274:
6272:
6269:
6267:
6264:
6262:
6259:
6258:
6257:
6256:
6252:
6251:
6246:
6243:
6241:
6238:
6236:
6233:
6231:
6228:
6226:
6223:
6221:
6218:
6217:
6216:
6215:
6211:
6210:
6205:
6202:
6201:
6200:
6199:
6195:
6194:
6192:
6190:(noncellular)
6186:
6183:
6181:
6177:
6174:
6172:
6168:
6165:
6163:Extracellular
6161:
6151:
6148:
6146:
6143:
6141:
6138:
6136:
6133:
6131:
6130:Ridaforolimus
6128:
6126:
6123:
6122:
6120:
6118:
6114:
6108:
6105:
6104:
6102:
6100:
6096:
6093:
6089:Intracellular
6087:
6075:
6072:
6071:
6070:
6069:
6065:
6063:
6060:
6058:
6055:
6053:
6050:
6049:
6047:
6045:
6041:
6035:
6032:
6030:
6027:
6026:
6021:
6018:
6016:
6013:
6011:
6008:
6006:
6003:
6002:
6001:
6000:
5996:
5992:
5988:
5987:
5985:
5982:
5976:
5972:
5964:
5961:
5960:
5959:
5958:
5954:
5953:
5948:
5947:Teriflunomide
5945:
5943:
5940:
5939:
5937:
5936:
5932:
5931:
5926:
5923:
5921:
5918:
5917:
5916:
5915:
5911:
5910:
5908:
5906:
5902:
5899:
5895:Intracellular
5893:
5888:
5884:
5880:
5873:
5868:
5866:
5861:
5859:
5854:
5853:
5850:
5834:
5832:
5829:
5828:
5826:
5823:
5820:
5817:
5815:
5812:
5811:
5807:
5797:
5794:
5792:
5789:
5787:
5784:
5782:
5779:
5777:
5774:
5772:
5769:
5767:
5764:
5762:
5759:
5757:
5754:
5752:
5749:
5747:
5744:
5742:
5739:
5737:
5734:
5732:
5729:
5727:
5724:
5722:
5719:
5716:
5713:
5711:
5707:
5706:
5702:
5700:
5699:Tabelecleucel
5697:
5695:
5692:
5690:
5687:
5685:
5682:
5680:
5677:
5675:
5672:
5670:
5667:
5665:
5662:
5660:
5659:Lurbinectedin
5657:
5655:
5652:
5650:
5647:
5645:
5642:
5640:
5637:
5635:
5632:
5630:
5627:
5625:
5622:
5620:
5617:
5615:
5612:
5610:
5607:
5605:
5602:
5600:
5597:
5595:
5592:
5590:
5587:
5585:
5582:
5580:
5577:
5575:
5572:
5570:
5567:
5565:
5562:
5560:
5557:
5555:
5552:
5550:
5547:
5545:
5542:
5539:
5535:
5531:
5530:
5526:
5524:
5521:
5519:
5516:
5514:
5511:
5510:
5508:
5504:
5497:
5493:
5492:
5488:
5485:
5481:
5480:
5476:
5473:
5469:
5468:
5464:
5463:
5461:
5459:
5455:
5448:
5445:
5443:
5440:
5438:
5435:
5433:
5430:
5428:
5424:
5422:
5418:
5415:
5412:
5410:
5407:
5405:
5402:
5400:
5397:
5395:
5391:
5390:
5386:
5383:
5380:
5378:
5375:
5373:
5370:
5367:
5365:
5361:
5360:
5356:
5353:
5349:
5348:
5344:
5341:
5337:
5336:
5332:
5329:
5325:
5324:
5320:
5316:
5313:
5311:
5308:
5306:
5303:
5301:
5298:
5297:
5296:
5295:
5291:
5288:
5285:
5283:
5280:
5278:
5275:
5273:
5270:
5268:
5264:
5263:
5260:
5256:
5253:
5249:
5248:
5244:
5243:
5241:
5239:
5235:
5232:
5228:
5221:
5218:
5216:
5213:
5211:
5208:
5206:
5202:
5201:
5197:
5195:
5192:
5190:
5187:
5185:
5182:
5180:
5177:
5176:
5174:
5172:
5168:
5164:
5149:
5146:
5144:
5141:
5139:
5136:
5134:
5130:
5127:
5126:
5124:
5122:
5121:Intercalation
5118:
5111:
5108:
5106:
5103:
5101:
5097:
5096:
5092:
5090:
5087:
5085:
5082:
5080:
5077:
5074:
5070:
5069:
5065:
5063:
5060:
5059:
5057:
5055:
5051:
5045:
5042:
5040:
5037:
5035:
5032:
5030:
5029:Dicycloplatin
5027:
5025:
5022:
5020:
5017:
5016:
5014:
5012:
5008:
5002:
4999:
4997:
4994:
4992:
4989:
4987:
4984:
4982:
4978:
4977:
4973:
4970:
4968:
4964:
4961:
4959:
4955:
4954:
4951:
4948:
4946:
4943:
4941:
4938:
4935:
4931:
4928:
4926:
4923:
4921:
4918:
4916:
4912:
4911:
4908:
4905:
4903:
4902:Prednimustine
4900:
4897:
4893:
4890:
4888:
4885:
4882:
4879:
4877:
4873:
4870:
4868:
4865:
4863:
4860:
4858:
4854:
4853:
4851:
4849:
4845:
4842:
4839:
4834:
4830:
4820:
4817:
4815:
4812:
4810:
4807:
4805:
4802:
4799:
4796:
4794:
4791:
4789:
4785:
4784:
4780:
4777:
4774:
4772:
4769:
4767:
4764:
4762:
4759:
4757:
4754:
4752:
4749:
4746:
4742:
4739:
4737:
4734:
4732:
4728:
4727:
4723:
4722:
4720:
4718:
4717:Intercalation
4714:
4710:
4703:
4700:
4698:
4694:
4693:
4689:
4688:
4686:
4684:
4680:
4673:
4670:
4668:
4665:
4663:
4660:
4658:
4655:
4653:
4650:
4648:
4645:
4643:
4640:
4638:
4635:
4633:
4630:
4628:
4625:
4623:
4619:
4618:
4614:
4613:
4611:
4609:
4605:
4602:
4599:
4594:
4590:
4579:
4575:
4574:
4570:
4569:
4567:
4565:
4561:
4554:
4551:
4549:
4545:
4544:
4540:
4539:
4535:
4531:
4530:
4526:
4525:
4521:
4520:+daunorubicin
4518:
4514:
4513:
4509:
4508:
4504:
4500:
4497:
4495:
4492:
4490:
4487:
4485:
4484:Doxifluridine
4482:
4480:
4477:
4475:
4471:
4470:
4466:
4465:
4463:
4461:
4457:
4450:
4447:
4445:
4441:
4440:
4436:
4434:
4433:Rabacfosadine
4431:
4429:
4426:
4423:
4420:
4418:
4415:
4413:
4409:
4408:
4404:
4400:
4399:
4395:
4391:
4390:
4386:
4385:
4383:
4381:
4377:
4370:
4367:
4365:
4361:
4360:
4356:
4353:
4350:
4348:
4345:
4343:
4340:
4338:
4334:
4333:
4329:
4328:
4326:
4324:
4320:
4317:
4314:
4309:
4303:
4300:
4296:
4292:
4281:
4277:
4276:
4272:
4269:
4266:
4264:
4261:
4259:
4256:
4254:
4251:
4249:
4246:
4244:
4240:
4239:
4235:
4234:
4232:
4228:
4221:
4218:
4216:
4213:
4211:
4208:
4206:
4203:
4201:
4197:
4196:
4192:
4191:
4189:
4186:
4181:
4178:
4175:
4170:
4166:
4162:
4157:
4153:
4149:
4141:
4136:
4134:
4129:
4127:
4122:
4121:
4118:
4106:
4103:
4101:
4098:
4097:
4095:
4091:
4085:
4082:
4080:
4077:
4075:
4072:
4070:
4067:
4065:
4062:
4060:
4057:
4055:
4054:Levothyroxine
4052:
4050:
4047:
4045:
4042:
4040:
4037:
4035:
4032:
4030:
4027:
4025:
4022:
4020:
4017:
4015:
4012:
4011:
4009:
4005:
4001:
3994:
3989:
3987:
3982:
3980:
3975:
3974:
3971:
3959:
3956:
3954:
3951:
3949:
3946:
3944:
3941:
3940:
3938:
3934:
3924:
3921:
3919:
3916:
3914:
3911:
3910:
3908:
3904:
3898:
3895:
3893:
3890:
3888:
3885:
3883:
3880:
3878:
3875:
3873:
3870:
3868:
3865:
3863:
3860:
3858:
3855:
3854:
3852:
3850:
3846:
3840:
3837:
3835:
3832:
3830:
3827:
3825:
3822:
3820:
3817:
3815:
3812:
3810:
3807:
3806:
3804:
3802:
3798:
3792:
3789:
3787:
3784:
3782:
3779:
3777:
3774:
3772:
3769:
3768:
3766:
3764:
3760:
3757:
3753:
3747:
3744:
3740:
3737:
3736:
3734:
3733:
3731:
3727:
3721:
3720:Teriflunomide
3718:
3716:
3713:
3711:
3708:
3706:
3703:
3700:
3696:
3693:
3691:
3688:
3686:
3683:
3681:
3678:
3676:
3673:
3671:
3668:
3666:
3663:
3661:
3658:
3656:
3653:
3651:
3648:
3646:
3643:
3641:
3638:
3636:
3633:
3631:
3628:
3627:
3625:
3621:
3615:
3612:
3608:
3605:
3603:
3600:
3598:
3595:
3594:
3593:Radiological
3592:
3588:
3585:
3584:
3582:
3578:
3575:
3573:
3570:
3569:
3567:
3566:
3563:
3560:
3556:
3553:
3552:
3551:
3548:
3547:
3545:
3541:
3535:
3532:
3530:
3527:
3525:
3522:
3520:
3517:
3515:
3512:
3510:
3507:
3505:
3502:
3500:
3497:
3495:
3492:
3490:
3487:
3485:
3482:
3481:
3479:
3477:
3473:
3469:
3461:
3456:
3454:
3449:
3447:
3442:
3441:
3438:
3427:
3421:
3413:
3409:
3404:
3399:
3395:
3391:
3387:
3383:
3379:
3372:
3369:
3364:
3357:
3354:
3349:
3343:
3335:
3331:
3327:
3323:
3318:
3313:
3309:
3305:
3301:
3294:
3292:
3290:
3286:
3281:
3275:
3267:
3263:
3258:
3253:
3249:
3245:
3241:
3237:
3233:
3226:
3223:
3218:
3212:
3204:
3200:
3196:
3192:
3188:
3184:
3176:
3173:
3168:
3162:
3154:
3150:
3145:
3140:
3136:
3132:
3128:
3124:
3120:
3113:
3110:
3105:
3099:
3083:
3079:
3075:
3068:
3065:
3060:
3052:
3049:
3044:
3038:
3030:
3026:
3022:
3018:
3013:
3008:
3004:
3000:
2996:
2989:
2987:
2983:
2978:
2971:
2968:
2963:
2957:
2949:
2945:
2941:
2937:
2933:
2929:
2925:
2921:
2914:
2911:
2906:
2900:
2892:
2888:
2884:
2880:
2876:
2872:
2868:
2864:
2856:
2854:
2850:
2845:
2839:
2831:
2827:
2822:
2817:
2813:
2809:
2805:
2798:
2795:
2790:
2784:
2776:
2772:
2768:
2764:
2759:
2754:
2750:
2746:
2742:
2735:
2733:
2731:
2727:
2722:
2718:
2713:
2708:
2704:
2700:
2696:
2692:
2688:
2681:
2678:
2673:
2669:
2665:
2661:
2657:
2653:
2649:
2645:
2638:
2635:
2630:
2626:
2621:
2616:
2612:
2608:
2604:
2600:
2596:
2589:
2586:
2581:
2577:
2571:
2568:
2556:
2552:
2545:
2542:
2537:
2533:
2527:
2524:
2519:
2515:
2509:
2506:
2501:
2495:
2487:
2483:
2478:
2473:
2469:
2465:
2461:
2457:
2453:
2446:
2443:
2438:
2431:
2429:
2425:
2421:
2406:
2402:
2395:
2389:
2387:
2385:
2381:
2376:
2372:
2365:
2362:
2349:
2345:
2341:
2335:
2332:
2327:
2323:
2316:
2313:
2308:
2304:
2297:
2294:
2289:
2283:
2280:
2275:
2271:
2264:
2262:
2258:
2242:
2238:
2231:
2225:
2222:
2206:
2202:
2195:
2189:
2187:
2183:
2178:
2174:
2170:
2163:
2160:
2155:
2148:
2141:
2138:
2133:
2129:
2124:
2119:
2115:
2111:
2107:
2103:
2099:
2092:
2089:
2084:
2080:
2075:
2070:
2066:
2062:
2058:
2054:
2050:
2043:
2040:
2035:
2029:
2021:
2017:
2012:
2007:
2003:
1999:
1995:
1991:
1987:
1980:
1978:
1976:
1972:
1967:
1961:
1953:
1949:
1945:
1941:
1937:
1933:
1926:
1923:
1918:
1914:
1910:
1906:
1902:
1898:
1894:
1890:
1883:
1881:
1877:
1872:
1868:
1863:
1858:
1853:
1848:
1844:
1840:
1836:
1829:
1826:
1821:
1817:
1812:
1807:
1803:
1799:
1795:
1791:
1787:
1780:
1778:
1774:
1770:
1766:
1762:
1757:
1752:
1748:
1744:
1740:
1733:
1730:
1725:
1721:
1717:
1711:
1708:
1695:
1691:
1685:
1683:
1681:
1679:
1675:
1669:
1664:
1660:
1656:
1650:
1647:
1642:
1638:
1633:
1628:
1624:
1620:
1616:
1612:
1608:
1601:
1598:
1593:
1589:
1585:
1581:
1577:
1573:
1569:
1565:
1558:
1556:
1554:
1552:
1550:
1548:
1544:
1539:
1532:
1526:
1524:
1522:
1520:
1518:
1516:
1514:
1512:
1510:
1508:
1506:
1504:
1502:
1498:
1485:
1481:
1477:
1471:
1469:
1465:
1452:
1448:
1444:
1438:
1435:
1430:
1426:
1421:
1416:
1412:
1408:
1404:
1400:
1396:
1389:
1387:
1385:
1383:
1381:
1379:
1377:
1375:
1373:
1371:
1367:
1354:
1347:
1341:
1339:
1337:
1335:
1331:
1326:
1322:
1318:
1314:
1310:
1306:
1302:
1298:
1291:
1288:
1275:
1271:
1265:
1262:
1249:
1248:
1247:Health Canada
1243:
1237:
1234:
1222:
1218:
1214:
1208:
1205:
1192:
1188:
1182:
1179:
1172:
1170:
1168:
1164:
1156:
1154:
1152:
1146:
1143:
1139:
1135:
1133:
1128:
1120:
1118:
1114:
1110:
1106:
1102:
1100:
1091:
1089:
1085:
1082:
1079:
1078:herpes zoster
1074:
1070:
1067:
1062:
1055:
1053:
1049:
1045:
1041:
1039:
1035:
1030:
1028:
1025:in 2006, and
1024:
1019:
1012:
1010:
1008:
1004:
1000:
996:
992:
988:
984:
980:
972:
970:
967:
965:
961:
957:
952:
950:
945:
941:
937:
930:
928:
924:
920:
916:
912:
910:
906:
900:
896:
889:
887:
885:
880:
878:
872:
870:
866:
862:
861:histiocytosis
853:
851:
849:
846:It is on the
844:
842:
838:
834:
830:
826:
823:
819:
815:
810:
808:
804:
800:
796:
792:
788:
778:
771:
762:
757:
753:
746:
737:
733:
726:
719:
715:
714:
712:
709:
704:
697:
695:
691:
661:
659:
655:
650:
646:
642:
639:
637:
635:ECHA InfoCard
631:
623:
619:
618:DTXSID8022828
615:
614:
612:
603:
599:
592:
588:
587:
585:
583:
579:
572:
568:
567:
565:
563:
559:
552:
548:
547:
545:
543:
539:
532:
528:
527:
525:
523:
519:
512:
508:
507:
505:
503:
499:
492:
488:
487:
485:
483:
479:
472:
468:
467:
465:
463:
459:
452:
448:
447:
445:
438:
434:
427:
423:
422:
420:
418:
414:
405:
401:
394:
389:
385:
383:
379:
374:
372:
365:
361:
357:
354:
353:intracellular
350:
348:
344:
340:
338:
334:
330:
326:
322:
320:
316:
312:
308:
302: Rx-only
295:
292:
280:
277:
267:
265:
256:
253:
243:
242:
240:
238:
234:
229:
222:
218:
213:
208:
203:
198:
193:
190:
189:
187:
185:
181:
177:
173:
169:
166:
164:
158:
152:
143:
142:
140:
138:
132:
125:
120:
111:
110:
108:
106:
102:
98:
94:
92:
88:
84:
80:
78:
74:
70:
66:
62:
58:
56:
52:
49:Clinical data
47:
43:
38:
30:
19:
7322:Orphan drugs
7279:Upadacitinib
7239:Satralizumab
7224:Ritlecitinib
7219:Risankizumab
7014:
6995:
6935:Teprotumumab
6885:Inebilizumab
6880:Fontolizumab
6860:Atorolimumab
6828:T-lymphocyte
6826:
6800:
6784:
6770:
6751:
6740:Lerdelimumab
6735:Bertilimumab
6727:
6715:
6694:
6680:
6666:
6652:
6633:
6614:
6598:Pascolizumab
6583:Obinutuzumab
6575:
6561:
6547:
6531:Clenoliximab
6523:
6507:Otelixizumab
6494:
6467:
6454:Lebrikizumab
6438:
6422:
6406:
6390:
6374:
6331:Satralizumab
6321:Risankizumab
6253:
6212:
6196:
6188:Serum target
6140:Temsirolimus
6066:
6057:Pomalidomide
6052:Lenalidomide
6010:Pimecrolimus
5989:
5963:Methotrexate
5955:
5933:
5920:Azathioprine
5912:
5897:(initiation)
5746:Tazemetostat
5710:Alitretinoin
5703:
5614:Estramustine
5594:Elsamitrucin
5584:Eflornithine
5538:Pegaspargase
5534:Asparaginase
5527:
5496:Testolactone
5489:
5477:
5465:
5419:
5404:Panobinostat
5387:
5357:
5345:
5333:
5321:
5292:
5257:
5245:
5198:
5194:Padeliporfin
5133:Dactinomycin
5129:Streptomyces
5110:Temozolomide
5105:Mitozolomide
5093:
5084:Mitobronitol
5073:Procarbazine
5066:
5054:Nonclassical
4979:
4956:
4950:Streptozocin
4915:Nitrosoureas
4913:
4887:Chlorambucil
4881:Trofosfamide
4867:Chlormethine
4862:Bendamustine
4855:
4793:Mitoxantrone
4788:Losoxantrone
4781:
4741:Daunorubicin
4724:
4690:
4627:Camptothecin
4615:
4571:
4541:
4527:
4510:
4494:Fluorouracil
4474:Capecitabine
4467:
4437:
4411:
4401:
4387:
4357:
4352:Pralatrexate
4342:Methotrexate
4330:
4273:
4236:
4193:
4069:Mitoxantrone
4028:
4000:Merck Serono
3801:Inflammatory
3680:Mitoxantrone
3639:
3514:Incontinence
3420:cite journal
3385:
3381:
3371:
3362:
3356:
3342:cite journal
3307:
3303:
3274:cite journal
3239:
3235:
3225:
3211:cite journal
3189:(1): 49–54.
3186:
3182:
3175:
3161:cite journal
3126:
3122:
3112:
3098:cite journal
3086:. Retrieved
3082:the original
3077:
3067:
3058:
3051:
3037:cite journal
3002:
2998:
2976:
2970:
2956:cite journal
2923:
2919:
2913:
2899:cite journal
2866:
2862:
2838:cite journal
2811:
2807:
2797:
2783:cite journal
2748:
2744:
2694:
2690:
2680:
2647:
2643:
2637:
2602:
2598:
2588:
2579:
2570:
2558:. Retrieved
2554:
2544:
2536:PR News Wire
2535:
2526:
2517:
2508:
2494:cite journal
2459:
2455:
2445:
2419:
2412:. Retrieved
2405:the original
2400:
2374:
2364:
2352:. Retrieved
2348:the original
2343:
2334:
2325:
2315:
2306:
2296:
2282:
2273:
2248:. Retrieved
2241:the original
2236:
2224:
2212:. Retrieved
2205:the original
2200:
2177:the original
2172:
2162:
2153:
2140:
2105:
2101:
2091:
2056:
2053:eBioMedicine
2052:
2042:
2028:cite journal
1993:
1989:
1960:cite journal
1935:
1931:
1925:
1892:
1888:
1842:
1838:
1828:
1793:
1789:
1768:
1746:
1742:
1732:
1724:the original
1719:
1710:
1698:. Retrieved
1693:
1668:10665/371090
1658:
1649:
1614:
1610:
1600:
1570:(1): 28–35.
1567:
1563:
1537:
1488:. Retrieved
1484:the original
1479:
1455:. Retrieved
1451:the original
1446:
1437:
1402:
1398:
1357:. Retrieved
1352:
1300:
1296:
1290:
1278:. Retrieved
1273:
1264:
1252:. Retrieved
1250:. 9 May 2018
1245:
1236:
1224:. Retrieved
1216:
1207:
1195:. Retrieved
1190:
1187:"Cladribine"
1181:
1160:
1147:
1140:
1136:
1124:
1115:
1111:
1107:
1103:
1098:
1095:
1086:
1083:
1075:
1071:
1063:
1059:
1050:
1046:
1042:
1034:cyclodextrin
1031:
1020:
1016:
995:septic shock
976:
968:
953:
942:had studied
934:
925:
921:
917:
913:
909:cytochrome c
901:
897:
893:
881:
873:
863:, including
857:
854:Medical uses
845:
811:
802:
790:
786:
785:
774:
571:CHEBI:567361
368:Elimination
359:
237:Legal status
231:Legal status
172:subcutaneous
105:License data
29:
7274:Tofacitinib
7214:Ravulizumab
7204:Pirfenidone
7194:Peficitinib
7094:Canakinumab
7084:Briakinumab
7074:Bimekizumab
7069:Baricitinib
7034:Aflibercept
6945:Vepalimomab
6940:Vapaliximab
6915:Rovelizumab
6905:Pexelizumab
6895:Morolimumab
6870:Cedelizumab
6855:Anifrolumab
6850:Alemtuzumab
6808:Basiliximab
6778:Tocilizumab
6764:Vedolizumab
6759:Natalizumab
6745:Metelimumab
6688:Gavilimomab
6646:Toralizumab
6641:Teneliximab
6627:Lumiliximab
6622:Gomiliximab
6608:Ublituximab
6588:Ocrelizumab
6541:Zanolimumab
6517:Visilizumab
6475:Secukinumab
6459:Ustekinumab
6430:Elsilimomab
6414:Faralimomab
6382:Mepolizumab
6366:Ustekinumab
6361:Tocilizumab
6336:Secukinumab
6286:Canakinumab
6276:Briakinumab
6271:Bimekizumab
6266:Basiliximab
6255:Interleukin
6245:Nerelimomab
6150:Zotarolimus
6091:(reception)
6062:Thalidomide
6020:Voclosporin
6005:Ciclosporin
5999:Calcineurin
5995:Cyclophilin
5942:Leflunomide
5938:inhibitors
5821:from market
5791:Vorasidenib
5771:Trabectedin
5756:Tiazofurine
5751:Tebentafusp
5736:Tagraxofusp
5694:Plitidepsin
5664:Mitoguazone
5604:Epacadostat
5574:Demecolcine
5518:Aflibercept
5491:Sex steroid
5364:Fuzuloparib
5305:Carfilzomib
5277:Palbociclib
5267:Abemaciclib
5220:Verteporfin
5184:Efaproxiral
5100:Dacarbazine
5062:Altretamine
5044:Satraplatin
5039:Oxaliplatin
5019:Carboplatin
4996:Triaziquone
4967:Mannosulfan
4945:Ranimustine
4925:Fotemustine
4766:Pirarubicin
4751:Doxorubicin
4745:+cytarabine
4731:Aclarubicin
4692:Podophyllum
4617:Camptotheca
4548:Azacitidine
4534:Gemcitabine
4489:Floxuridine
4422:Fludarabine
4417:Clofarabine
4403:Halogenated
4394:Pentostatin
4369:Raltitrexed
4337:Aminopterin
4280:Ixabepilone
4275:Epothilones
4243:Cabazitaxel
4220:Vinorelbine
4205:Vincristine
4200:Vinblastine
4185:microtubule
4100:Merck Group
3695:Ocrelizumab
3690:Natalizumab
3635:Alemtuzumab
3310:: 157–167.
3005:: 168–174.
2697:(4): e360.
2462:(6): e158.
2108:(4): e360.
1845:: 1654754.
1359:27 November
1132:lymphopenia
983:neutrophils
964:orphan drug
814:lymphocytes
701: g·mol
641:100.164.726
391:Identifiers
351:Mostly via
168:Intravenous
91:MedlinePlus
64:Other names
55:Trade names
7316:Categories
7264:Sutimlimab
7259:Spesolimab
7244:Siltuximab
7184:Olokizumab
7174:Ixekizumab
7169:Itacitinib
7159:Guselkumab
7154:Fingolimod
7149:Filgotinib
7099:Crovalimab
7089:Brodalumab
7079:Blisibimod
7044:Rilonacept
7027:Opinercept
7022:Etanercept
7008:Belatacept
6959:Polyclonal
6920:Siplizumab
6910:Reslizumab
6900:Ofatumumab
6890:Maslimomab
6875:Emapalumab
6818:Inolimomab
6813:Daclizumab
6792:Odulimomab
6707:Ruplizumab
6702:Frexalimab
6660:Aselizumab
6555:Efalizumab
6512:Teplizumab
6408:Interferon
6398:Omalizumab
6351:Spesolimab
6341:Siltuximab
6316:Rilonacept
6311:Olokizumab
6301:Ixekizumab
6296:Guselkumab
6291:Daclizumab
6281:Brodalumab
6240:Infliximab
6225:Afelimomab
6220:Adalimumab
6204:Eculizumab
6180:Monoclonal
6171:Antibodies
6145:Umirolimus
6125:Everolimus
6074:Apremilast
6034:Gusperimus
6015:Tacrolimus
5983:inhibitors
5975:Macrolides
5957:antifolate
5786:Verdinexor
5781:Venetoclax
5684:Oblimersen
5679:Navitoclax
5654:Lucanthone
5649:Lonidamine
5639:Lifileucel
5634:Ivosidenib
5629:Imetelstat
5599:Enasidenib
5589:Elesclomol
5554:Bexarotene
5549:Belzutifan
5484:Bexarotene
5472:Atrasentan
5447:Umbralisib
5442:Idelalisib
5432:Copanlisib
5414:Vorinostat
5409:Romidepsin
5399:Entinostat
5394:Belinostat
5352:Masoprocol
5340:Tiazofurin
5328:Anagrelide
5300:Bortezomib
5287:Seliciclib
5282:Ribociclib
5262:inhibitors
5252:Tipifarnib
5215:Temoporfin
5210:Talaporfin
5148:Plicamycin
5143:Mitomycins
5089:Pipobroman
5068:Hydrazines
5034:Nedaplatin
4986:Carboquone
4981:Aziridines
4972:Treosulfan
4920:Carmustine
4907:Uramustine
4876:Ifosfamide
4848:Alkylating
4809:Bisantrene
4798:Pixantrone
4771:Valrubicin
4761:Idarubicin
4756:Epirubicin
4702:Teniposide
4657:Lurtotecan
4652:Irinotecan
4553:Decitabine
4517:Cytarabine
4460:Pyrimidine
4449:Tioguanine
4439:Thiopurine
4428:Nelarabine
4412:Cladribine
4364:Pemetrexed
4347:Pemetrexed
4323:Folic acid
4263:Paclitaxel
4215:Vinflunine
4034:Efalizumab
4029:Cladribine
4019:Bisoprolol
3849:Hereditary
3763:Autoimmune
3739:Daclizumab
3675:Laquinimod
3655:Fingolimod
3640:Cladribine
3614:Frexalimab
3499:Dysarthria
3489:Depression
1938:(1): 1–8.
1226:22 October
1173:References
1151:malignancy
1027:Merck KGaA
822:nucleoside
787:Cladribine
706:3D model (
694:Molar mass
591:ChEMBL1619
531:47M74X9YT5
502:ChemSpider
462:IUPHAR/BPS
417:CAS Number
400:IUPAC name
347:Metabolism
202:parenteral
174:(liquid),
124:Cladribine
34:Cladribine
7254:Sirukumab
7249:Siponimod
7234:Sarilumab
7209:Ponesimod
7179:Netakimab
7164:Iptacopan
7144:Etrasimod
7104:Danicopan
7039:Alefacept
7003:Abatacept
6925:Talizumab
6865:Begelomab
6721:Belimumab
6674:Galiximab
6603:Rituximab
6569:Erlizumab
6536:Keliximab
6346:Sirukumab
6326:Sarilumab
6306:Netakimab
6235:Golimumab
6135:Sirolimus
5831:Phase III
5819:Withdrawn
5796:Vosaroxin
5776:Veliparib
5731:Sotorasib
5721:Selinexor
5715:Tretinoin
5705:Retinoids
5619:Glasdegib
5564:Celecoxib
5513:Adagrasib
5437:Duvelisib
5427:Alpelisib
5382:Rucaparib
5369:Niraparib
5310:Oprozomib
5272:Alvocidib
5138:Bleomycin
5095:Triazenes
5079:Etoglucid
5024:Cisplatin
4940:Nimustine
4934:Semustine
4930:Lomustine
4892:Melphalan
4819:Menogaril
4814:Crisnatol
4804:Amsacrine
4776:Zorubicin
4736:Amrubicin
4697:Etoposide
4672:Topotecan
4667:Silatecan
4662:Rubitecan
4647:Gimatecan
4632:Cositecan
4622:Belotecan
4298:inhibitor
4268:Tesetaxel
4258:Ortataxel
4253:Larotaxel
4248:Docetaxel
4210:Vindesine
4064:Metformin
4024:Cetuximab
3715:Siponimod
3710:Ponesimod
3568:Clinical
3519:Nystagmus
3504:Dysphagia
2560:22 August
2456:Neurology
2414:21 August
2354:21 August
2250:21 August
2214:21 August
2102:Neurology
2059:: 41–50.
1917:207508023
1490:19 August
1280:26 August
1191:Drugs.com
1007:platelets
837:apoptosis
803:Mavenclad
791:Leustatin
426:4291-63-8
382:Excretion
370:half-life
161:Routes of
135:Pregnancy
83:Monograph
77:Drugs.com
7306:Medicine
7189:Ozanimod
7064:Avacopan
7057:Unsorted
6843:Unsorted
6753:Integrin
6486:Cellular
6261:Anakinra
6107:Anakinra
6029:Abetimus
5881: /
5669:Mitotane
5609:Eribulin
5377:Olaparib
5315:Ixazomib
4991:Thiotepa
4963:Busulfan
4642:Exatecan
4479:Carmofur
4187:assembly
4150: /
4079:Serostim
4014:Avelumab
4007:Products
3705:Ozanimod
3494:Diplopia
3412:26109102
3334:81873347
3326:30885374
3266:28140753
3203:25876451
3153:29716436
3029:83461539
3021:30885375
2940:23263473
2891:20149620
2883:21397565
2830:20089960
2767:28870107
2721:28626781
2664:29634596
2629:30944586
2486:26468472
2132:28626781
2083:28161400
2020:29550884
1952:27463204
1909:21463108
1871:32256946
1820:33247831
1765:26913480
1694:DailyMed
1657:(2023).
1641:24652320
1592:43201228
1584:21242742
1429:29168160
1325:32926069
1254:13 April
1157:Research
1099:Post-hoc
985:(called
777:(verify)
482:DrugBank
200:) (
184:ATC code
178:(tablet)
176:by mouth
137:category
119:DailyMed
7332:Purines
6985:-cept (
4598:S phase
4499:Tegafur
4313:S phase
4238:Taxanes
4174:M phase
4093:Related
3735:Former
3509:Fatigue
3403:4807901
3365:: A965.
3257:5818021
3144:6460686
3088:14 July
3078:Ectrims
3061:: 0542.
2977:Ectrims
2948:8934723
2775:1910070
2712:5459792
2672:4736668
2620:6440030
2549:Merck.
2477:4592538
2123:5459792
2074:5474520
2011:5937883
1862:7103043
1811:7708385
1700:4 March
1632:4198068
1420:5722776
1317:9068927
1197:4 March
1003:anaemic
658:Formula
491:DB00242
437:PubChem
386:Urinary
376:phases.
356:kinases
293:Rx-only
290:WARNING
262::
209: (
207:L04AA40
194: (
192:L01BB04
150: D
121::
97:a693015
7292:Portal
6997:CTLA-4
6987:Fusion
6488:target
6469:IL-17A
6446:, and
5979:other
5814:WHO-EM
5423:(Pi3K)
4380:Purine
4183:Block
4105:Serono
4074:Saizen
3484:Ataxia
3410:
3400:
3332:
3324:
3264:
3254:
3201:
3151:
3141:
3027:
3019:
2979:: 549.
2946:
2938:
2889:
2881:
2828:
2773:
2765:
2719:
2709:
2670:
2662:
2627:
2617:
2484:
2474:
2130:
2120:
2081:
2071:
2018:
2008:
1950:
1915:
1907:
1869:
1859:
1818:
1808:
1763:
1639:
1629:
1590:
1582:
1457:1 July
1427:
1417:
1323:
1315:
981:, and
732:SMILES
699:285.69
582:ChEMBL
551:D01370
329:orally
323:100% (
287:
274:
264:℞-only
250:
117:
6786:LFA-1
6696:CD154
6549:CD11a
6448:IL-23
6444:IL-13
6440:IL-12
6044:IMiDs
5335:IMPDI
5230:Other
3936:Other
3906:Other
3330:S2CID
3025:S2CID
2944:S2CID
2887:S2CID
2771:S2CID
2668:S2CID
2408:(PDF)
2397:(PDF)
2244:(PDF)
2233:(PDF)
2208:(PDF)
2197:(PDF)
2150:(PDF)
1913:S2CID
1790:Drugs
1588:S2CID
1534:(PDF)
1349:(PDF)
1321:S2CID
752:InChI
708:JSmol
562:ChEBI
511:19105
451:20279
6716:BLyS
6668:CD80
6635:CD40
6616:CD23
6577:CD20
6563:CD18
6424:IL-6
6117:mTOR
5991:FKBP
5981:IL-2
5421:PIKI
5389:HDAC
4838:CCNS
3529:Pain
3426:link
3408:PMID
3348:link
3322:PMID
3280:link
3262:PMID
3217:link
3199:PMID
3167:link
3149:PMID
3104:link
3090:2021
3043:link
3017:PMID
2962:link
2936:PMID
2905:link
2879:PMID
2844:link
2826:PMID
2789:link
2763:PMID
2717:PMID
2660:PMID
2625:PMID
2562:2017
2500:link
2482:PMID
2416:2016
2356:2016
2252:2016
2216:2016
2128:PMID
2079:PMID
2034:link
2016:PMID
1966:link
1948:PMID
1905:PMID
1867:PMID
1843:2020
1816:PMID
1761:PMID
1702:2020
1637:PMID
1580:PMID
1492:2016
1459:2024
1425:PMID
1361:2014
1313:PMID
1282:2024
1256:2024
1228:2023
1199:2020
1023:Teva
938:and
867:and
542:KEGG
522:UNII
471:4799
325:i.v.
313:data
219:for
217:oral
73:AHFS
18:2CDA
6729:CAT
6525:CD4
6496:CD3
6214:TNF
5887:L04
5467:ERA
5323:PhI
5294:PrI
5259:CDK
5171:PDT
4169:MIs
4165:SPs
4156:L01
3398:PMC
3390:doi
3312:doi
3252:PMC
3244:doi
3191:doi
3139:PMC
3131:doi
3059:EAN
3007:doi
2928:doi
2924:260
2871:doi
2816:doi
2812:362
2753:doi
2707:PMC
2699:doi
2652:doi
2615:PMC
2607:doi
2472:PMC
2464:doi
2118:PMC
2110:doi
2069:PMC
2061:doi
2006:PMC
1998:doi
1994:265
1940:doi
1897:doi
1857:PMC
1847:doi
1806:PMC
1798:doi
1751:doi
1747:173
1663:hdl
1627:PMC
1619:doi
1572:doi
1415:PMC
1407:doi
1305:doi
1221:FDA
905:p53
607:EPA
441:CID
276:POM
215:) (
212:WHO
197:WHO
7318::
6442:,
5827::
5347:LI
5247:FI
4713:II
4683:II
4505:))
3422:}}
3418:{{
3406:.
3396:.
3384:.
3380:.
3344:}}
3340:{{
3328:.
3320:.
3308:29
3306:.
3302:.
3288:^
3276:}}
3272:{{
3260:.
3250:.
3240:24
3238:.
3234:.
3213:}}
3209:{{
3197:.
3185:.
3163:}}
3159:{{
3147:.
3137:.
3127:25
3125:.
3121:.
3100:}}
3096:{{
3076:.
3039:}}
3035:{{
3023:.
3015:.
3003:29
3001:.
2997:.
2985:^
2958:}}
2954:{{
2942:.
2934:.
2922:.
2901:}}
2897:{{
2885:.
2877:.
2867:10
2865:.
2852:^
2840:}}
2836:{{
2824:.
2810:.
2806:.
2785:}}
2781:{{
2769:.
2761:.
2749:24
2747:.
2743:.
2729:^
2715:.
2705:.
2693:.
2689:.
2666:.
2658:.
2648:31
2646:.
2623:.
2613:.
2603:12
2601:.
2597:.
2578:.
2553:.
2534:.
2516:.
2496:}}
2492:{{
2480:.
2470:.
2458:.
2454:.
2427:^
2418:.
2399:.
2383:^
2373:.
2342:.
2324:.
2305:.
2272:.
2260:^
2235:.
2199:.
2185:^
2171:.
2152:.
2126:.
2116:.
2104:.
2100:.
2077:.
2067:.
2057:16
2055:.
2051:.
2030:}}
2026:{{
2014:.
2004:.
1992:.
1988:.
1974:^
1962:}}
1958:{{
1946:.
1934:.
1911:.
1903:.
1893:52
1891:.
1879:^
1865:.
1855:.
1841:.
1837:.
1814:.
1804:.
1794:80
1792:.
1788:.
1776:^
1767:.
1759:.
1745:.
1741:.
1718:.
1692:.
1677:^
1635:.
1625:.
1615:15
1613:.
1609:.
1586:.
1578:.
1568:34
1566:.
1546:^
1536:.
1500:^
1478:.
1467:^
1445:.
1423:.
1413:.
1403:14
1401:.
1397:.
1369:^
1351:.
1333:^
1319:.
1311:.
1301:32
1299:.
1272:.
1244:.
1219:.
1215:.
1189:.
1169:.
871:.
850:.
809:.
675:Cl
672:12
666:10
298:EU
283:US
270:UK
259:CA
252:S4
246:AU
170:,
146:AU
114:US
7294::
7140:)
7136:(
6989:)
6835:)
6831:(
6594:)
6590:(
5997:/
5993:/
5977:/
5889:)
5885:(
5871:e
5864:t
5857:v
5717:)
5708:(
5540:)
5536:/
5532:(
5498:)
5494:(
5486:)
5482:(
5474:)
5470:(
5449:)
5425:(
5416:)
5392:(
5384:)
5362:(
5354:)
5350:(
5342:)
5338:(
5330:)
5326:(
5289:)
5265:(
5254:)
5250:(
5222:)
5203:(
5169:/
5150:)
5131:(
5112:)
5098:(
5075:)
5071:(
4983::
4974:)
4965:(
4960::
4936:)
4932:(
4917::
4898:)
4894:(
4883:)
4874:(
4859::
4840:)
4836:(
4800:)
4786:(
4778:)
4747:)
4743:(
4729:(
4715:+
4704:)
4695:(
4674:)
4620:(
4608:I
4600:)
4596:(
4580:)
4576:(
4555:)
4546:(
4536:)
4532:(
4522:)
4515:(
4501:(
4472:(
4451:)
4442:(
4424:)
4410:(
4405:/
4396:)
4392:(
4371:)
4362:(
4354:)
4335:(
4315:)
4311:(
4282:)
4278:(
4270:)
4241:(
4222:)
4198:(
4176:)
4172:(
4167:/
4158:)
4154:(
4139:e
4132:t
4125:v
3992:e
3985:t
3978:v
3701:)
3697:(
3459:e
3452:t
3445:v
3428:)
3414:.
3392::
3386:7
3350:)
3336:.
3314::
3282:)
3268:.
3246::
3219:)
3205:.
3193::
3187:1
3169:)
3155:.
3133::
3106:)
3092:.
3045:)
3031:.
3009::
2964:)
2950:.
2930::
2907:)
2893:.
2873::
2846:)
2832:.
2818::
2791:)
2777:.
2755::
2723:.
2701::
2695:4
2674:.
2654::
2631:.
2609::
2564:.
2520:.
2502:)
2488:.
2466::
2460:2
2439:.
2377:.
2358:.
2328:.
2309:.
2276:.
2254:.
2218:.
2156:.
2134:.
2112::
2106:4
2085:.
2063::
2036:)
2022:.
2000::
1968:)
1954:.
1942::
1936:5
1919:.
1899::
1873:.
1849::
1822:.
1800::
1753::
1704:.
1665::
1643:.
1621::
1594:.
1574::
1494:.
1461:.
1431:.
1409::
1363:.
1327:.
1307::
1284:.
1258:.
1230:.
1201:.
710:)
687:3
684:O
681:5
678:N
669:H
663:C
609:)
605:(
331:)
300::
285::
272::
248::
223:)
204:)
148::
75:/
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.