237:
162:
571:
391:
35:
1042:
man. ... The lack of effect of administration of neomycin to one patient indicates that the hydroxylation occurs in body tissues. ... a major portion of the β-hydroxylation of hydroxyamphetamine occurs in non-adrenal tissue. Unfortunately, at the present time one cannot be completely certain that the hydroxylation of hydroxyamphetamine in vivo is accomplished by the same enzyme which converts dopamine to noradrenaline.
1268:
1189:
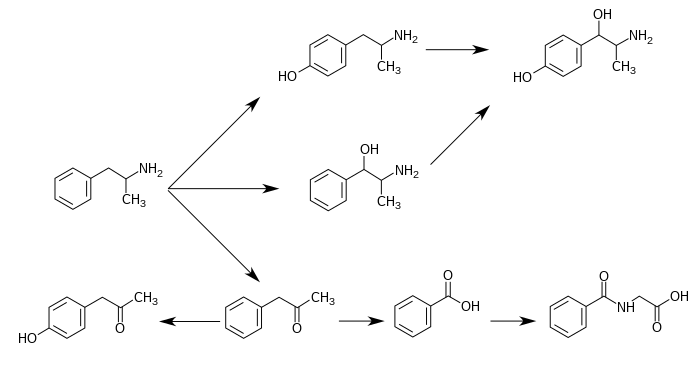
The observed lack of a significant accumulation of PHN in brain following the intraventricular administration of (+)-amphetamine and the formation of appreciable amounts of PHN from (+)-POH in brain tissue in vivo supports the view that the aromatic hydroxylation of amphetamine following its systemic
1136:
The biologic significance of the different levels of serum DβH activity was studied in two ways. First, in vivo ability to β-hydroxylate the synthetic substrate hydroxyamphetamine was compared in two subjects with low serum DβH activity and two subjects with average activity. ... In one study,
1089:
Figure 1. Glycine conjugation of benzoic acid. The glycine conjugation pathway consists of two steps. First benzoate is ligated to CoASH to form the high-energy benzoyl-CoA thioester. This reaction is catalyzed by the HXM-A and HXM-B medium-chain acid:CoA ligases and requires energy in the form of
1041:
Hydroxyamphetamine was administered orally to five human subjects ... Since conversion of hydroxyamphetamine to hydroxynorephedrine occurs in vitro by the action of dopamine-β-oxidase, a simple method is suggested for measuring the activity of this enzyme and the effect of its inhibitors in
790:
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class
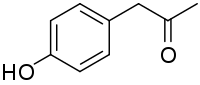
1137:
hydroxyamphetamine (Paredrine), a synthetic substrate for DβH, was administered to subjects with either low or average levels of serum DβH activity. The percent of the drug hydroxylated to hydroxynorephedrine was comparable in all subjects (6.5-9.62) (Table 3).
1090:
ATP. ... The benzoyl-CoA is then conjugated to glycine by GLYAT to form hippuric acid, releasing CoASH. In addition to the factors listed in the boxes, the levels of ATP, CoASH, and glycine may influence the overall rate of the glycine conjugation pathway.
1187:-hydroxylation and β-hydroxylation reactions is important in species where aromatic hydroxylation of amphetamine is the predominant pathway of metabolism. Following systemic administration of amphetamine to rats, POH has been found in urine and in plasma.
1190:
administration occurs predominantly in the periphery, and that POH is then transported through the blood-brain barrier, taken up by noradrenergic neurones in brain where (+)-POH is converted in the storage vesicles by dopamine β-hydroxylase to PHN.
1054:
Badenhorst CP, van der Sluis R, Erasmus E, van Dijk AA (September 2013). "Glycine conjugation: importance in metabolism, the role of glycine N-acyltransferase, and factors that influence interindividual variation".
934:
Cashman JR, Xiong YN, Xu L, Janowsky A (March 1999). "N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication".
791:(39). ... The phase 1 metabolism of amphetamine analogs is catalyzed by two systems: cytochrome P450 and flavin monooxygenase. ... Amphetamine can also undergo aromatic hydroxylation to
961:
Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G (September 2002). "Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection".
674:
Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G (September 2002). "Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection".
276:
1102:
Horwitz D, Alexander RW, Lovenberg W, Keiser HR (May 1973). "Human serum dopamine-β-hydroxylase. Relationship to hypertension and sympathetic activity".
1149:
Freeman JJ, Sulser F (December 1974). "Formation of p-hydroxynorephedrine in brain following intraventricular administration of p-hydroxyamphetamine".
1202:
Matsuda LA, Hanson GR, Gibb JW (December 1989). "Neurochemical effects of amphetamine metabolites on central dopaminergic and serotonergic systems".
1309:
1338:
783:
531:
251:
1348:
1343:
1183:-hydroxynorephedrine (PHN) may contribute to the pharmacological profile of the parent drug. ... The location of the
348:
194:
119:
1302:
215:
883:"Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism"
799:-hydroxynorephedrine. Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
559:
516:
509:
709:
371:
in humans. When it occurs as a metabolite of amphetamine, it is produced directly from the inactive metabolite
1255:
555:
778:(7th ed.). Philadelphia, US: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648.
738:
390:
1228:-OHNor is well documented and dopamine-β hydroxylase present in noradrenergic neurons could easily convert
157:
1295:
795:-hydroxyamphetamine. ... Subsequent oxidation at the benzylic position by DA β-hydroxylase affords
448:
441:
47:
232:
434:
85:
1251:
1127:
1080:
922:
629:; however, other evidence from animal studies suggests that this reaction is catalyzed by DBH in
1211:
1166:
1119:
1072:
1032:
978:
943:
912:
837:
779:
771:
691:
1279:
139:
1333:
1328:
1158:
1111:
1064:
1022:
1014:
970:
902:
894:
827:
683:
630:
299:
203:
575:
In humans, 4-hydroxyphenylacetone occurs as a metabolite of amphetamine and phenylacetone.
236:
161:
95:
1175:
In species where aromatic hydroxylation of amphetamine is the major metabolic pathway,
1027:
1018:
1002:
907:
882:
342:
34:
974:
832:
687:
17:
1322:
1162:
898:
420:
406:
372:
364:
150:
1131:
1084:
621:
metabolism in humans suggests that a different enzyme may mediate the conversion of
183:
413:
1068:
812:
617:. Evidence from studies that measured the effect of serum DBH concentrations on
427:
368:
1003:"Dopamine-beta-oxidase activity in man, using hydroxyamphetamine as substrate"
327:
130:
1267:
1115:
1076:
1036:
982:
947:
916:
695:
1215:
1170:
1123:
841:
1275:
607:
765:
763:
613:
170:
483:
476:
341:
Except where otherwise noted, data are given for materials in their
813:"Dopamine-beta-hydroxylase. Stereochemical course of the reaction"
118:
108:
570:
923:
Table 5: N-containing drugs and xenobiotics oxygenated by FMO
772:"Phenylisopropylamine stimulants: amphetamine-related agents"
260:
InChI=1S/C9H10O2/c1-7(10)6-8-2-4-9(11)5-3-8/h2-5,11H,6H2,1H3
220:
853:
Dopamine-β-hydroxylase catalyzed the removal of the pro-
1283:
857:
hydrogen atom and the production of 1-norephedrine, (2
733:
731:
1204:
936:
774:. In Lemke TL, Williams DA, Roche VF, Zito W (eds.).
378:
1057:Expert Opinion on Drug Metabolism & Toxicology
963:Journal of Pharmaceutical and Biomedical Analysis
1007:British Journal of Pharmacology and Chemotherapy
182:
611:and it is presumed to be metabolized similarly
94:
996:
994:
992:
669:
667:
1303:
1236:-OHNor after intraventricular administration.
748:. Shire US Inc. December 2013. pp. 12–13
8:
865:)-2-amino-1-hydroxyl-1-phenylpropane, from
385:Metabolic pathways of amphetamine in humans
1310:
1296:
1001:Sjoerdsma A, von Studnitz W (April 1963).
746:United States Food and Drug Administration
633:within noradrenergic neurons in the brain.
235:
160:
138:
26:
1254:at the U.S. National Library of Medicine
1026:
906:
831:
202:
776:Foye's principles of medicinal chemistry
663:
646:
591:
281:
256:
231:
601:has been shown to be metabolized into
151:
881:Krueger SK, Williams DE (June 2005).
739:"Adderall XR Prescribing Information"
263:Key: VWMVAQHMFFZQGD-UHFFFAOYSA-N
7:
1264:
1262:
605:by dopamine beta-hydroxylase (DBH)
173:
1282:. You can help Knowledge (XXG) by
1019:10.1111/j.1476-5381.1963.tb01467.x
25:
1266:
899:10.1016/j.pharmthera.2005.01.001
569:
389:
311:
33:
887:Pharmacology & Therapeutics
820:Journal of Biological Chemistry
345:(at 25 °C , 100 kPa).
52:1-(4-Hydroxyphenyl)propan-2-one
1179:-hydroxyamphetamine (POH) and
363:is the para-hydroxy analog of
317:
305:
1:
1339:Recreational drug metabolites
975:10.1016/S0731-7085(02)00330-8
833:10.1016/S0021-9258(19)43051-2
688:10.1016/S0731-7085(02)00330-8
1163:10.1016/0028-3908(74)90069-0
1069:10.1517/17425255.2013.796929
367:, an inactive metabolite of
1365:
1261:
811:Taylor KB (January 1974).
339:
292:
272:
247:
78:
58:
46:
41:
32:
1256:Medical Subject Headings
710:"4-Hydroxyphenylacetone"
1349:Aromatic compound stubs
1116:10.1161/01.RES.32.5.594
28:4-Hydroxyphenylacetone
1344:Human drug metabolites
1274:This article about an
1252:4-hydroxyphenylacetone
676:J. Pharm. Biomed. Anal
400:4-Hydroxyphenylacetone
361:4-Hydroxyphenylacetone
18:4-hydroxyphenylacetone
627:4-hydroxynorephedrine
603:4-hydroxynorephedrine
449:4-Hydroxynorephedrine
284:CC(=O)CC1=CC=C(C=C1)O
72:-Hydroxyphenylacetone
65:-Hydroxyphenylacetone
1104:Circulation Research
623:4-hydroxyamphetamine
619:4-hydroxyamphetamine
599:4-Hydroxyamphetamine
442:4-Hydroxyamphetamine
48:Preferred IUPAC name
770:Glennon RA (2013).
335: g·mol
29:
1220:The metabolism of
716:. PubChem Compound
349:Infobox references
27:
1291:
1290:
1157:(12): 1187–1190.
1151:Neuropharmacology
631:synaptic vesicles
582:
581:
357:Chemical compound
355:
354:
216:CompTox Dashboard
120:Interactive image
16:(Redirected from
1356:
1312:
1305:
1298:
1270:
1263:
1239:
1238:
1199:
1193:
1192:
1146:
1140:
1139:
1099:
1093:
1092:
1063:(9): 1139–1153.
1051:
1045:
1044:
1030:
998:
987:
986:
958:
952:
951:
942:(3): 1251–1260.
931:
925:
920:
910:
878:
872:
871:
850:
848:
835:
817:
808:
802:
801:
767:
758:
757:
755:
753:
743:
735:
726:
725:
723:
721:
706:
700:
699:
671:
651:
634:
628:
624:
620:
604:
600:
596:
573:
572:
562:
551:
544:
539:
534:
527:
520:
512:
505:
498:
491:
486:
479:
472:
465:
458:
451:
444:
437:
430:
423:
416:
409:
402:
393:
379:
334:
319:
313:
307:
300:Chemical formula
240:
239:
224:
222:
206:
186:
175:
164:
153:
142:
122:
98:
73:
66:
37:
30:
21:
1364:
1363:
1359:
1358:
1357:
1355:
1354:
1353:
1319:
1318:
1317:
1316:
1248:
1243:
1242:
1201:
1200:
1196:
1188:
1148:
1147:
1143:
1101:
1100:
1096:
1053:
1052:
1048:
1000:
999:
990:
960:
959:
955:
933:
932:
928:
921:
880:
879:
875:
846:
844:
815:
810:
809:
805:
786:
769:
768:
761:
751:
749:
741:
737:
736:
729:
719:
717:
708:
707:
703:
673:
672:
665:
660:
655:
654:
648:
643:
641:Reference notes
638:
637:
626:
622:
618:
602:
598:
597:
593:
588:
583:
578:
577:
576:
574:
566:
565:
564:
563:
558:
554:
552:
549:
547:
545:
542:
540:
537:
535:
530:
528:
525:
523:
521:
519:
515:
513:
508:
506:
503:
501:
499:
496:
494:
492:
489:
487:
482:
480:
475:
473:
470:
468:
466:
463:
461:
459:
456:
454:
452:
447:
445:
440:
438:
433:
431:
426:
424:
419:
417:
412:
410:
405:
403:
398:
394:
386:
358:
351:
346:
332:
322:
316:
310:
302:
288:
285:
280:
279:
268:
265:
264:
261:
255:
254:
243:
225:
218:
209:
189:
176:
145:
125:
112:
101:
88:
74:
68:
61:
54:
53:
23:
22:
15:
12:
11:
5:
1362:
1360:
1352:
1351:
1346:
1341:
1336:
1331:
1321:
1320:
1315:
1314:
1307:
1300:
1292:
1289:
1288:
1278:compound is a
1271:
1260:
1259:
1247:
1246:External links
1244:
1241:
1240:
1210:(3): 901–908.
1194:
1141:
1110:(5): 594–599.
1094:
1046:
1013:(2): 278–284.
988:
969:(2): 247–255.
953:
926:
893:(3): 357–387.
873:
826:(2): 454–458.
803:
784:
759:
727:
701:
662:
661:
659:
656:
653:
652:
645:
644:
642:
639:
636:
635:
590:
589:
587:
584:
580:
579:
568:
567:
553:
546:
541:
536:
529:
522:
514:
507:
500:
493:
488:
481:
474:
467:
460:
453:
446:
439:
432:
425:
418:
411:
404:
397:
396:
395:
388:
387:
384:
383:
382:
377:
356:
353:
352:
347:
343:standard state
340:
337:
336:
330:
324:
323:
320:
314:
308:
303:
298:
295:
294:
290:
289:
287:
286:
283:
275:
274:
273:
270:
269:
267:
266:
262:
259:
258:
250:
249:
248:
245:
244:
242:
241:
233:DTXSID40427095
228:
226:
214:
211:
210:
208:
207:
199:
197:
191:
190:
188:
187:
179:
177:
169:
166:
165:
155:
147:
146:
144:
143:
135:
133:
127:
126:
124:
123:
115:
113:
106:
103:
102:
100:
99:
91:
89:
84:
81:
80:
76:
75:
60:
56:
55:
51:
50:
44:
43:
39:
38:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1361:
1350:
1347:
1345:
1342:
1340:
1337:
1335:
1332:
1330:
1327:
1326:
1324:
1313:
1308:
1306:
1301:
1299:
1294:
1293:
1287:
1285:
1281:
1277:
1272:
1269:
1265:
1257:
1253:
1250:
1249:
1245:
1237:
1235:
1231:
1227:
1223:
1217:
1213:
1209:
1205:
1198:
1195:
1191:
1186:
1182:
1178:
1172:
1168:
1164:
1160:
1156:
1152:
1145:
1142:
1138:
1133:
1129:
1125:
1121:
1117:
1113:
1109:
1105:
1098:
1095:
1091:
1086:
1082:
1078:
1074:
1070:
1066:
1062:
1058:
1050:
1047:
1043:
1038:
1034:
1029:
1024:
1020:
1016:
1012:
1008:
1004:
997:
995:
993:
989:
984:
980:
976:
972:
968:
964:
957:
954:
949:
945:
941:
937:
930:
927:
924:
918:
914:
909:
904:
900:
896:
892:
888:
884:
877:
874:
870:
869:-amphetamine.
868:
864:
860:
856:
843:
839:
834:
829:
825:
821:
814:
807:
804:
800:
798:
794:
787:
785:9781609133450
781:
777:
773:
766:
764:
760:
747:
740:
734:
732:
728:
715:
711:
705:
702:
697:
693:
689:
685:
682:(2): 247–55.
681:
677:
670:
668:
664:
657:
650:
647:
640:
632:
616:
615:
610:
609:
595:
592:
585:
561:
557:
533:
518:
511:
504:Hydroxylation
497:Hydroxylation
485:
478:
471:Hydroxylation
464:Hydroxylation
457:Hydroxylation
450:
443:
436:
429:
422:
421:Hippuric acid
415:
408:
407:Phenylacetone
401:
392:
381:
380:
376:
374:
373:phenylacetone
370:
366:
365:phenylacetone
362:
350:
344:
338:
331:
329:
326:
325:
304:
301:
297:
296:
291:
282:
278:
271:
257:
253:
246:
238:
234:
230:
229:
227:
217:
213:
212:
205:
201:
200:
198:
196:
193:
192:
185:
181:
180:
178:
172:
168:
167:
163:
159:
156:
154:
152:ECHA InfoCard
149:
148:
141:
137:
136:
134:
132:
129:
128:
121:
117:
116:
114:
110:
105:
104:
97:
93:
92:
90:
87:
83:
82:
77:
71:
64:
57:
49:
45:
40:
36:
31:
19:
1284:expanding it
1273:
1233:
1229:
1225:
1221:
1219:
1207:
1203:
1197:
1184:
1180:
1176:
1174:
1154:
1150:
1144:
1135:
1107:
1103:
1097:
1088:
1060:
1056:
1049:
1040:
1010:
1006:
966:
962:
956:
939:
935:
929:
890:
886:
876:
866:
862:
858:
854:
852:
845:. Retrieved
823:
819:
806:
796:
792:
789:
775:
750:. Retrieved
745:
718:. Retrieved
713:
704:
679:
675:
649:
612:
606:
594:
543:unidentified
490:unidentified
435:Norephedrine
414:Benzoic acid
399:
360:
359:
79:Identifiers
69:
62:
59:Other names
752:30 December
550:Conjugation
526:Deamination
428:Amphetamine
369:amphetamine
293:Properties
158:100.129.975
1323:Categories
847:6 November
720:25 October
658:References
328:Molar mass
204:7K79N2OO7F
131:ChemSpider
107:3D model (
86:CAS Number
556:XM-ligase
538:Oxidation
524:Oxidative
1276:aromatic
1232:-OHA to
1224:-OHA to
1132:28641000
1085:23738007
1077:23650932
1037:13977820
983:12191709
948:10027866
917:15922018
696:12191709
608:in vitro
96:770-39-8
1334:Phenols
1329:Ketones
1216:2600821
1171:4457764
1124:4713201
1028:1703637
908:1828602
842:4809526
614:in vivo
548:Glycine
333:150.177
184:7019274
171:PubChem
140:5382241
1258:(MeSH)
1214:
1169:
1130:
1122:
1083:
1075:
1035:
1025:
981:
946:
915:
905:
840:
782:
694:
484:CYP2D6
477:CYP2D6
277:SMILES
42:Names
1128:S2CID
1081:S2CID
816:(PDF)
742:(PDF)
586:Notes
560:GLYAT
502:Beta-
495:Beta-
469:Para-
462:Para-
455:Para-
252:InChI
109:JSmol
1280:stub
1212:PMID
1167:PMID
1120:PMID
1073:PMID
1033:PMID
979:PMID
944:PMID
913:PMID
849:2014
838:PMID
780:ISBN
754:2013
722:2013
714:NCBI
692:PMID
532:FMO3
195:UNII
70:para
1208:251
1159:doi
1112:doi
1065:doi
1023:PMC
1015:doi
971:doi
940:288
903:PMC
895:doi
891:106
828:doi
824:249
684:doi
625:to
517:DBH
510:DBH
221:EPA
174:CID
1325::
1218:.
1206:.
1173:.
1165:.
1155:13
1153:.
1134:.
1126:.
1118:.
1108:32
1106:.
1087:.
1079:.
1071:.
1059:.
1039:.
1031:.
1021:.
1011:20
1009:.
1005:.
991:^
977:.
967:30
965:.
938:.
911:.
901:.
889:.
885:.
861:,1
851:.
836:.
822:.
818:.
788:.
762:^
744:.
730:^
712:.
690:.
680:30
678:.
666:^
315:10
67:;
1311:e
1304:t
1297:v
1286:.
1234:p
1230:p
1226:p
1222:p
1185:p
1181:p
1177:p
1161::
1114::
1067::
1061:9
1017::
985:.
973::
950:.
919:.
897::
867:d
863:R
859:S
855:R
830::
797:p
793:p
756:.
724:.
698:.
686::
375:.
321:2
318:O
312:H
309:9
306:C
223:)
219:(
111:)
63:p
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.