752:
69:
713:
736:
88:
56:
769:
757:
1151:
Crucially, a similar buffer operates in the oceans. It is a major factor in climate change and the long-term carbon cycle, due to the large number of marine organisms (especially coral) which are made of calcium carbonate. Increased solubility of carbonate through increased temperatures results in
900:
In solution the equilibrium between carbonate, bicarbonate, carbon dioxide and carbonic acid is sensitive to pH, temperature, and pressure. Although di- and trivalent carbonates have low solubility, bicarbonate salts are far more soluble. This difference is related to the disparate
2879:
970:
within a larger molecule that contains a carbon atom bound to three oxygen atoms, one of which is double bonded. These compounds are also known as organocarbonates or carbonate esters, and have the general formula
2838:
719:
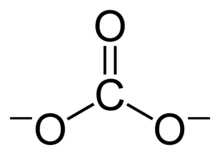
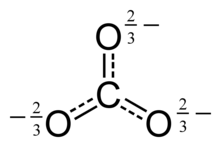
This structure is incompatible with the observed symmetry of the ion, which implies that the three bonds are the same length and that the three oxygen atoms are equivalent. As in the case of the
2567:
Milewski, Jarosław; Wejrzanowski, Tomasz; Fung, Kuan-Zong; Szczśniak, Arkadiusz; Ćwieka, Karol; Tsai, Shu-Yi; Dybiński, Olaf; Skibiński, Jakub; Tang, Jhih-Yu; Szabłowski, Łukasz (2021-04-21).
3455:
751:
610:, and more. New applications of alkali metal carbonates include: thermal energy storage, catalysis and electrolyte both in fuel cell technology as well as in electrosynthesis of
814:
2265:
show evidence for carbonates in space, where aqueous alteration similar to that on Earth is unlikely. Other minerals have been proposed which would fit the observations.
2361:
2347:
2900:
Carbonate/bicarbonate/carbonic acid equilibrium in water: pH of solutions, buffer capacity, titration and species distribution vs. pH computed with a free spreadsheet
302:
3448:
2399:
712:
2272:
have not been found on Mars via remote sensing or in situ missions, even though
Martian meteorites contain small amounts. Groundwater may have existed at
3238:
2945:
1101:, causing the equilibrium of the first reaction to try to restore the level of carbonic acid by reacting bicarbonate with a hydrogen ion, an example of
2682:
3280:
1168:
available is on a geological scale and substantial quantities may eventually be redissolved into the sea and released to the atmosphere, increasing
3266:
2745:
Kemper, F., Molster, F.J., Jager, C. and Waters, L.B.F.M. (2001) The mineral composition and spatial distribution of the dust ejecta of NGC 6302.
3441:
735:
2705:
2636:
1105:. The result is to make the blood more alkaline (raise pH). By the same principle, when the pH is too high, the kidneys excrete bicarbonate (
2473:"Temperature dependence of high-temperature corrosion on nickel-based alloy in molten carbonates for concentrated solar power applications"
1215:
843:
carbonates are water-soluble salts, but carbonates of 2+ and 3+ ions are often poorly soluble in water. Of the insoluble metal carbonates,
3232:
2820:
2352:
776:
Metal carbonates generally decompose on heating, liberating carbon dioxide leaving behind an oxide of the metal. This process is called
2653:
2369:
267:
2471:
Lambrecht, Mickaël; García-Martín, Gustavo; de Miguel, María Teresa; Lasanta, María Isabel; Pérez, Francisco Javier (2023-08-01).
3652:
3408:
3252:
756:
707:
of the carbonate ion has two (long) single bonds to negative oxygen atoms, and one short double bond to a neutral oxygen atom.
3322:
2938:
1102:
380:
244:
2569:"Supporting ionic conductivity of Li2CO3/K2CO3 molten carbonate electrolyte by using yttria stabilized zirconia matrix"
2416:"K2CO3–Li2CO3 molten carbonate mixtures and their nanofluids for thermal energy storage: An overview of the literature"
3464:
1987:
1156:
and increased concentration of atmospheric carbon dioxide. This, in turn, increases Earth temperature. The amount of
188:
68:
3294:
2357:
940:, is in equilibrium with carbonic acid – the equilibrium lies strongly towards carbon dioxide. Thus
2839:"Overview of the Opportunity Mars Exploration Rover Mission to Meridiani Planum: Eagle Crater to Purgatory Ripple"
2931:
2725:
3352:
2094:
2062:
2003:
1208:
3369:
2613:
Anodic generation of hydrogen peroxide in continuous flow, DOI: 10.1039/D2GC02575B (Paper) Green Chem., 2022,
2158:
2022:
1781:
1702:
1532:
2142:
2110:
2078:
2046:
2038:
1861:
1548:
1524:
2182:
2126:
1821:
1572:
2522:"Simple methods for the synthesis of N -substituted acryl amides using Na 2 CO 3 /SiO 2 or NaHSO 4 /SiO 2"
1615:
1384:
1596:
1971:
1829:
1485:
1408:
727:
136:
74:
2568:
2415:
916:. In strongly basic conditions, the carbonate ion predominates, while in weakly basic conditions, the
3133:
2853:
2778:
2521:
2484:
1849:
1608:
1588:
1564:
1331:
1145:
913:
124:
3719:
3482:
3203:
3189:
3175:
3147:
2373:
2328:
1955:
1762:
1671:
1501:
1473:
1420:
1400:
1283:
1275:
1201:
1184:
991:
828:
580:
154:
35:
2627:
594:, have been used since antiquity for cleaning and preservation, as well as for the manufacture of
3724:
3537:
3433:
3308:
3161:
3119:
3094:
3088:
3084:
2812:
2596:
2549:
2453:
2294:
2269:
1659:
1556:
1463:
1444:
1376:
1354:
1315:
987:
983:
945:
840:
832:
654:
550:
531:
480:
768:
3582:
3562:
3422:
3228:
3034:
2804:
2763:
2701:
2588:
2541:
2502:
2445:
2365:
1750:
1722:
1493:
1455:
1436:
1343:
1303:
1263:
963:
844:
836:
820:
611:
508:
3554:
3532:
3516:
3508:
3059:
3014:
3004:
2871:
2861:
2794:
2786:
2580:
2533:
2492:
2435:
2427:
2277:
2259:
2197:
1884:
1770:
1710:
1364:
1291:
967:
941:
909:
824:
565:
500:
487:(which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion,
461:
397:
325:
55:
1193:
253:
208:
3594:
3570:
3524:
3500:
3487:
3105:
3024:
2994:
2968:
2393:
2323:
1299:
1011:
957:
861:
704:
644:
632:
599:
598:. Carbonates are widely used in industry, such as in iron smelting, as a raw material for
436:
164:
42:
31:
2657:
2857:
2782:
2488:
3683:
3678:
3542:
3492:
3388:
3347:
3045:
2973:
2255:
1841:
1631:
921:
902:
868:
743:
666:
603:
484:
465:
420:
374:
360:
3713:
3688:
3393:
2600:
2553:
2457:
2388:
2289:
1240:
785:
720:
697:
662:
607:
401:
2899:
2677:
2575:. International Workshop of Molten Carbonates & Related Topics 2019 (IWMC2019).
499:. Carbonate minerals are extremely varied and ubiquitous in chemically precipitated
3416:
3364:
2905:
2816:
2584:
2273:
468:
gas under pressure or by dissolving carbonate or bicarbonate salts into the water.
2537:
2520:
Hayakawa, Mamiko; Tashiro, Kenshiro; Sumiya, Daiki; Aoyama, Tadashi (2023-06-18).
233:
87:
3636:
2700:(Seventh, Global ed.). Harlow, England: Pearson. pp. 607–608, 666–673.
2497:
2472:
2431:
817:
feature metal ions covalently bonded to carbonate in a variety of bonding modes.
3693:
3626:
3412:
3398:
2258:
is strong evidence for the presence of liquid water. Recent observations of the
995:
917:
777:
670:
457:
453:
365:
2414:
Navarrete, N.; Nithiyanantham, U.; Hernández, L.; Mondragón, R. (2022-03-01).
1118:
857:
658:
476:
347:
199:
2592:
2545:
2506:
2449:
912:, carbonate, bicarbonate, carbon dioxide, and carbonic acid participate in a
3673:
3657:
3631:
2954:
2799:
2790:
853:
789:
519:
2808:
464:
and other carbonated beverages – either by the addition of
17:
2866:
2262:
561:
546:
2440:
1121:(or Krebs–Henseleit ornithine cycle). By removing the bicarbonate, more
905:
of solids composed of mono- vs dianions, as well as mono- vs dications.
3621:
3616:
3610:
2318:
1153:
723:
523:
504:
472:
220:
2875:
860:
is rich in this material, giving rise to the need for infrastructural
742:
This resonance can be summarized by a model with fractional bonds and
3467:
2733:
IPCC Special Report on the Ocean and
Cryosphere in a Changing Climate
640:
636:
109:
99:
373:
Except where otherwise noted, data are given for materials in their
767:
595:
527:
187:
177:
78:
2392:
2721:
3437:
2927:
1197:
2629:
Nomenclature of
Inorganic Chemistry IUPAC Recommendations 2005
2923:
948:
is weakly basic, while carbon dioxide itself is a weak acid.
456:: the process of raising the concentrations of carbonate and
2254:
It is generally thought that the presence of carbonates in
1007:
2764:"Pyroclastic Activity at Home Plate in Gusev Crater, Mars"
856:, it accumulates in and impedes flow through pipes.
815:
Transition metal carbonate and bicarbonate complexes
788:, CaO, which is obtained by roasting limestone in a
3666:
3645:
3603:
3475:
3381:
3340:
2987:
2961:
920:ion is prevalent. In more acid conditions, aqueous
772:
Stalactites and stalagmites are carbonate minerals.
2348:International Union of Pure and Applied Chemistry
867:Acidification of carbonates generally liberates
813:, carbonate is a ligand for many metal cations.
232:
163:
2356:(IUPAC Recommendations 2005). Cambridge (UK):
3449:
2939:
1209:
452:The term is also used as a verb, to describe
8:
2654:"Chemical of the Week -- Biological Buffers"
2403:(11th ed.). Cambridge University Press.
435:. The word "carbonate" may also refer to a
3456:
3442:
3434:
2946:
2932:
2924:
1216:
1202:
1194:
207:
47:
2865:
2798:
2683:The Merck Manual of Diagnosis and Therapy
2496:
2439:
479:, the term "carbonate" can refer both to
252:
3329:
3325:
3315:
3311:
3301:
3297:
3287:
3283:
3273:
3269:
3259:
3255:
3245:
3241:
3223:
3219:
3210:
3206:
3196:
3192:
3182:
3178:
3168:
3164:
3154:
3150:
3140:
3136:
3126:
3122:
3112:
3108:
3097:
3079:
3075:
3066:
3062:
3052:
3048:
3037:
3027:
3017:
3007:
2997:
2976:
2846:Journal of Geophysical Research: Planets
2698:Human physiology. An integrated approach
2573:International Journal of Hydrogen Energy
2383:
2381:
2311:
2304:
2300:
1171:
1139:
1132:
1128:
1097:
1093:
1085:
1077:
1066:
1062:
1053:
1049:
1045:
1041:
1033:
1029:
1014:to stabilise it in the range 7.37–7.43:
978:
935:
927:
889:
885:
881:
877:
847:
802:
798:
692:
688:
657:. It has a molecular mass of 60.01
618:
614:
590:
586:
575:
571:
556:
541:
537:
514:
445:
411:
407:
2340:
1006:Three reversible reactions control the
932:, is the main form, which, with water,
726:ion, the symmetry can be achieved by a
415:, characterized by the presence of the
307:
276:InChI=1S/CH2O3/c2-1(3)4/h(H2,2,3,4)/p-2
272:
2420:Solar Energy Materials and Solar Cells
1253:
897:Thus, scale can be removed with acid.
286:InChI=1/CH2O3/c2-1(3)4/h(H2,2,3,4)/p-2
1944:
982:. Important organocarbonates include
852:is important because, in the form of
439:, an organic compound containing the
279:Key: BVKZGUZCCUSVTD-UHFFFAOYSA-L
7:
2837:Squyres, S. W.; et al. (2006).
2762:Squyres, S. W.; et al. (2007).
1236:
3233:Ethylenetetracarboxylic dianhydride
2353:Nomenclature of Inorganic Chemistry
809:As illustrated by its affinity for
606:manufacture, in the composition of
289:Key: BVKZGUZCCUSVTD-NUQVWONBAE
223:
2678:Acid–Base Regulation and Disorders
631:The carbonate ion is the simplest
522:(as well as the main component of
25:
2696:Silverthorn, Dee Unglaub (2016).
1125:is generated from carbonic acid (
784:, the Latin name of quicklime or
685:, which is the conjugate base of
3653:Graphite intercalation compounds
2885:from the original on 2017-08-08.
2826:from the original on 2017-09-22.
2315:, a hypothetic unstable molecule
994:, and the phosgene replacement,
966:a carbonate can also refer to a
755:
750:
734:
711:
534:, a calcium-magnesium carbonate
86:
67:
54:
34:. For the town in Colorado, see
2642:from the original on 2017-05-18
671:hydrogencarbonate (bicarbonate)
377:(at 25 °C , 100 kPa).
2585:10.1016/j.ijhydene.2020.12.073
1010:balance of blood and act as a
27:Salt or ester of carbonic acid
1:
3382:Compounds derived from oxides
2538:10.1080/00397911.2023.2201454
1117:) into urine as urea via the
2747:Astronomy & Astrophysics
2498:10.1016/j.corsci.2023.111262
2432:10.1016/j.solmat.2021.111525
30:For organic carbonates, see
1152:lower production of marine
518:, the chief constituent of
77:structure of the carbonate
3741:
2726:"Summary for Policymakers"
1946:
1182:
955:
40:
29:
1255:
1233:
1224:Compounds containing the
1090:, which in turn consumes
639:atom surrounded by three
460:ions in water to produce
371:
318:
298:
263:
147:
135:
123:
118:
85:
66:
53:
2526:Synthetic Communications
1103:Le Châtelier's principle
730:among three structures:
41:Not to be confused with
2791:10.1126/science.1139045
2400:Encyclopædia Britannica
986:, the cyclic compounds
3465:Inorganic compounds of
2635:, IUPAC, p. 137,
2250:Presence outside Earth
773:
568:("soda" or "natron"),
503:. The most common are
771:
635:. It consists of one
627:Structure and bonding
137:Systematic IUPAC name
2867:10.1029/2006JE002771
2686:Professional Edition
1146:cellular respiration
1136:), which comes from
661:and carries a total
125:Preferred IUPAC name
2858:2006JGRE..11112S12S
2783:2007Sci...316..738S
2579:(28): 14977–14987.
2489:2023Corro.22011262L
2374:Electronic version.
2329:Sodium percarbonate
1190:Carbonate overview:
1185:Category:Carbonates
992:propylene carbonate
914:dynamic equilibrium
764:Chemical properties
581:potassium carbonate
355: g·mol
50:
36:Carbonate, Colorado
3667:Oxides and related
3423:Peroxydicarbonates
3089:1,3-Dioxetanedione
3085:1,2-Dioxetanedione
2394:"Carbonates"
2295:Orthocarbonic acid
2270:carbonate deposits
1175:levels even more.
988:ethylene carbonate
984:dimethyl carbonate
952:Organic carbonates
946:sodium bicarbonate
774:
655:molecular symmetry
647:arrangement, with
623:in aqueous media.
551:iron(II) carbonate
481:carbonate minerals
381:Infobox references
48:
3707:
3706:
3431:
3430:
3229:Cyclohexanehexone
2777:(5825): 738–742.
2707:978-1-292-09493-9
2477:Corrosion Science
2246:
2245:
2240:
2239:
964:organic chemistry
665:of −2. It is the
509:calcium carbonate
423:with the formula
389:Chemical compound
387:
386:
189:Interactive image
141:Trioxidocarbonate
16:(Redirected from
3732:
3470:and related ions
3458:
3451:
3444:
3435:
3332:
3318:
3304:
3290:
3276:
3262:
3248:
3226:
3213:
3199:
3185:
3171:
3157:
3143:
3129:
3115:
3101:
3082:
3069:
3055:
3041:
3030:
3020:
3010:
3000:
2979:
2948:
2941:
2934:
2925:
2920:
2918:
2916:
2887:
2886:
2884:
2869:
2843:
2834:
2828:
2827:
2825:
2802:
2800:2060/20070016011
2768:
2759:
2753:
2743:
2737:
2736:
2735:. pp. 3–35.
2730:
2718:
2712:
2711:
2693:
2687:
2675:
2669:
2668:
2666:
2665:
2656:. Archived from
2650:
2644:
2643:
2641:
2634:
2624:
2618:
2611:
2605:
2604:
2564:
2558:
2557:
2517:
2511:
2510:
2500:
2468:
2462:
2461:
2443:
2411:
2405:
2404:
2396:
2385:
2376:
2345:
2314:
2307:
2278:Meridiani Planum
2260:planetary nebula
1237:
1218:
1211:
1204:
1195:
1174:
1167:
1166:
1165:
1162:
1143:
1135:
1124:
1116:
1115:
1114:
1111:
1100:
1089:
1081:
1070:
1057:
1036:
1027:
1026:
1023:
981:
974:
968:functional group
942:sodium carbonate
939:
931:
910:aqueous solution
903:lattice energies
893:
850:
812:
805:
759:
754:
738:
715:
695:
684:
683:
682:
679:
621:
593:
578:
566:Sodium carbonate
559:
544:
517:
501:sedimentary rock
498:
497:
496:
493:
462:carbonated water
448:
434:
433:
432:
429:
414:
354:
341:
340:
339:
336:
326:Chemical formula
256:
236:
225:
211:
191:
167:
107:
97:
90:
71:
58:
51:
21:
3740:
3739:
3735:
3734:
3733:
3731:
3730:
3729:
3710:
3709:
3708:
3703:
3684:Metal carbonyls
3662:
3641:
3599:
3590:
3586:
3578:
3574:
3566:
3558:
3550:
3546:
3528:
3520:
3512:
3504:
3496:
3471:
3462:
3432:
3427:
3389:Metal carbonyls
3377:
3373:
3360:
3356:
3336:
3331:
3327:
3323:
3317:
3313:
3309:
3303:
3299:
3295:
3289:
3285:
3281:
3275:
3271:
3267:
3261:
3257:
3253:
3247:
3243:
3239:
3225:
3221:
3217:
3212:
3208:
3204:
3198:
3194:
3190:
3184:
3180:
3176:
3170:
3166:
3162:
3156:
3152:
3148:
3142:
3138:
3134:
3128:
3124:
3120:
3114:
3110:
3106:
3099:
3095:
3081:
3077:
3073:
3068:
3064:
3060:
3054:
3050:
3046:
3039:
3035:
3029:
3025:
3019:
3015:
3009:
3005:
2999:
2995:
2983:
2978:
2974:
2957:
2952:
2914:
2912:
2904:
2896:
2891:
2890:
2882:
2841:
2836:
2835:
2831:
2823:
2766:
2761:
2760:
2756:
2744:
2740:
2728:
2720:
2719:
2715:
2708:
2695:
2694:
2690:
2676:
2672:
2663:
2661:
2652:
2651:
2647:
2639:
2632:
2626:
2625:
2621:
2612:
2608:
2566:
2565:
2561:
2532:(12): 883–892.
2519:
2518:
2514:
2470:
2469:
2465:
2413:
2412:
2408:
2387:
2386:
2379:
2346:
2342:
2337:
2324:Peroxocarbonate
2313:
2309:
2306:
2302:
2298:
2286:
2268:Until recently
2252:
2247:
2242:
2241:
2205:
2201:
2190:
2186:
2170:
2166:
2162:
2154:
2150:
2146:
2138:
2134:
2130:
2122:
2118:
2114:
2106:
2102:
2098:
2090:
2086:
2082:
2074:
2070:
2066:
2058:
2054:
2050:
2045:
2042:
2034:
2030:
2026:
2015:
2011:
2007:
1999:
1995:
1991:
1983:
1979:
1975:
1967:
1963:
1959:
1888:
1869:
1865:
1857:
1853:
1845:
1837:
1833:
1825:
1793:
1789:
1785:
1774:
1766:
1761:
1758:
1754:
1726:
1718:
1714:
1706:
1675:
1667:
1663:
1635:
1627:
1623:
1619:
1612:
1607:
1604:
1600:
1592:
1584:
1580:
1576:
1571:
1568:
1560:
1552:
1544:
1540:
1536:
1531:
1528:
1509:
1505:
1500:
1497:
1489:
1484:
1481:
1477:
1459:
1448:
1443:
1440:
1432:
1428:
1424:
1416:
1412:
1407:
1404:
1396:
1392:
1388:
1383:
1380:
1375:
1372:
1368:
1347:
1342:
1339:
1335:
1330:
1327:
1323:
1319:
1311:
1307:
1302:
1295:
1287:
1279:
1274:
1271:
1267:
1248:
1244:
1229:
1222:
1187:
1181:
1179:Carbonate salts
1173:
1169:
1163:
1160:
1159:
1157:
1141:
1137:
1134:
1130:
1126:
1122:
1112:
1109:
1108:
1106:
1099:
1095:
1091:
1087:
1083:
1079:
1075:
1068:
1064:
1060:
1055:
1051:
1047:
1043:
1039:
1035:
1031:
1024:
1021:
1020:
1018:
1004:
980:
976:
972:
960:
958:Carbonate ester
954:
937:
933:
929:
925:
891:
887:
883:
879:
875:
862:water softening
849:
845:
810:
804:
800:
796:
766:
705:Lewis structure
694:
690:
686:
680:
677:
676:
674:
653:
645:trigonal planar
633:oxocarbon anion
629:
620:
616:
612:
600:Portland cement
592:
588:
584:
577:
573:
569:
560:, an important
558:
554:
543:
539:
535:
516:
512:
494:
491:
490:
488:
447:
443:
441:carbonate group
437:carbonate ester
430:
427:
426:
424:
413:
409:
405:
390:
383:
378:
352:
337:
334:
333:
331:
328:
314:
311:
306:
305:
294:
291:
290:
287:
281:
280:
277:
271:
270:
259:
239:
226:
214:
194:
181:
170:
157:
143:
142:
131:
130:
114:
113:
105:
103:
95:
93:Carbonate anion
91:
81:
72:
62:
61:Carbonate anion
59:
46:
43:Carbon trioxide
39:
32:Carbonate ester
28:
23:
22:
15:
12:
11:
5:
3738:
3736:
3728:
3727:
3722:
3712:
3711:
3705:
3704:
3702:
3701:
3696:
3691:
3686:
3681:
3676:
3670:
3668:
3664:
3663:
3661:
3660:
3655:
3649:
3647:
3646:Nanostructures
3643:
3642:
3640:
3639:
3634:
3629:
3624:
3619:
3614:
3607:
3605:
3601:
3600:
3598:
3597:
3592:
3588:
3584:
3580:
3576:
3572:
3568:
3564:
3560:
3556:
3552:
3548:
3544:
3540:
3535:
3530:
3526:
3522:
3518:
3514:
3510:
3506:
3502:
3498:
3494:
3490:
3485:
3479:
3477:
3473:
3472:
3463:
3461:
3460:
3453:
3446:
3438:
3429:
3428:
3426:
3425:
3420:
3409:Polycarbonates
3406:
3401:
3396:
3391:
3385:
3383:
3379:
3378:
3376:
3375:
3371:
3367:
3362:
3358:
3354:
3350:
3348:Graphite oxide
3344:
3342:
3338:
3337:
3335:
3334:
3320:
3306:
3292:
3278:
3264:
3250:
3236:
3215:
3201:
3187:
3173:
3159:
3145:
3131:
3117:
3103:
3092:
3071:
3057:
3043:
3032:
3022:
3012:
3002:
2991:
2989:
2985:
2984:
2982:
2981:
2971:
2965:
2963:
2959:
2958:
2953:
2951:
2950:
2943:
2936:
2928:
2922:
2921:
2910:Dictionary.com
2902:
2895:
2894:External links
2892:
2889:
2888:
2829:
2754:
2738:
2713:
2706:
2688:
2670:
2645:
2619:
2606:
2559:
2512:
2463:
2406:
2391:, ed. (1911).
2389:Chisholm, Hugh
2377:
2339:
2338:
2336:
2333:
2332:
2331:
2326:
2321:
2316:
2292:
2290:Cap carbonates
2285:
2282:
2251:
2248:
2244:
2243:
2238:
2237:
2234:
2231:
2228:
2225:
2222:
2219:
2216:
2213:
2210:
2207:
2203:
2199:
2195:
2192:
2188:
2184:
2180:
2177:
2173:
2172:
2168:
2164:
2160:
2156:
2152:
2148:
2144:
2140:
2136:
2132:
2128:
2124:
2120:
2116:
2112:
2108:
2104:
2100:
2096:
2092:
2088:
2084:
2080:
2076:
2072:
2068:
2064:
2060:
2056:
2052:
2048:
2040:
2036:
2032:
2028:
2024:
2020:
2017:
2013:
2009:
2005:
2001:
1997:
1993:
1989:
1985:
1981:
1977:
1973:
1969:
1965:
1961:
1957:
1953:
1949:
1948:
1945:
1942:
1941:
1938:
1935:
1932:
1929:
1926:
1923:
1920:
1917:
1914:
1911:
1908:
1905:
1902:
1899:
1896:
1893:
1890:
1886:
1882:
1878:
1877:
1874:
1871:
1867:
1863:
1859:
1855:
1851:
1847:
1843:
1839:
1835:
1831:
1827:
1823:
1819:
1816:
1813:
1810:
1807:
1804:
1801:
1798:
1795:
1791:
1787:
1783:
1779:
1776:
1772:
1768:
1764:
1756:
1752:
1747:
1746:
1743:
1740:
1737:
1734:
1731:
1728:
1724:
1720:
1716:
1712:
1708:
1704:
1700:
1697:
1694:
1691:
1688:
1685:
1682:
1679:
1677:
1673:
1669:
1665:
1661:
1656:
1655:
1652:
1649:
1646:
1643:
1640:
1637:
1633:
1629:
1625:
1621:
1617:
1610:
1602:
1598:
1594:
1590:
1586:
1582:
1578:
1574:
1566:
1562:
1558:
1554:
1550:
1546:
1542:
1538:
1534:
1526:
1522:
1519:
1516:
1513:
1511:
1507:
1503:
1495:
1491:
1487:
1479:
1475:
1470:
1469:
1466:
1461:
1457:
1453:
1450:
1446:
1438:
1434:
1430:
1426:
1422:
1418:
1414:
1410:
1402:
1398:
1394:
1390:
1386:
1378:
1370:
1366:
1361:
1360:
1357:
1352:
1349:
1345:
1337:
1333:
1325:
1321:
1317:
1313:
1309:
1305:
1297:
1293:
1289:
1285:
1281:
1277:
1269:
1265:
1260:
1259:
1256:
1254:
1252:
1250:
1246:
1242:
1235:
1234:
1231:
1230:
1223:
1221:
1220:
1213:
1206:
1198:
1192:
1191:
1180:
1177:
1072:
1071:
1058:
1037:
1003:
1000:
973:R−O−C(=O)−O−R′
956:Main article:
953:
950:
922:carbon dioxide
895:
894:
880:+ 2 HCl → CaCl
869:carbon dioxide
807:
806:
765:
762:
761:
760:
740:
739:
717:
716:
667:conjugate base
651:
628:
625:
608:ceramic glazes
485:carbonate rock
466:carbon dioxide
421:polyatomic ion
388:
385:
384:
379:
375:standard state
372:
369:
368:
363:
361:Conjugate acid
357:
356:
350:
344:
343:
329:
324:
321:
320:
316:
315:
313:
312:
309:
301:
300:
299:
296:
295:
293:
292:
288:
285:
284:
282:
278:
275:
274:
266:
265:
264:
261:
260:
258:
257:
249:
247:
241:
240:
238:
237:
229:
227:
219:
216:
215:
213:
212:
204:
202:
196:
195:
193:
192:
184:
182:
175:
172:
171:
169:
168:
160:
158:
153:
150:
149:
145:
144:
140:
139:
133:
132:
128:
127:
121:
120:
116:
115:
104:
94:
92:
83:
82:
73:
64:
63:
60:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
3737:
3726:
3723:
3721:
3718:
3717:
3715:
3700:
3697:
3695:
3692:
3690:
3689:Carbonic acid
3687:
3685:
3682:
3680:
3677:
3675:
3672:
3671:
3669:
3665:
3659:
3656:
3654:
3651:
3650:
3648:
3644:
3638:
3637:Thiofulminate
3635:
3633:
3630:
3628:
3625:
3623:
3620:
3618:
3615:
3612:
3609:
3608:
3606:
3602:
3596:
3593:
3591:
3581:
3579:
3569:
3567:
3561:
3559:
3553:
3551:
3541:
3539:
3536:
3534:
3531:
3529:
3523:
3521:
3515:
3513:
3507:
3505:
3499:
3497:
3491:
3489:
3486:
3484:
3481:
3480:
3478:
3474:
3469:
3466:
3459:
3454:
3452:
3447:
3445:
3440:
3439:
3436:
3424:
3421:
3418:
3417:Tricarbonates
3414:
3410:
3407:
3405:
3402:
3400:
3397:
3395:
3394:Carbonic acid
3392:
3390:
3387:
3386:
3384:
3380:
3374:
3368:
3366:
3363:
3361:
3351:
3349:
3346:
3345:
3343:
3339:
3333:
3321:
3319:
3307:
3305:
3293:
3291:
3279:
3277:
3265:
3263:
3251:
3249:
3237:
3234:
3230:
3216:
3214:
3202:
3200:
3188:
3186:
3174:
3172:
3160:
3158:
3146:
3144:
3132:
3130:
3118:
3116:
3104:
3102:
3093:
3090:
3086:
3072:
3070:
3058:
3056:
3044:
3042:
3033:
3031:
3023:
3021:
3013:
3011:
3003:
3001:
2993:
2992:
2990:
2988:Exotic oxides
2986:
2980:
2972:
2970:
2967:
2966:
2964:
2962:Common oxides
2960:
2956:
2949:
2944:
2942:
2937:
2935:
2930:
2929:
2926:
2911:
2907:
2903:
2901:
2898:
2897:
2893:
2881:
2877:
2873:
2868:
2863:
2859:
2855:
2851:
2847:
2840:
2833:
2830:
2822:
2818:
2814:
2810:
2806:
2801:
2796:
2792:
2788:
2784:
2780:
2776:
2772:
2765:
2758:
2755:
2751:
2748:
2742:
2739:
2734:
2727:
2723:
2717:
2714:
2709:
2703:
2699:
2692:
2689:
2685:
2684:
2679:
2674:
2671:
2660:on 2011-07-21
2659:
2655:
2649:
2646:
2638:
2631:
2630:
2623:
2620:
2616:
2610:
2607:
2602:
2598:
2594:
2590:
2586:
2582:
2578:
2574:
2570:
2563:
2560:
2555:
2551:
2547:
2543:
2539:
2535:
2531:
2527:
2523:
2516:
2513:
2508:
2504:
2499:
2494:
2490:
2486:
2482:
2478:
2474:
2467:
2464:
2459:
2455:
2451:
2447:
2442:
2437:
2433:
2429:
2425:
2421:
2417:
2410:
2407:
2402:
2401:
2395:
2390:
2384:
2382:
2378:
2375:
2371:
2370:0-85404-438-8
2367:
2363:
2359:
2355:
2354:
2349:
2344:
2341:
2334:
2330:
2327:
2325:
2322:
2320:
2317:
2296:
2293:
2291:
2288:
2287:
2283:
2281:
2279:
2275:
2271:
2266:
2264:
2261:
2257:
2249:
2235:
2232:
2229:
2226:
2223:
2220:
2217:
2214:
2211:
2208:
2206:
2196:
2193:
2191:
2181:
2178:
2175:
2174:
2171:
2157:
2155:
2141:
2139:
2125:
2123:
2109:
2107:
2093:
2091:
2077:
2075:
2061:
2059:
2043:
2037:
2035:
2021:
2018:
2016:
2002:
2000:
1986:
1984:
1970:
1968:
1954:
1951:
1950:
1943:
1939:
1936:
1933:
1930:
1927:
1924:
1921:
1918:
1915:
1912:
1909:
1906:
1903:
1900:
1897:
1894:
1891:
1889:
1883:
1880:
1879:
1875:
1872:
1870:
1860:
1858:
1848:
1846:
1840:
1838:
1828:
1826:
1820:
1817:
1814:
1811:
1808:
1805:
1802:
1799:
1796:
1794:
1780:
1777:
1775:
1769:
1767:
1759:
1749:
1748:
1744:
1741:
1738:
1735:
1732:
1729:
1727:
1721:
1719:
1709:
1707:
1701:
1698:
1695:
1692:
1689:
1686:
1683:
1680:
1678:
1676:
1670:
1668:
1658:
1657:
1653:
1650:
1647:
1644:
1641:
1638:
1636:
1630:
1628:
1613:
1605:
1595:
1593:
1587:
1585:
1569:
1563:
1561:
1555:
1553:
1547:
1545:
1529:
1523:
1520:
1517:
1514:
1512:
1510:
1498:
1492:
1490:
1482:
1472:
1471:
1467:
1465:
1462:
1460:
1454:
1451:
1449:
1441:
1435:
1433:
1419:
1417:
1405:
1399:
1397:
1381:
1373:
1363:
1362:
1358:
1356:
1353:
1350:
1348:
1340:
1328:
1314:
1312:
1301:
1298:
1296:
1290:
1288:
1282:
1280:
1272:
1262:
1261:
1257:
1251:
1249:
1239:
1238:
1232:
1227:
1219:
1214:
1212:
1207:
1205:
1200:
1199:
1196:
1189:
1188:
1186:
1178:
1176:
1155:
1149:
1147:
1120:
1104:
1059:
1038:
1017:
1016:
1015:
1013:
1009:
1001:
999:
997:
993:
989:
985:
969:
965:
959:
951:
949:
947:
943:
923:
919:
915:
911:
906:
904:
898:
874:
873:
872:
870:
865:
863:
859:
855:
851:
842:
838:
834:
830:
826:
822:
818:
816:
795:
794:
793:
791:
787:
786:calcium oxide
783:
779:
770:
763:
758:
753:
749:
748:
747:
745:
737:
733:
732:
731:
729:
725:
722:
721:isoelectronic
714:
710:
709:
708:
706:
701:
699:
698:carbonic acid
672:
668:
664:
663:formal charge
660:
656:
650:
646:
642:
638:
634:
626:
624:
622:
609:
605:
601:
597:
582:
567:
563:
552:
548:
533:
529:
525:
521:
510:
506:
502:
486:
482:
478:
474:
469:
467:
463:
459:
455:
450:
442:
438:
422:
418:
417:carbonate ion
403:
402:carbonic acid
399:
395:
382:
376:
370:
367:
364:
362:
359:
358:
351:
349:
346:
345:
330:
327:
323:
322:
317:
308:
304:
297:
283:
273:
269:
262:
255:
251:
250:
248:
246:
243:
242:
235:
231:
230:
228:
222:
218:
217:
210:
206:
205:
203:
201:
198:
197:
190:
186:
185:
183:
179:
174:
173:
166:
162:
161:
159:
156:
152:
151:
146:
138:
134:
126:
122:
117:
111:
101:
89:
84:
80:
76:
70:
65:
57:
52:
44:
37:
33:
19:
3698:
3694:Bicarbonates
3413:Dicarbonates
3403:
3399:Bicarbonates
2913:. Retrieved
2909:
2852:(E12): n/a.
2849:
2845:
2832:
2774:
2770:
2757:
2749:
2746:
2741:
2732:
2716:
2697:
2691:
2681:
2673:
2662:. Retrieved
2658:the original
2648:
2628:
2622:
2614:
2609:
2576:
2572:
2562:
2529:
2525:
2515:
2480:
2476:
2466:
2441:10234/196651
2423:
2419:
2409:
2398:
2351:
2343:
2267:
2253:
1225:
1150:
1144:produced by
1073:
1005:
961:
907:
899:
896:
866:
819:
808:
781:
775:
741:
718:
702:
648:
643:atoms, in a
630:
583:("potash"),
530:skeletons);
470:
451:
440:
416:
393:
391:
148:Identifiers
3627:Thiocyanate
3604:Carbon ions
2906:"Carbonate"
2617:, 7931-7940
1300:(RO)(R'O)CO
996:triphosgene
918:bicarbonate
778:calcination
744:delocalized
526:shells and
458:bicarbonate
454:carbonation
366:Bicarbonate
319:Properties
3720:Carbonates
3714:Categories
3699:Carbonates
3658:Fullerides
3404:Carbonates
2955:Oxocarbons
2876:1893/17165
2752:, 679–690.
2664:2010-09-05
2483:: 111262.
2426:: 111525.
2335:References
1183:See also:
1119:urea cycle
944:is basic,
858:Hard water
801:→ CaO + CO
477:mineralogy
348:Molar mass
254:7UJQ5OPE7D
200:ChemSpider
176:3D model (
155:CAS Number
49:Carbonate
18:Carbonates
3725:Oxyanions
3632:Fulminate
3476:Compounds
2601:234180559
2593:0360-3199
2554:258197818
2546:0039-7911
2507:0010-938X
2458:245455194
2450:0927-0248
1226:carbonate
1082:depletes
1065:(aq) ⇌ CO
829:potassium
790:lime kiln
746:charges:
728:resonance
520:limestone
394:carbonate
165:3812-32-6
129:Carbonate
3679:Nitrides
3611:Carbides
3341:Polymers
2880:Archived
2821:Archived
2809:17478719
2724:(2019).
2637:archived
2350:(2005).
2284:See also
2263:NGC 6302
1074:Exhaled
1052:(aq) + H
841:ammonium
833:rubidium
780:, after
562:iron ore
547:siderite
532:dolomite
444:O=C(−O−)
75:Resonant
3622:Cyanate
3617:Cyanide
2915:5 April
2854:Bibcode
2817:9687521
2779:Bibcode
2771:Science
2485:Bibcode
2319:Oxalate
1947:
1154:calcite
1019:H + HCO
837:caesium
821:Lithium
724:nitrate
669:of the
536:CaMg(CO
524:mollusc
505:calcite
473:geology
342:
310:C(=O)()
221:PubChem
3674:Oxides
3468:carbon
2815:
2807:
2704:
2599:
2591:
2552:
2544:
2505:
2456:
2448:
2368:
1502:Ca(HCO
1409:Mg(HCO
1012:buffer
1002:Buffer
839:, and
825:sodium
641:oxygen
637:carbon
579:, and
545:; and
353:60.008
303:SMILES
119:Names
110:Oxygen
108:
106:
100:Carbon
98:
96:
2883:(PDF)
2842:(PDF)
2824:(PDF)
2813:S2CID
2767:(PDF)
2729:(PDF)
2640:(PDF)
2633:(PDF)
2597:S2CID
2550:S2CID
2454:S2CID
2362:IUPAC
2310:C(OH)
2308:, or
2274:Gusev
2183:Th(CO
1862:Po(CO
1850:(BiO)
1763:CsHCO
1377:NaHCO
1276:LiHCO
1228:group
977:RR′CO
975:, or
854:scale
673:ion,
659:g/mol
596:glass
549:, or
528:coral
396:is a
268:InChI
234:19660
209:18519
178:JSmol
79:anion
3613:, ,
3415:and
3231:and
3087:and
2917:2014
2805:PMID
2722:IPCC
2702:ISBN
2589:ISSN
2542:ISSN
2503:ISSN
2446:ISSN
2366:ISBN
2276:and
2256:rock
2039:EuCO
1885:RaCO
1842:PbCO
1822:HgCO
1771:BaCO
1723:CdCO
1703:PdCO
1672:SrCO
1632:ZnCO
1624:(OH)
1609:CuCO
1589:NiCO
1565:CoCO
1557:FeCO
1549:MnCO
1525:CrCO
1494:CaCO
1486:KHCO
1445:+SiO
1437:SiCO
1401:MgCO
1389:H(CO
1284:BeCO
1088:(aq)
1048:⇌ CO
990:and
930:(aq)
884:+ CO
876:CaCO
846:CaCO
797:CaCO
782:calx
703:The
604:lime
602:and
555:FeCO
513:CaCO
483:and
475:and
419:, a
398:salt
245:UNII
3595:SiC
3563:CSe
3533:COS
2872:hdl
2862:doi
2850:111
2795:hdl
2787:doi
2775:316
2750:394
2680:at
2581:doi
2534:doi
2493:doi
2481:220
2436:hdl
2428:doi
2424:236
2372:.
2358:RSC
2236:No
2233:Md
2230:Fm
2227:Es
2224:Cf
2221:Bk
2218:Cm
2215:Am
2212:Pu
2209:Np
2194:Pa
2179:Ac
2176:**
2163:(CO
2147:(CO
2131:(CO
2115:(CO
2099:(CO
2083:(CO
2067:(CO
2051:(CO
2027:(CO
2019:Pm
2008:(CO
1992:(CO
1976:(CO
1960:(CO
1940:Og
1937:Ts
1934:Lv
1931:Mc
1928:Fl
1925:Nh
1922:Cn
1919:Rg
1916:Ds
1913:Mt
1910:Hs
1907:Bh
1904:Sg
1901:Db
1898:Rf
1895:Lr
1892:**
1881:Fr
1876:Rn
1873:At
1818:Au
1815:Pt
1812:Ir
1809:Os
1806:Re
1800:Ta
1797:Hf
1786:(CO
1745:Xe
1739:Te
1736:Sb
1733:Sn
1730:In
1699:Rh
1696:Ru
1693:Tc
1690:Mo
1687:Nb
1684:Zr
1654:Kr
1651:Br
1648:Se
1645:As
1642:Ge
1639:Ga
1577:(CO
1537:(CO
1518:Ti
1515:Sc
1468:Ar
1464:+Cl
1456:+SO
1425:(CO
1359:Ne
1344:+NO
1336:HCO
1316:(NH
1292:+BO
1258:He
1142:(g)
1107:HCO
1080:(g)
1069:(g)
1028:⇌ H
962:In
908:In
888:+ H
675:HCO
507:or
471:In
400:of
224:CID
112:, O
102:, C
3716::
3555:CS
3538:CS
3525:CO
3517:CO
3509:CO
3501:CO
3493:CO
3488:CO
3483:CF
3370:CO
3365:CO
3330:12
3326:12
3312:12
3298:12
3288:10
3284:10
3270:10
3026:CO
3016:CO
3006:CO
2996:CO
2975:CO
2969:CO
2908:.
2878:.
2870:.
2860:.
2848:.
2844:.
2819:.
2811:.
2803:.
2793:.
2785:.
2773:.
2769:.
2731:.
2615:24
2595:.
2587:.
2577:46
2571:.
2548:.
2540:.
2530:53
2528:.
2524:.
2501:.
2491:.
2479:.
2475:.
2452:.
2444:.
2434:.
2422:.
2418:.
2397:.
2380:^
2364:.
2303:CO
2297:,
2280:.
2202:CO
2198:UO
2159:Yb
2143:Tm
2127:Er
2111:Ho
2095:Dy
2079:Tb
2063:Gd
2047:Eu
2023:Sm
2004:Nd
1988:Pr
1972:Ce
1956:La
1952:*
1854:CO
1834:CO
1830:Tl
1803:W
1782:Lu
1778:*
1755:CO
1751:Cs
1742:I
1715:CO
1711:Ag
1681:Y
1664:CO
1660:Rb
1620:CO
1616:Cu
1614:,
1601:CO
1597:Cu
1573:Co
1533:Cr
1521:V
1478:CO
1452:P
1421:Al
1385:Na
1369:CO
1365:Na
1355:+F
1351:O
1332:NH
1324:CO
1304:+C
1268:CO
1264:Li
1245:CO
1170:CO
1161:2−
1158:CO
1148:.
1138:CO
1131:CO
1096:CO
1084:CO
1076:CO
1061:CO
1044:CO
1032:CO
1008:pH
998:.
926:CO
924:,
871::
864:.
835:,
831:,
827:,
823:,
811:Ca
792::
700:.
696:,
691:CO
652:3h
589:CO
574:CO
570:Na
564:.
553:,
511:,
492:2−
489:CO
449:.
428:2−
425:CO
410:CO
404:,
392:A
335:2−
332:CO
3589:2
3587:S
3585:3
3583:C
3577:2
3575:O
3573:3
3571:C
3565:2
3557:2
3549:2
3547:S
3545:2
3543:C
3527:6
3519:5
3511:4
3503:3
3495:2
3457:e
3450:t
3443:v
3419:)
3411:(
3372:2
3359:2
3357:O
3355:3
3353:C
3328:O
3324:C
3316:9
3314:O
3310:C
3302:6
3300:O
3296:C
3286:O
3282:C
3274:8
3272:O
3268:C
3260:9
3258:O
3256:9
3254:C
3246:8
3244:O
3242:8
3240:C
3235:)
3227:(
3224:6
3222:O
3220:6
3218:C
3211:5
3209:O
3207:5
3205:C
3197:2
3195:O
3193:5
3191:C
3183:6
3181:O
3179:4
3177:C
3169:4
3167:O
3165:4
3163:C
3155:2
3153:O
3151:4
3149:C
3141:6
3139:O
3137:3
3135:C
3127:3
3125:O
3123:3
3121:C
3113:2
3111:O
3109:3
3107:C
3100:O
3098:3
3096:C
3091:)
3083:(
3080:4
3078:O
3076:2
3074:C
3067:3
3065:O
3063:2
3061:C
3053:2
3051:O
3049:2
3047:C
3040:O
3038:2
3036:C
3028:6
3018:5
3008:4
2998:3
2977:2
2947:e
2940:t
2933:v
2919:.
2874::
2864::
2856::
2797::
2789::
2781::
2710:.
2667:.
2603:.
2583::
2556:.
2536::
2509:.
2495::
2487::
2460:.
2438::
2430::
2360:–
2312:4
2305:4
2301:4
2299:H
2204:3
2200:2
2189:2
2187:)
2185:3
2169:3
2167:)
2165:3
2161:2
2153:3
2151:)
2149:3
2145:2
2137:3
2135:)
2133:3
2129:2
2121:3
2119:)
2117:3
2113:2
2105:3
2103:)
2101:3
2097:2
2089:3
2087:)
2085:3
2081:2
2073:3
2071:)
2069:3
2065:2
2057:3
2055:)
2053:3
2049:2
2044:,
2041:3
2033:3
2031:)
2029:3
2025:2
2014:3
2012:)
2010:3
2006:2
1998:3
1996:)
1994:3
1990:2
1982:3
1980:)
1978:3
1974:2
1966:3
1964:)
1962:3
1958:2
1887:3
1868:2
1866:)
1864:3
1856:3
1852:2
1844:3
1836:3
1832:2
1824:3
1792:3
1790:)
1788:3
1784:2
1773:3
1765:3
1760:,
1757:3
1753:2
1725:3
1717:3
1713:2
1705:3
1674:3
1666:3
1662:2
1634:3
1626:2
1622:3
1618:2
1611:3
1606:,
1603:3
1599:2
1591:3
1583:3
1581:)
1579:3
1575:2
1570:,
1567:3
1559:3
1551:3
1543:3
1541:)
1539:3
1535:2
1530:,
1527:3
1508:2
1506:)
1504:3
1499:,
1496:3
1488:3
1483:,
1480:3
1476:2
1474:K
1458:4
1447:4
1442:,
1439:4
1431:3
1429:)
1427:3
1423:2
1415:2
1413:)
1411:3
1406:,
1403:3
1395:2
1393:)
1391:3
1387:3
1382:,
1379:3
1374:,
1371:3
1367:2
1346:3
1341:,
1338:3
1334:4
1329:,
1326:3
1322:2
1320:)
1318:4
1310:4
1308:O
1306:2
1294:3
1286:3
1278:3
1273:,
1270:3
1266:2
1247:3
1243:2
1241:H
1217:e
1210:t
1203:v
1172:2
1164:3
1140:2
1133:3
1129:2
1127:H
1123:H
1113:3
1110:−
1098:3
1094:2
1092:H
1086:2
1078:2
1067:2
1063:2
1056:O
1054:2
1050:2
1046:3
1042:2
1040:H
1034:3
1030:2
1025:3
1022:−
979:3
938:O
936:2
934:H
928:2
892:O
890:2
886:2
882:2
878:3
848:3
803:2
799:3
693:3
689:2
687:H
681:3
678:−
649:D
619:2
617:O
615:2
613:H
591:3
587:2
585:K
576:3
572:2
557:3
542:2
540:)
538:3
515:3
495:3
446:2
431:3
412:3
408:2
406:H
338:3
180:)
45:.
38:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.