2538:
1219:. Together, the hydroxyl and carbonyl group form the functional group carboxyl. Carboxylic acids usually exist as dimers in nonpolar media due to their tendency to "self-associate". Smaller carboxylic acids (1 to 5 carbons) are soluble in water, whereas bigger carboxylic acids have limited solubility due to the increasing hydrophobic nature of the alkyl chain. These longer chain acids tend to be soluble in less-polar solvents such as ethers and alcohols. Aqueous sodium hydroxide and carboxylic acids, even hydrophobic ones, react to yield water-soluble sodium salts. For example,
2250:
63:
55:
1254:
47:
1229:
2599:
alkoxide dianion, which is protonated upon workup to give the hydrate of a ketone. Because most ketone hydrates are unstable relative to their corresponding ketones, the equilibrium between the two is shifted heavily in favor of the ketone. For example, the equilibrium constant for the formation of
1876:
Oxidation of hydrocarbons using air. For simple alkanes, this method is inexpensive but not selective enough to be useful. Allylic and benzylic compounds undergo more selective oxidations. Alkyl groups on a benzene ring are oxidized to the carboxylic acid, regardless of its chain length.
1732:, because the negative charge is delocalized over the two oxygen atoms, increasing the stability of the anion. Each of the carbon–oxygen bonds in the carboxylate anion has a partial double-bond character. The carbonyl carbon's partial positive charge is also weakened by the -/
2388:
salt. Heating the salt to above 100 °C will drive off water and lead to the formation of the amide. This method of synthesizing amides is industrially important, and has laboratory applications as well. In the presence of a strong acid catalyst, carboxylic acids can
2537:
2472:-butoxy)aluminum hydride to afford an aldehyde in a one pot procedure. This procedure is known to tolerate reactive carbonyl functionalities such as ketone as well as moderately reactive ester, olefin, nitrile, and halide moieties.
2594:
Carboxylic acids react with
Grignard reagents and organolithiums to form ketones. The first equivalent of nucleophile acts as a base and deprotonates the acid. A second equivalent will attack the carbonyl group to create a
2393:
to form acid anhydrides. The condensation produces water, however, which can hydrolyze the anhydride back to the starting carboxylic acids. Thus, the formation of the anhydride via condensation is an equilibrium process.
2468:) is a highly chemoselective agent for carboxylic acid reduction. It selectively activates the carboxylic acid to give the carboxymethyleneammonium salt, which can be reduced by a mild reductant like lithium tris(
2383:
Converting a carboxylic acid to an amide is possible, but not straightforward. Instead of acting as a nucleophile, an amine will react as a base in the presence of a carboxylic acid to give the ammonium
7331:
2065:. The method is more suitable for laboratory conditions than the industrial use of air, which is "greener" because it yields less inorganic side products such as chromium or manganese oxides.
1794:
Many carboxylic acids are produced industrially on a large scale. They are also frequently found in nature. Esters of fatty acids are the main components of lipids and polyamides of
2409:
can be used to convert an acid to an ester. While esterification reactions with diazomethane often give quantitative yields, diazomethane is only useful for forming methyl esters.
6447:
2368:, but this conversion typically does not occur by direct reaction of the carboxylic acid and the amine. Instead esters are typical precursors to amides. The conversion of
6392:
2839:
2038:
7160:
6502:
6652:
5286:
7381:
2736:
is an electrolytic, decarboxylative dimerization reaction. It gets rid of the carboxyl groups of two acid molecules, and joins the remaining fragments together.
7155:
201:) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the
6257:
4981:
1240:
Carboxylic acids tend to have higher boiling points than water, because of their greater surface areas and their tendency to form stabilized dimers through
6027:
4178:
3219:
2622:
1272:
6827:
4771:
6922:
5026:
6902:
6397:
5564:
1805:
Carboxylic acids are used in the production of polymers, pharmaceuticals, solvents, and food additives. Industrially important carboxylic acids include
5445:
5001:
6992:
4138:
6747:
4214:
4143:
5579:
7225:
6682:
7175:
6787:
6767:
6727:
5534:
7321:
7246:
7130:
5742:
5146:
4477:
2910:
7316:
7145:
6802:
6657:
6287:
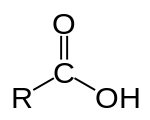
6132:
5369:
2034:
Preparative methods for small scale reactions for research or for production of fine chemicals often employ expensive consumable reagents.
6492:
5982:
5657:
7396:
7180:
6202:
2509:
is activated towards nucleophilic attack and has a good leaving group, setting it apart from a normal carboxylic acid. In the next step,
1930:
Carbonylation coupled to the addition of water. This method is effective and versatile for alkenes that generate secondary and tertiary
1854:
In general, industrial routes to carboxylic acids differ from those used on a smaller scale because they require specialized equipment.
1786:
hydrogen appears in the 10–13 ppm region, although it is often either broadened or not observed owing to exchange with traces of water.
6757:
7391:
7105:
6967:
6722:
2924:
7281:
6752:
6667:
6637:
6617:
6482:
6477:
5852:
5777:
5420:
5374:
5241:
4502:
7220:
2893:
7386:
7346:
7296:
6772:
6522:
6452:
4941:
6982:
6587:
5480:
5201:
6972:
4512:
7140:
6897:
6847:
5637:
5569:
5460:
5036:
4791:
4716:
4497:
2654:
1244:. For boiling to occur, either the dimer bonds must be broken or the entire dimer arrangement must be vaporized, increasing the
6852:
6662:
6137:
6047:
4171:
3212:
2754:
1822:
7426:
7210:
7150:
6552:
6527:
6437:
6017:
5897:
4931:
4427:
7311:
6797:
6592:
4861:
3001:
1862:
for the production of acetic acid. Formic acid is prepared by a different carbonylation pathway, also starting from methanol.
7416:
7002:
6512:
6022:
5967:
5812:
5772:
5604:
5359:
5076:
4926:
7376:
6937:
6892:
6382:
6237:
4042:
7411:
7326:
7185:
7100:
6997:
6072:
5727:
5395:
4806:
4367:
7301:
7276:
7261:
6957:
6822:
6777:
6542:
6087:
5937:
5151:
4831:
4776:
1332:, only 0.001% of the acid are dissociated (i.e. 10 moles out of 1 mol). Electron-withdrawing substituents, such as
7306:
7251:
6782:
6197:
5912:
5907:
5400:
5216:
5206:
4921:
4781:
4731:
4726:
4701:
4607:
2662:
7361:
6962:
6882:
6497:
6462:
6307:
5732:
5692:
5589:
5364:
5116:
5061:
4661:
4372:
4362:
4337:
2704:
2223:
2003:
7336:
7037:
6842:
6277:
5842:
5817:
5757:
5349:
5056:
4706:
2233:
2014:
4891:
3106:
Milligan, D. E.; Jacox, M. E. (1971). "Infrared
Spectrum and Structure of Intermediates in Reaction of OH with CO".
213:. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another parent structure, such as
7480:
6627:
6162:
5614:
4836:
4801:
4397:
4332:
4164:
3615:
3205:
2750:
1779:
1385:
7195:
6817:
5877:
5802:
5326:
5161:
4846:
4622:
4582:
4327:
2618:
1996:
obtained from plant or animal oils. These methods of synthesizing some long-chain carboxylic acids are related to
7475:
7436:
7341:
7075:
7047:
7017:
6932:
6862:
6792:
6712:
6612:
6572:
6267:
5887:
5186:
5181:
4643:
4507:
2673:
2579:
2430:
2422:
267:
7401:
7271:
7135:
6977:
6837:
6357:
5331:
4881:
4841:
4592:
2208:
Many reactions produce carboxylic acids but are used only in specific cases or are mainly of academic interest.
7291:
6887:
6857:
6732:
6687:
6517:
6427:
6242:
6232:
6062:
5619:
5559:
5524:
5311:
5271:
5046:
4916:
4432:
4422:
4352:
3652:
2795:
2726:
2685:
2543:
1287:
1245:
1176:
6867:
5847:
4871:
4417:
4297:
2647:
1181:
containing a doubly unsaturated carbon chain attached via an ether bond to a fatty acid, found in some plants
7080:
7371:
7230:
7022:
6947:
6927:
6647:
6597:
6457:
6422:
6362:
6292:
5594:
5574:
5306:
5226:
5121:
5081:
5051:
4986:
4856:
4766:
4756:
4632:
4342:
4125:
2719:
1017:
medium to long-chain saturated and unsaturated monocarboxylic acids, with even number of carbons; examples:
2725:
Organolithium reagents (>2 equiv) react with carboxylic acids to give a dilithium 1,1-diolate, a stable
1869:
with air using cobalt and manganese catalysts. The required aldehydes are readily obtained from alkenes by
7110:
6832:
6582:
6562:
6537:
6487:
6402:
6377:
6332:
6302:
6282:
6252:
6217:
6172:
6147:
6122:
6007:
5932:
5712:
5405:
5341:
5141:
4866:
4786:
4472:
4447:
4224:
4219:
4025:
2551:
2402:
2377:
2077:
2058:
1228:
210:
7446:
6192:
4816:
3192:
7032:
6987:
6702:
6672:
6642:
6577:
6557:
6472:
6467:
6432:
6387:
6372:
6367:
6347:
6337:
6272:
6262:
6142:
5662:
5465:
5041:
4996:
4826:
4562:
4282:
4244:
4132:
4020:
2434:
2390:
1761:
1348:
1079:
1022:
4492:
4487:
62:
7085:
2498:
can be used to convert carboxylic acids to their corresponding acyl chlorides. First, carboxylic acid
2249:
7215:
7165:
7115:
7095:
6942:
6917:
6632:
6622:
6507:
6322:
6317:
6247:
6032:
5832:
5792:
5722:
5687:
5642:
5609:
5475:
5450:
5430:
5251:
5211:
5171:
5136:
5066:
4821:
4691:
4666:
4204:
4101:
3546:
3115:
2640:
2558:) will also convert carboxylic acids to acid chlorides, by a similar mechanism. One equivalent of PCl
2229:
2081:
2050:
1729:
1601:
1532:
1198:
1018:
7431:
7421:
7406:
7052:
7027:
7012:
7007:
6737:
6692:
6677:
6567:
6547:
6442:
6327:
6312:
6157:
6102:
6092:
6082:
6057:
5822:
5697:
5672:
5584:
5440:
5425:
5410:
5266:
5231:
5176:
4946:
4796:
4741:
4612:
4527:
4387:
4312:
3407:
2959:
2708:
2217:
1921:
1651:
1578:
1509:
4457:
2604:
hydrate from acetone is only 0.002. The carboxylic group is the most acidic in organic compounds.
7170:
7120:
7090:
6952:
6742:
6532:
6417:
6352:
6342:
6107:
6037:
6002:
5997:
5977:
5972:
5917:
5827:
5677:
5539:
5529:
5435:
5221:
5166:
5096:
5016:
4911:
4811:
4746:
4671:
4517:
4382:
4317:
2733:
2712:
2290:
1954:
is catalyzed by strong acids. Hydrocarboxylations involve the simultaneous addition of water and
1166:
1130:
1109:
271:
4302:
2661:
at the alpha position can have the chain shortened by one carbon. The inverse procedure is the
54:
6907:
6227:
6112:
6077:
6042:
5987:
5942:
5902:
5857:
5837:
5787:
5782:
5752:
5737:
5647:
5554:
5490:
5455:
5281:
5156:
5031:
4956:
4936:
4851:
4686:
4681:
4627:
4537:
4442:
4402:
4357:
4239:
4234:
4199:
4091:
4061:
3819:
3441:
3063:
2997:
2920:
2889:
2800:
2530:
2445:
2438:
2171:
2147:
1886:
1838:
1555:
1486:
1417:
1258:
1156:
1087:
1064:
773:
71:
7441:
7286:
7256:
7200:
7125:
7057:
6812:
6762:
6607:
6412:
6187:
6182:
6127:
6117:
5892:
5702:
5682:
5652:
5549:
5485:
5470:
5301:
5256:
5246:
5236:
5131:
5111:
5106:
5091:
5086:
4966:
4961:
4901:
4886:
4876:
4721:
4711:
4577:
4567:
4557:
4467:
4462:
4437:
4377:
4229:
4188:
3796:
3290:
3228:
3165:
3123:
3072:
3027:
2989:
2853:
2658:
2629:
2495:
2483:
2266:
2254:
2155:
2103:
1870:
1321:
1053:
910:
3188:– freeware for calculations, data analysis, simulation, and distribution diagram generation
7351:
7042:
6877:
6872:
6167:
6152:
6097:
6052:
6012:
5962:
5927:
5922:
5867:
5862:
5797:
5747:
5667:
5495:
5379:
5354:
5316:
5291:
5276:
5261:
5196:
5071:
5021:
5011:
4991:
4951:
4761:
4751:
4736:
4532:
4452:
4277:
4272:
4015:
3774:
3769:
3752:
3735:
3285:
2868:
2571:
2062:
2046:
1955:
1333:
705:
626:
561:
446:
270:
in nature, is not generally classed as one of the carboxylic acids, despite that it has a
31:
4322:
4292:
3018:
Perry C. Reeves (1977). "Carboxylation of
Aromatic Compounds: Ferrocenecarboxylic Acid".
2871:. Organic Chemistry IUPAC Nomenclature. Rules C-4 Carboxylic Acids and Their Derivatives.
3119:
7356:
7266:
7205:
6297:
6207:
6177:
5952:
5807:
5544:
5321:
5191:
5006:
4976:
4676:
4572:
4347:
4209:
4086:
4081:
3957:
3952:
3947:
3740:
3707:
3491:
3473:
3463:
2815:
2770:
2518:
2349:
2298:
2294:
2117:
2018:
1859:
1834:
1253:
1223:
has a low solubility in water (0.2 g/L), but its sodium salt is very soluble in water.
1123:
1075:
946:
636:
353:
249:
7469:
7366:
7067:
6912:
6807:
6602:
5992:
5957:
5947:
5882:
5872:
5762:
5599:
5415:
5126:
5101:
4971:
4617:
4602:
4587:
4482:
4412:
4392:
4307:
4106:
4054:
3985:
3871:
3861:
3856:
3846:
3841:
3791:
3786:
3702:
3697:
3687:
3541:
3496:
3458:
3446:
3417:
3295:
3149:
Jeevarajan, A. S.; Carmichael, I.; Fessenden, R. W. (1990). "ESR Measurement of the p
2775:
2689:
2525:. Chloride ion can remove the proton on the carbonyl group, giving the acyl chloride
2487:
2426:
2167:
2107:
2054:
1943:
1898:
1725:
1241:
1220:
1216:
1148:
1140:
1136:
841:
806:
738:
526:
493:
316:
263:
214:
143:
115:
38:
3187:
3156:
of
Carboxyl Radical and Ab Initio Calculation of the Carbon-13 Hyperfine Constant".
2849:
6407:
5767:
5519:
5296:
4896:
4696:
4547:
4542:
4407:
4262:
4037:
3924:
3919:
3896:
3647:
3486:
3412:
3349:
3344:
3322:
3278:
3263:
3253:
2746:
2666:
2614:
2406:
2397:
Under acid-catalyzed conditions, carboxylic acids will react with alcohols to form
2163:
1993:
1976:
1959:
1939:
1910:
1878:
1810:
1624:
1366:
1276:
1119:
1101:
1071:
1057:
996:
874:
457:
421:
386:
202:
178:
162:
79:
2482:
The hydroxyl group on carboxylic acids may be replaced with a chlorine atom using
2441:, will reduce carboxylic acids to ketones along with transfer of the alkyl group.
134:, or other groups. Carboxylic acids occur widely. Important examples include the
4906:
4552:
4522:
4287:
4096:
4049:
4010:
3891:
3779:
3764:
3759:
3747:
3312:
3307:
3273:
3268:
3258:
3236:
3058:
2844:
2810:
2758:
2681:
2503:
2385:
2345:
2282:
1997:
1935:
1931:
1830:
1818:
1814:
1806:
1764:. They exhibit a sharp band associated with vibration of the C=O carbonyl bond (
1701:
1686:
1436:
1398:
1329:
1304:
1144:
1115:
1093:
694:
669:
622:
597:
586:
326:
305:
253:
206:
147:
91:
1748:
of carboxylic acids tend to have fruity, pleasant odours, and many are used in
1082:– the class of compounds where a phenyl group is attached to a carboxylic acid
7190:
6717:
6067:
4156:
4005:
3996:
3876:
3831:
3727:
3692:
3682:
3622:
3558:
3481:
3429:
2805:
2785:
2369:
2073:
1826:
1795:
1030:
1012:
971:
375:
139:
135:
46:
3076:
3031:
2993:
2848:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
3972:
3886:
3851:
3836:
3824:
3667:
3642:
3451:
2916:
2857:
2491:
2361:
1968:
1291:
1283:
1043:
936:
899:
2517:, a chlorosulfite. The tetrahedral intermediate collapses with the loss of
2940:
2665:, where an acid is converted into acyl halide, which is then reacted with
30:"COOH" redirects here. For the Bulgarian DJ and producer Ivan Shopov, see
17:
4597:
4267:
3980:
3934:
3901:
3597:
3503:
3377:
3332:
3317:
2696:
2353:
2274:
2270:
2213:
2042:
1866:
1783:
1325:
1209:
1202:
831:
551:
252:
and its conjugate base, respectively. For example, the conjugate base of
131:
3169:
173:-recommended names also exist; in this system, carboxylic acids have an
4257:
3942:
3866:
3717:
3712:
3677:
3662:
3657:
3627:
3610:
3434:
3361:
3327:
2601:
2596:
2562:
can react with three equivalents of acid, producing one equivalent of H
2373:
2286:
2088:
2022:
2007:
1951:
1947:
1914:
1882:
1799:
1749:
1459:
1036:
343:
257:
123:
3127:
1778:
band appears as a broad peak in the 2500 to 3000 cm region. By H
4030:
3962:
3806:
3515:
3508:
3402:
3383:
3372:
3356:
3302:
2677:
2341:
2337:
2278:
2237:
2069:
1906:
1894:
1745:
1300:
1275:
because they are proton (H) donors. They are the most common type of
1049:
763:
411:
3197:
3911:
3881:
3814:
3672:
3637:
3632:
3605:
3553:
3520:
3424:
3248:
2790:
2780:
2700:
2633:
2418:
2398:
2365:
2357:
2248:
2096:
2092:
1317:
1252:
1097:
1074:– the sodium salt of benzoic acid is used as a food preservative;
170:
150:
119:
2502:
attacks thionyl chloride, and chloride ion leaves. The resulting
1358:
of 0.23). Electron-donating substituents give weaker acids (the p
3339:
2984:
Riemenschneider, Wilhelm (2002). "Carboxylic Acids, Aliphatic".
2187:
2177:
482:
127:
4641:
4160:
3201:
2405:
reaction, which is also an equilibrium process. Alternatively,
1171:
containing a hydroxy group beyond the first or second position
94:. The general formula of a carboxylic acid is often written as
2176:
Base-catalyzed cleavage of non-enolizable ketones, especially
2513:
is attacked by chloride ion to give tetrahedral intermediate
1809:(component of vinegar, precursor to solvents and coatings),
1347:
of acetic acid is 4.76 whereas trifluoroacetic acid, with a
1135:
containing a hydroxy group in the first position; examples:
278:
Straight-chain, saturated carboxylic acids (alkanoic acids)
2757:
spectroscopy. The carboxyl group tends to dimerise to form
3059:"Reduction of carboxylic acids to aldehydes: 6-Ooxdecanal"
2657:, a carboxylic acid on an aliphatic chain having a simple
2578:
reacts with carboxylic acids in a 1:1 ratio, and produces
2650:
converts an amino acid to the corresponding amino ketone.
2336:
Widely practiced reactions convert carboxylic acids into
2669:
to give one additional methylene in the aliphatic chain.
1728:
of carboxylic acids gives carboxylate anions; these are
1078:– a beta-hydroxy type found in many skin-care products;
236:) of a carboxylic acid is usually named with the suffix
2729:
which decomposes to give a ketone upon acidic workup.
2006:
of ethanol. This method is used in the production of
1201:. Because they are both hydrogen-bond acceptors (the
7332:
Erlenmeyer–Plöchl azlactone and amino-acid synthesis
7239:
7066:
6701:
6216:
5711:
5628:
5508:
5388:
5340:
4650:
4074:
3994:
3971:
3933:
3910:
3805:
3726:
3596:
3573:
3529:
3472:
3395:
3370:
3235:
2613:As with all carbonyl compounds, the protons on the
2376:is a significant biochemical process that requires
1841:(polymers). Important carboxylate salts are soaps.
1821:(a flavor and preservative in food and beverages),
1760:Carboxylic acids are readily identified as such by
1365:of formic acid is 3.75 whereas acetic acid, with a
1324:
solution. For example, at room temperature, in a 1-
2621:. Thus, the α-carbon is easily halogenated in the
2521:and chloride ion, giving protonated acyl chloride
2228:Involving the generation of benzoic acids are the
1771:) between 1680 and 1725 cm. A characteristic
1161:containing a hydroxy group in the second position
161:Carboxylic acids are commonly identified by their
6393:Divinylcyclopropane-cycloheptadiene rearrangement
1070:containing at least one aromatic ring; examples:
1048:acids of biochemical significance that contain a
2364:. Likewise, carboxylic acids are converted into
1858:Carbonylation of alcohols as illustrated by the
1744:Carboxylic acids often have strong sour odours.
1147:(2-hydroxypropanoic acid) – found in sour milk,
2574:, in addition to the desired acid chloride. PCl
2490:. In nature, carboxylic acids are converted to
2429:, or using hydride transferring agents such as
1946:, the addition of water and carbon monoxide to
6653:Thermal rearrangement of aromatic hydrocarbons
5287:Thermal rearrangement of aromatic hydrocarbons
2986:Ullmann's Encyclopedia of Industrial Chemistry
7382:Lectka enantioselective beta-lactam synthesis
4172:
3213:
2586:) and hydrogen chloride (HCl) as byproducts.
2456:-Dimethyl(chloromethylene)ammonium chloride;
8:
7161:Inverse electron-demand Diels–Alder reaction
4982:Heterogeneous metal catalyzed cross-coupling
2433:. Strong alkyl transferring agents, such as
1114:containing three carboxyl groups; examples:
27:Organic compound containing a –C(=O)OH group
6503:Lobry de Bruyn–Van Ekenstein transformation
2680:that catalyze these reactions are known as
2639:Carboxylic acids are decarboxylated in the
1927:Base-catalyzed dehydrogenation of alcohols.
7063:
5337:
4638:
4179:
4165:
4157:
3593:
3392:
3220:
3206:
3198:
2722:carboxylic acids are converted to ketones.
2360:is widely used, e.g. in the production of
1909:are illustrative large-scale conversions.
1092:containing two carboxyl groups; examples:
6993:Petrenko-Kritschenko piperidone synthesis
6448:Fritsch–Buttenberg–Wiechell rearrangement
2879:
2877:
240:, in keeping with the general pattern of
7156:Intramolecular Diels–Alder cycloaddition
2463:
2459:
2332:Conversion to esters, amides, anhydrides
2323:
2319:
2315:
2311:
2307:
2269:to form carboxylate salts, in which the
2195:
2191:
2151:
2119:
1982:
1978:
1972:
1736:negative charges on the 2 oxygen atoms.
1673:
1669:
1665:
1661:
1638:
1634:
1630:
1611:
1607:
1588:
1584:
1565:
1561:
1542:
1538:
1519:
1515:
1496:
1492:
1473:
1469:
1465:
1446:
1442:
1423:
1404:
1378:
1294:
1002:
978:
964:
960:
956:
928:
924:
920:
892:
888:
884:
859:
855:
851:
824:
820:
816:
791:
787:
783:
756:
752:
748:
723:
719:
715:
687:
683:
679:
654:
650:
646:
615:
611:
607:
579:
575:
571:
544:
540:
536:
511:
507:
503:
475:
471:
467:
439:
435:
431:
404:
400:
396:
367:
363:
336:
276:
196:
192:
188:
184:
104:
61:
53:
45:
3043:
3041:
2832:
2099:, usually with acid- or base-catalysis.
1958:. Such reactions are sometimes called "
1716:) (second dissociation of oxalic acid)
7176:Metal-centered cycloaddition reactions
6828:Debus–Radziszewski imidazole synthesis
4772:Bodroux–Chichibabin aldehyde synthesis
66:3D structure of a carboxylic acid
7322:Diazoalkane 1,3-dipolar cycloaddition
7226:Vinylcyclopropane (5+2) cycloaddition
7131:Diazoalkane 1,3-dipolar cycloaddition
6903:Hurd–Mori 1,2,3-thiadiazole synthesis
6398:Dowd–Beckwith ring-expansion reaction
5565:Hurd–Mori 1,2,3-thiadiazole synthesis
4478:LFER solvent coefficients (data page)
2912:CRC Handbook of Chemistry and Physics
2277:(–OH) group is replaced with a metal
1813:(precursors to polymers, adhesives),
7:
6133:Sharpless asymmetric dihydroxylation
5370:Methoxymethylenetriphenylphosphorane
2590:Reactions with carbanion equivalents
6258:Allen–Millar–Trippett rearrangement
3147: = −0.2 ± 0.1.
2884:Morrison, R.T.; Boyd, R.N. (1992).
1286:, meaning that they only partially
7397:Nitrone-olefin (3+2) cycloaddition
7392:Niementowski quinazoline synthesis
7181:Nitrone-olefin (3+2) cycloaddition
7106:Azide-alkyne Huisgen cycloaddition
6968:Niementowski quinazoline synthesis
6723:Azide-alkyne Huisgen cycloaddition
6028:Meerwein–Ponndorf–Verley reduction
5580:Leimgruber–Batcho indole synthesis
3186:Carboxylic acids pH and titration
2845:Compendium of Chemical Terminology
2749:, •COOH, only exists briefly. The
2623:Hell–Volhard–Zelinsky halogenation
2222:Rearrangement of diketones in the
25:
7221:Trimethylenemethane cycloaddition
6923:Johnson–Corey–Chaykovsky reaction
6788:Cadogan–Sundberg indole synthesis
6768:Bohlmann–Rahtz pyridine synthesis
6728:Baeyer–Emmerling indole synthesis
5535:Cadogan–Sundberg indole synthesis
5027:Johnson–Corey–Chaykovsky reaction
3057:Fujisawa, Tamotsu; Sato, Toshio.
2960:"The C=O Bond, Part VIII: Review"
2753:of •COOH has been measured using
7317:Cook–Heilbron thiazole synthesis
7146:Hexadehydro Diels–Alder reaction
6973:Niementowski quinoline synthesis
6803:Cook–Heilbron thiazole synthesis
6748:Bischler–Möhlau indole synthesis
6658:Tiffeneau–Demjanov rearrangement
6288:Baker–Venkataraman rearrangement
5446:Horner–Wadsworth–Emmons reaction
5117:Mizoroki-Heck vs. Reductive Heck
5002:Horner–Wadsworth–Emmons reaction
4513:Neighbouring group participation
2909:Haynes, William M., ed. (2011).
2695:Carboxylic acids are reduced to
2536:
1227:
1208:) and hydrogen-bond donors (the
993:unsaturated monocarboxylic acids
374:Preservative for stored grains,
6853:Fiesselmann thiophene synthesis
6683:Westphalen–Lettré rearrangement
6663:Vinylcyclopropane rearrangement
6493:Kornblum–DeLaMare rearrangement
6138:Epoxidation of allylic alcohols
6048:Noyori asymmetric hydrogenation
5983:Kornblum–DeLaMare rearrangement
5658:Gallagher–Hollander degradation
2888:(6th ed.). Prentice Hall.
2755:electron paramagnetic resonance
2707:, via the acid chloride in the
2421:, most carboxylic acids can be
1823:ethylenediaminetetraacetic acid
1282:Carboxylic acids are typically
7312:Chichibabin pyridine synthesis
6798:Chichibabin pyridine synthesis
6758:Blum–Ittah aziridine synthesis
6593:Ring expansion and contraction
4862:Cross dehydrogenative coupling
274:that looks like a COOH group.
50:Structure of a carboxylic acid
1:
7282:Bischler–Napieralski reaction
7240:Heterocycle forming reactions
6893:Hemetsberger indole synthesis
6753:Bischler–Napieralski reaction
6668:Wagner–Meerwein rearrangement
6638:Sommelet–Hauser rearrangement
6618:Seyferth–Gilbert homologation
6483:Ireland–Claisen rearrangement
6478:Hofmann–Martius rearrangement
6238:2,3-sigmatropic rearrangement
5853:Corey–Winter olefin synthesis
5778:Barton–McCombie deoxygenation
5421:Corey–Winter olefin synthesis
5375:Seyferth–Gilbert homologation
5242:Seyferth–Gilbert homologation
3158:Journal of Physical Chemistry
2711:and via the thioester in the
2632:converts carboxylic acids to
2039:Oxidation of primary alcohols
1811:acrylic and methacrylic acids
866:Pheromone in various animals
165:. They often have the suffix
146:of a carboxylic acid gives a
7387:Lehmstedt–Tanasescu reaction
7347:Gabriel–Colman rearrangement
7302:Bucherer carbazole synthesis
7297:Borsche–Drechsel cyclization
7277:Bernthsen acridine synthesis
7262:Bamberger triazine synthesis
7247:Algar–Flynn–Oyamada reaction
6958:Nazarov cyclization reaction
6823:De Kimpe aziridine synthesis
6778:Bucherer carbazole synthesis
6773:Borsche–Drechsel cyclization
6543:Nazarov cyclization reaction
6523:Meyer–Schuster rearrangement
6453:Gabriel–Colman rearrangement
6203:Wolffenstein–Böters reaction
6088:Reduction of nitro compounds
5938:Grundmann aldehyde synthesis
5743:Algar–Flynn–Oyamada reaction
5152:Olefin conversion technology
5147:Nozaki–Hiyama–Kishi reaction
4942:Gabriel–Colman rearrangement
4832:Claisen-Schmidt condensation
4777:Bouveault aldehyde synthesis
2265:Carboxylic acids react with
1340:, give stronger acids (the p
1248:requirements significantly.
1215:), they also participate in
1096:the monomer used to produce
1007:, used in polymer synthesis
7362:Hantzsch pyridine synthesis
7141:Enone–alkene cycloadditions
6963:Nenitzescu indole synthesis
6883:Hantzsch pyridine synthesis
6848:Ferrario–Ackermann reaction
6498:Kowalski ester homologation
6463:Halogen dance rearrangement
6308:Benzilic acid rearrangement
5733:Akabori amino-acid reaction
5693:Von Braun amide degradation
5638:Barbier–Wieland degradation
5590:Nenitzescu indole synthesis
5570:Kharasch–Sosnovsky reaction
5461:Julia–Kocienski olefination
5365:Kowalski ester homologation
5062:Kowalski ester homologation
5037:Julia–Kocienski olefination
4792:Cadiot–Chodkiewicz coupling
4717:Aza-Baylis–Hillman reaction
4662:Acetoacetic ester synthesis
4373:Dynamic binding (chemistry)
4363:Conrotatory and disrotatory
4338:Charge remote fragmentation
3108:Journal of Chemical Physics
2655:Barbier–Wieland degradation
2232:from nitrobenzenes and the
2224:benzilic acid rearrangement
1798:are the main components of
1790:Occurrence and applications
1349:trifluoromethyl substituent
7497:
7427:Robinson–Gabriel synthesis
7377:Kröhnke pyridine synthesis
7211:Retro-Diels–Alder reaction
7151:Imine Diels–Alder reaction
6938:Kröhnke pyridine synthesis
6553:Newman–Kwart rearrangement
6528:Mislow–Evans rearrangement
6438:Fischer–Hepp rearrangement
6383:Di-π-methane rearrangement
6163:Stephen aldehyde synthesis
5898:Eschweiler–Clarke reaction
5615:Williamson ether synthesis
4932:Fujiwara–Moritani reaction
4837:Combes quinoline synthesis
4802:Carbonyl olefin metathesis
4503:More O'Ferrall–Jencks plot
4428:Grunwald–Winstein equation
4398:Electron-withdrawing group
4333:Catalytic resonance theory
3085:, vol. 8, p. 498
2751:acid dissociation constant
2478:Conversion to acyl halides
2166:followed by hydrolysis of
1920:Oxidation of ethene using
1104:– a family of sugar acids
1025:(nutritional supplements)
268:bicarbonate buffer systems
36:
29:
7437:Urech hydantoin synthesis
7417:Pomeranz–Fritsch reaction
7342:Fischer oxazole synthesis
7076:1,3-Dipolar cycloaddition
7048:Urech hydantoin synthesis
7018:Reissert indole synthesis
7003:Pomeranz–Fritsch reaction
6933:Knorr quinoline synthesis
6863:Fischer oxazole synthesis
6793:Camps quinoline synthesis
6713:1,3-Dipolar cycloaddition
6613:Semipinacol rearrangement
6588:Ramberg–Bäcklund reaction
6573:Piancatelli rearrangement
6513:McFadyen–Stevens reaction
6268:Alpha-ketol rearrangement
6023:McFadyen–Stevens reaction
5968:Kiliani–Fischer synthesis
5888:Elbs persulfate oxidation
5813:Bouveault–Blanc reduction
5773:Baeyer–Villiger oxidation
5605:Schotten–Baumann reaction
5481:Ramberg–Bäcklund reaction
5360:Kiliani–Fischer synthesis
5202:Ramberg–Bäcklund reaction
5187:Pinacol coupling reaction
5182:Piancatelli rearrangement
5077:Liebeskind–Srogl coupling
4927:Fujimoto–Belleau reaction
4644:List of organic reactions
4508:Negative hyperconjugation
4253:
4195:
4115:
2945:Human Metabolome Database
2919:. pp. 5–94 to 5–98.
2674:oxidative decarboxylation
2619:keto–enol tautomerization
2580:phosphorus(V) oxychloride
2431:lithium aluminium hydride
2212:Disproportionation of an
902:, waxes, soaps, and oils
157:Examples and nomenclature
7412:Pictet–Spengler reaction
7327:Einhorn–Brunner reaction
7292:Boger pyridine synthesis
7186:Oxo-Diels–Alder reaction
7101:Aza-Diels–Alder reaction
6998:Pictet–Spengler reaction
6898:Hofmann–Löffler reaction
6888:Hegedus indole synthesis
6858:Fischer indole synthesis
6733:Bartoli indole synthesis
6688:Willgerodt rearrangement
6518:McLafferty rearrangement
6428:Ferrier carbocyclization
6243:2,3-Wittig rearrangement
6233:1,2-Wittig rearrangement
6073:Parikh–Doering oxidation
6063:Oxygen rebound mechanism
5728:Adkins–Peterson reaction
5620:Yamaguchi esterification
5560:Hegedus indole synthesis
5525:Bartoli indole synthesis
5396:Bamford–Stevens reaction
5312:Weinreb ketone synthesis
5272:Stork enamine alkylation
5047:Knoevenagel condensation
4917:Ferrier carbocyclization
4807:Castro–Stephens coupling
4433:Hammett acidity function
4423:Free-energy relationship
4368:Curtin–Hammett principle
4353:Conformational isomerism
3077:10.15227/orgsyn.066.0121
3032:10.15227/orgsyn.056.0028
2994:10.1002/14356007.a05_235
2796:List of carboxylic acids
2727:tetrahedral intermediate
2544:Phosphorus(III) chloride
1246:enthalpy of vaporization
1177:Divinylether fatty acids
205:even if there are other
37:Not to be confused with
7372:Knorr pyrrole synthesis
7307:Bucherer–Bergs reaction
7252:Allan–Robinson reaction
7231:Wagner-Jauregg reaction
7023:Ring-closing metathesis
6948:Larock indole synthesis
6928:Knorr pyrrole synthesis
6783:Bucherer–Bergs reaction
6648:Stieglitz rearrangement
6628:Skattebøl rearrangement
6598:Ring-closing metathesis
6458:Group transfer reaction
6423:Favorskii rearrangement
6363:Cornforth rearrangement
6293:Bamberger rearrangement
6198:Wolff–Kishner reduction
6018:Markó–Lam deoxygenation
5913:Fleming–Tamao oxidation
5908:Fischer–Tropsch process
5595:Oxymercuration reaction
5575:Knorr pyrrole synthesis
5401:Barton–Kellogg reaction
5307:Wagner-Jauregg reaction
5227:Ring-closing metathesis
5217:Reimer–Tiemann reaction
5207:Rauhut–Currier reaction
5122:Nef isocyanide reaction
5082:Malonic ester synthesis
5052:Knorr pyrrole synthesis
4987:High dilution principle
4922:Friedel–Crafts reaction
4857:Cross-coupling reaction
4782:Bucherer–Bergs reaction
4767:Blanc chloromethylation
4757:Blaise ketone synthesis
4732:Baylis–Hillman reaction
4727:Barton–Kellogg reaction
4702:Allan–Robinson reaction
4608:Woodward–Hoffmann rules
4343:Charge-transfer complex
4126:chemical classification
3047:Wade 2010, pp. 964–965.
2988:. Weinheim: Wiley-VCH.
2858:10.1351/goldbook.C00852
2720:ketonic decarboxylation
2663:Arndt–Eistert synthesis
2356:. Their conversion to
1693:) (first dissociation)
1035:the building-blocks of
980:Other carboxylic acids
378:, milk, butter, cheese
297:Common location or use
220:The carboxylate anion (
114:with R referring to an
7337:Feist–Benary synthesis
7111:Bradsher cycloaddition
7081:4+4 Photocycloaddition
7038:Simmons–Smith reaction
6983:Paternò–Büchi reaction
6843:Feist–Benary synthesis
6833:Dieckmann condensation
6583:Pummerer rearrangement
6563:Oxy-Cope rearrangement
6538:Myers allene synthesis
6488:Jacobsen rearrangement
6403:Electrocyclic reaction
6378:Demjanov rearrangement
6333:Buchner ring expansion
6303:Beckmann rearrangement
6283:Aza-Cope rearrangement
6278:Arndt–Eistert reaction
6253:Alkyne zipper reaction
6173:Transfer hydrogenation
6148:Sharpless oxyamination
6123:Selenoxide elimination
6008:Lombardo methylenation
5933:Griesbaum coozonolysis
5843:Corey–Itsuno reduction
5818:Boyland–Sims oxidation
5758:Angeli–Rimini reaction
5406:Boord olefin synthesis
5350:Arndt–Eistert reaction
5342:Homologation reactions
5142:Nitro-Mannich reaction
5057:Kolbe–Schmitt reaction
4867:Cross-coupling partner
4787:Buchner ring expansion
4707:Arndt–Eistert reaction
4473:Kinetic isotope effect
4220:Rearrangement reaction
2552:phosphorus(V) chloride
2403:Fischer esterification
2293:(baking soda) to form
2257:
2234:Kolbe–Schmitt reaction
2078:potassium permanganate
2068:Oxidative cleavage of
2059:potassium permanganate
2015:Kolbe–Schmitt reaction
1261:
935:Fats, vegetable oils,
211:3-chloropropanoic acid
67:
59:
51:
7196:Pauson–Khand reaction
7033:Sharpless epoxidation
6988:Pechmann condensation
6868:Friedländer synthesis
6818:Davis–Beirut reaction
6673:Wallach rearrangement
6643:Stevens rearrangement
6578:Pinacol rearrangement
6558:Overman rearrangement
6473:Hofmann rearrangement
6468:Hayashi rearrangement
6433:Ferrier rearrangement
6388:Dimroth rearrangement
6373:Curtius rearrangement
6368:Criegee rearrangement
6348:Claisen rearrangement
6338:Carroll rearrangement
6273:Amadori rearrangement
6263:Allylic rearrangement
6143:Sharpless epoxidation
5878:Dess–Martin oxidation
5803:Bohn–Schmidt reaction
5663:Hofmann rearrangement
5466:Kauffmann olefination
5389:Olefination reactions
5327:Wurtz–Fittig reaction
5162:Palladium–NHC complex
5042:Kauffmann olefination
4997:Homologation reaction
4847:Corey–House synthesis
4827:Claisen rearrangement
4623:Yukawa–Tsuno equation
4583:Swain–Lupton equation
4563:Spherical aromaticity
4498:Möbius–Hückel concept
4283:Aromatic ring current
4245:Substitution reaction
4133:chemical nomenclature
2941:"Chlorocarbonic acid"
2608:Specialized reactions
2252:
2204:Less-common reactions
1837:(food preservative),
1796:aminocarboxylic acids
1762:infrared spectroscopy
1271:Carboxylic acids are
1256:
1197:Carboxylic acids are
1080:phenyl alkanoic acids
1023:eicosapentaenoic acid
999:(2-propenoic acid) –
177:suffix. For example,
65:
57:
49:
7402:Paal–Knorr synthesis
7272:Barton–Zard reaction
7216:Staudinger synthesis
7166:Ketene cycloaddition
7136:Diels–Alder reaction
7116:Cheletropic reaction
7096:Alkyne trimerisation
6978:Paal–Knorr synthesis
6943:Kulinkovich reaction
6918:Jacobsen epoxidation
6838:Diels–Alder reaction
6633:Smiles rearrangement
6623:Sigmatropic reaction
6508:Lossen rearrangement
6358:Corey–Fuchs reaction
6323:Boekelheide reaction
6318:Bergmann degradation
6248:Achmatowicz reaction
6033:Methionine sulfoxide
5833:Clemmensen reduction
5793:Bergmann degradation
5723:Acyloin condensation
5688:Strecker degradation
5643:Bergmann degradation
5610:Ullmann condensation
5476:Peterson olefination
5451:Hydrazone iodination
5431:Elimination reaction
5332:Zincke–Suhl reaction
5252:Sonogashira coupling
5212:Reformatsky reaction
5172:Peterson olefination
5137:Nierenstein reaction
5067:Kulinkovich reaction
4882:Diels–Alder reaction
4842:Corey–Fuchs reaction
4822:Claisen condensation
4692:Alkyne trimerisation
4667:Acyloin condensation
4633:Σ-bishomoaromaticity
4593:Thorpe–Ingold effect
4205:Elimination reaction
2869:Recommendations 1979
2641:Hunsdiecker reaction
2230:von Richter reaction
2082:potassium dichromate
2051:potassium dichromate
2017:provides a route to
1730:resonance stabilized
1602:Trichloroacetic acid
1533:Trifluoroacetic acid
1273:Brønsted–Lowry acids
1019:docosahexaenoic acid
697:and hand wash soaps
7422:Prilezhaev reaction
7407:Pellizzari reaction
7086:(4+3) cycloaddition
7053:Van Leusen reaction
7028:Robinson annulation
7013:Pschorr cyclization
7008:Prilezhaev reaction
6738:Bergman cyclization
6693:Wolff rearrangement
6678:Weerman degradation
6568:Pericyclic reaction
6548:Neber rearrangement
6443:Fries rearrangement
6328:Brook rearrangement
6313:Bergman cyclization
6158:Staudinger reaction
6103:Rosenmund reduction
6093:Reductive amination
6058:Oppenauer oxidation
5848:Corey–Kim oxidation
5823:Cannizzaro reaction
5698:Weerman degradation
5673:Isosaccharinic acid
5585:Mukaiyama hydration
5441:Hofmann elimination
5426:Dehydrohalogenation
5411:Chugaev elimination
5232:Robinson annulation
5177:Pfitzinger reaction
4947:Gattermann reaction
4892:Wulff–Dötz reaction
4872:Dakin–West reaction
4797:Carbonyl allylation
4742:Bergman cyclization
4528:Kennedy J. P. Orton
4448:Hammond's postulate
4418:Flippin–Lodge angle
4388:Electromeric effect
4313:Beta-silicon effect
4298:Baker–Nathan effect
3589:not C, H or O)
3170:10.1021/j100367a033
3120:1971JChPh..54..927M
2709:Rosenmund reduction
2672:Many acids undergo
2648:Dakin–West reaction
2261:Acid-base reactions
2218:Cannizzaro reaction
1922:silicotungstic acid
1825:(chelating agent),
1652:2-Nitrobenzoic acid
1579:Dichloroacetic acid
1510:Difluoroacetic acid
1188:Physical properties
1167:Omega hydroxy acids
1131:Alpha hydroxy acids
1110:Tricarboxylic acids
981:
279:
7171:McCormack reaction
7121:Conia-ene reaction
6953:Madelung synthesis
6743:Biginelli reaction
6533:Mumm rearrangement
6418:Favorskii reaction
6353:Cope rearrangement
6343:Chan rearrangement
6108:Rubottom oxidation
6038:Miyaura borylation
6003:Lipid peroxidation
5998:Lindgren oxidation
5978:Kornblum oxidation
5973:Kolbe electrolysis
5918:Fukuyama reduction
5828:Carbonyl reduction
5678:Marker degradation
5540:Diazonium compound
5530:Boudouard reaction
5509:Carbon-heteroatom
5436:Grieco elimination
5222:Rieche formylation
5167:Passerini reaction
5097:Meerwein arylation
5017:Hydroxymethylation
4912:Favorskii reaction
4812:Chan rearrangement
4747:Biginelli reaction
4672:Aldol condensation
4518:2-Norbornyl cation
4493:Möbius aromaticity
4488:Markovnikov's rule
4383:Effective molarity
4328:Bürgi–Dunitz angle
4318:Bicycloaromaticity
4031:Hypervalent iodine
3096:Wade 2010, p. 838.
2734:Kolbe electrolysis
2713:Fukuyama reduction
2617:are labile due to
2437:compounds but not
2291:sodium bicarbonate
2258:
2030:Laboratory methods
1913:is generated from
1782:spectrometry, the
1367:methyl substituent
1262:
1157:Beta hydroxy acids
1088:Dicarboxylic acids
979:
846:Heptadecanoic acid
778:Pentadecanoic acid
743:Tetradecanoic acid
661:Anti-fungal agent
277:
266:, which occurs in
68:
60:
52:
7481:Functional groups
7463:
7462:
7459:
7458:
7455:
7454:
7447:Wohl–Aue reaction
7091:6+4 Cycloaddition
6908:Iodolactonization
6228:1,2-rearrangement
6193:Wohl–Aue reaction
6113:Sabatier reaction
6078:Pinnick oxidation
6043:Mozingo reduction
5988:Leuckart reaction
5943:Haloform reaction
5858:Criegee oxidation
5838:Collins oxidation
5788:Benkeser reaction
5783:Bechamp reduction
5753:Andrussow process
5738:Alcohol oxidation
5648:Edman degradation
5555:Haloform reaction
5504:
5503:
5491:Takai olefination
5456:Julia olefination
5282:Takai olefination
5157:Olefin metathesis
5032:Julia olefination
4957:Grignard reaction
4937:Fukuyama coupling
4852:Coupling reaction
4817:Chan–Lam coupling
4687:Alkyne metathesis
4682:Alkane metathesis
4538:Phosphaethynolate
4443:George S. Hammond
4403:Electronic effect
4358:Conjugated system
4240:Stereospecificity
4235:Stereoselectivity
4200:Addition reaction
4189:organic reactions
4154:
4153:
4092:Sulfenyl chloride
4070:
4069:
3569:
3568:
3388:(only C, H and O)
3229:Functional groups
3128:10.1063/1.1675022
3083:Collected Volumes
3064:Organic Syntheses
2915:(92nd ed.).
2886:Organic Chemistry
2801:Dicarboxylic acid
2688: 6.4.1) and
2446:Vilsmaier reagent
2439:Grignard reagents
2346:carboxylate salts
2255:organic reactions
2172:haloform reaction
2102:Carbonation of a
1887:terephthalic acid
1850:Industrial routes
1839:terephthalic acid
1723:
1722:
1691:HO−C(=O)−C(=O)−OH
1556:Chloroacetic acid
1487:Fluoroacetic acid
1418:Chloroformic acid
1185:
1184:
1052:group; examples:
977:
976:
915:Nonadecanoic acid
879:Octadecanoic acid
811:Hexadecanoic acid
774:Pentadecylic acid
730:Plant metabolite
294:Chemical formula
90:) attached to an
72:organic chemistry
58:Carboxylate anion
16:(Redirected from
7488:
7476:Carboxylic acids
7442:Wenker synthesis
7432:Stollé synthesis
7287:Bobbitt reaction
7257:Auwers synthesis
7201:Povarov reaction
7126:Cyclopropanation
7064:
7058:Wenker synthesis
6813:Darzens reaction
6763:Bobbitt reaction
6608:Schmidt reaction
6413:Enyne metathesis
6188:Whiting reaction
6183:Wharton reaction
6128:Shapiro reaction
6118:Sarett oxidation
6083:Prévost reaction
5893:Emde degradation
5703:Wohl degradation
5683:Ruff degradation
5653:Emde degradation
5550:Grignard reagent
5486:Shapiro reaction
5471:McMurry reaction
5338:
5302:Ullmann reaction
5267:Stollé synthesis
5257:Stetter reaction
5247:Shapiro reaction
5237:Sakurai reaction
5132:Negishi coupling
5112:Minisci reaction
5107:Michael reaction
5092:McMurry reaction
5087:Mannich reaction
4967:Hammick reaction
4962:Grignard reagent
4902:Enyne metathesis
4887:Doebner reaction
4877:Darzens reaction
4722:Barbier reaction
4712:Auwers synthesis
4639:
4613:Woodward's rules
4578:Superaromaticity
4568:Spiroaromaticity
4468:Inductive effect
4463:Hyperconjugation
4438:Hammett equation
4378:Edwards equation
4230:Regioselectivity
4181:
4174:
4167:
4158:
4121:
4026:Trifluoromethoxy
3594:
3590:
3393:
3389:
3242:
3222:
3215:
3208:
3199:
3174:
3173:
3164:(4): 1372–1376.
3138:
3132:
3131:
3103:
3097:
3094:
3088:
3086:
3079:
3054:
3048:
3045:
3036:
3035:
3015:
3009:
3007:
2981:
2975:
2974:
2972:
2970:
2955:
2949:
2948:
2937:
2931:
2930:
2906:
2900:
2899:
2881:
2872:
2866:
2860:
2850:carboxylic acids
2837:
2741:Carboxyl radical
2692:(EC 4.1.1).
2659:methylene bridge
2630:Schmidt reaction
2540:
2496:Thionyl chloride
2484:thionyl chloride
2467:
2327:
2253:Carboxylic acid
2199:
2158:
2145:
2144:
2141:
2133:
2131:
2130:
2127:
2104:Grignard reagent
1987:
1871:hydroformylation
1756:Characterization
1715:
1714:
1713:
1710:
1702:Hydrogen oxalate
1692:
1677:
1642:
1615:
1592:
1569:
1546:
1523:
1500:
1477:
1450:
1427:
1408:
1382:Carboxylic acid
1379:
1315:
1314:
1313:
1310:
1298:
1257:Carboxylic acid
1231:
1217:hydrogen bonding
1214:
1207:
1151:– found in wine
1067:carboxylic acids
1054:acetoacetic acid
1006:
982:
968:
932:
911:Nonadecylic acid
896:
863:
828:
795:
760:
727:
710:Tridecanoic acid
691:
658:
619:
583:
548:
515:
479:
443:
408:
371:
340:
280:
235:
234:
233:
230:
223:
200:
113:
108:
98:
89:
82:that contains a
21:
7496:
7495:
7491:
7490:
7489:
7487:
7486:
7485:
7466:
7465:
7464:
7451:
7352:Gewald reaction
7235:
7062:
7043:Skraup reaction
6878:Graham reaction
6873:Gewald reaction
6704:
6697:
6219:
6212:
6168:Swern oxidation
6153:Stahl oxidation
6098:Riley oxidation
6053:Omega oxidation
6013:Luche reduction
5963:Jones oxidation
5928:Glycol cleavage
5923:Ganem oxidation
5868:Davis oxidation
5863:Dakin oxidation
5798:Birch reduction
5748:Amide reduction
5714:
5707:
5668:Hooker reaction
5630:
5624:
5512:
5510:
5500:
5496:Wittig reaction
5384:
5380:Wittig reaction
5355:Hooker reaction
5336:
5317:Wittig reaction
5292:Thorpe reaction
5277:Suzuki reaction
5262:Stille reaction
5197:Quelet reaction
5072:Kumada coupling
5022:Ivanov reaction
5012:Hydrovinylation
4992:Hiyama coupling
4952:Glaser coupling
4762:Blaise reaction
4752:Bingel reaction
4737:Benary reaction
4654:
4652:
4646:
4637:
4533:Passive binding
4453:Homoaromaticity
4303:Baldwin's rules
4278:Antiaromaticity
4273:Anomeric effect
4249:
4191:
4185:
4155:
4150:
4119:
4111:
4066:
4021:Trichloromethyl
4016:Trifluoromethyl
3990:
3967:
3929:
3906:
3801:
3770:Phosphine oxide
3722:
3588:
3586:
3585:
3583:
3581:
3579:
3577:
3575:
3565:
3525:
3468:
3387:
3386:
3381:
3376:
3366:
3240:
3239:
3231:
3226:
3183:
3178:
3177:
3155:
3148:
3146:
3139:
3135:
3105:
3104:
3100:
3095:
3091:
3081:
3056:
3055:
3051:
3046:
3039:
3017:
3016:
3012:
3004:
2983:
2982:
2978:
2968:
2966:
2957:
2956:
2952:
2939:
2938:
2934:
2927:
2908:
2907:
2903:
2896:
2883:
2882:
2875:
2867:
2863:
2838:
2834:
2829:
2821:
2767:
2743:
2610:
2592:
2585:
2577:
2572:phosphorus acid
2569:
2565:
2561:
2557:
2549:
2529:with a loss of
2480:
2465:
2461:
2457:
2425:to alcohols by
2415:
2334:
2325:
2321:
2317:
2313:
2309:
2305:
2281:. For example,
2263:
2247:
2206:
2197:
2193:
2185:
2153:
2142:
2139:
2138:
2136:
2128:
2125:
2124:
2121:
2115:
2063:sodium chlorite
2032:
2021:, precursor to
1984:
1980:
1974:
1967:
1960:Reppe chemistry
1852:
1847:
1792:
1777:
1770:
1758:
1742:
1735:
1711:
1708:
1707:
1705:
1690:
1675:
1671:
1667:
1663:
1659:
1640:
1636:
1632:
1628:
1613:
1609:
1605:
1590:
1586:
1582:
1567:
1563:
1559:
1544:
1540:
1536:
1521:
1517:
1513:
1498:
1494:
1490:
1475:
1471:
1467:
1463:
1448:
1444:
1440:
1425:
1421:
1406:
1402:
1392:
1375:
1364:
1357:
1346:
1337:
1311:
1308:
1307:
1305:
1296:
1292:
1269:
1238:
1212:
1205:
1195:
1190:
1004:
1000:
985:Compound class
966:
962:
958:
954:
930:
926:
922:
918:
894:
890:
886:
882:
861:
857:
853:
849:
826:
822:
818:
814:
793:
789:
785:
781:
758:
754:
750:
746:
725:
721:
717:
713:
706:Tridecylic acid
689:
685:
681:
677:
674:Dodecanoic acid
656:
652:
648:
644:
641:Undecanoic acid
627:Palm kernel oil
617:
613:
609:
605:
581:
577:
573:
569:
562:Pelargonic acid
546:
542:
538:
534:
513:
509:
505:
501:
477:
473:
469:
465:
441:
437:
433:
429:
406:
402:
398:
394:
369:
365:
361:
338:
334:
284:
231:
228:
227:
225:
221:
198:
194:
190:
186:
182:
159:
111:
110:, sometimes as
106:
102:
96:
87:
76:carboxylic acid
42:
35:
32:Cooh (musician)
28:
23:
22:
15:
12:
11:
5:
7494:
7492:
7484:
7483:
7478:
7468:
7467:
7461:
7460:
7457:
7456:
7453:
7452:
7450:
7449:
7444:
7439:
7434:
7429:
7424:
7419:
7414:
7409:
7404:
7399:
7394:
7389:
7384:
7379:
7374:
7369:
7364:
7359:
7357:Hantzsch ester
7354:
7349:
7344:
7339:
7334:
7329:
7324:
7319:
7314:
7309:
7304:
7299:
7294:
7289:
7284:
7279:
7274:
7269:
7267:Banert cascade
7264:
7259:
7254:
7249:
7243:
7241:
7237:
7236:
7234:
7233:
7228:
7223:
7218:
7213:
7208:
7206:Prato reaction
7203:
7198:
7193:
7188:
7183:
7178:
7173:
7168:
7163:
7158:
7153:
7148:
7143:
7138:
7133:
7128:
7123:
7118:
7113:
7108:
7103:
7098:
7093:
7088:
7083:
7078:
7072:
7070:
7061:
7060:
7055:
7050:
7045:
7040:
7035:
7030:
7025:
7020:
7015:
7010:
7005:
7000:
6995:
6990:
6985:
6980:
6975:
6970:
6965:
6960:
6955:
6950:
6945:
6940:
6935:
6930:
6925:
6920:
6915:
6910:
6905:
6900:
6895:
6890:
6885:
6880:
6875:
6870:
6865:
6860:
6855:
6850:
6845:
6840:
6835:
6830:
6825:
6820:
6815:
6810:
6805:
6800:
6795:
6790:
6785:
6780:
6775:
6770:
6765:
6760:
6755:
6750:
6745:
6740:
6735:
6730:
6725:
6720:
6715:
6709:
6707:
6699:
6698:
6696:
6695:
6690:
6685:
6680:
6675:
6670:
6665:
6660:
6655:
6650:
6645:
6640:
6635:
6630:
6625:
6620:
6615:
6610:
6605:
6600:
6595:
6590:
6585:
6580:
6575:
6570:
6565:
6560:
6555:
6550:
6545:
6540:
6535:
6530:
6525:
6520:
6515:
6510:
6505:
6500:
6495:
6490:
6485:
6480:
6475:
6470:
6465:
6460:
6455:
6450:
6445:
6440:
6435:
6430:
6425:
6420:
6415:
6410:
6405:
6400:
6395:
6390:
6385:
6380:
6375:
6370:
6365:
6360:
6355:
6350:
6345:
6340:
6335:
6330:
6325:
6320:
6315:
6310:
6305:
6300:
6298:Banert cascade
6295:
6290:
6285:
6280:
6275:
6270:
6265:
6260:
6255:
6250:
6245:
6240:
6235:
6230:
6224:
6222:
6218:Rearrangement
6214:
6213:
6211:
6210:
6208:Zinin reaction
6205:
6200:
6195:
6190:
6185:
6180:
6178:Wacker process
6175:
6170:
6165:
6160:
6155:
6150:
6145:
6140:
6135:
6130:
6125:
6120:
6115:
6110:
6105:
6100:
6095:
6090:
6085:
6080:
6075:
6070:
6065:
6060:
6055:
6050:
6045:
6040:
6035:
6030:
6025:
6020:
6015:
6010:
6005:
6000:
5995:
5990:
5985:
5980:
5975:
5970:
5965:
5960:
5955:
5953:Hydrogenolysis
5950:
5945:
5940:
5935:
5930:
5925:
5920:
5915:
5910:
5905:
5903:Étard reaction
5900:
5895:
5890:
5885:
5880:
5875:
5870:
5865:
5860:
5855:
5850:
5845:
5840:
5835:
5830:
5825:
5820:
5815:
5810:
5808:Bosch reaction
5805:
5800:
5795:
5790:
5785:
5780:
5775:
5770:
5765:
5760:
5755:
5750:
5745:
5740:
5735:
5730:
5725:
5719:
5717:
5713:Organic redox
5709:
5708:
5706:
5705:
5700:
5695:
5690:
5685:
5680:
5675:
5670:
5665:
5660:
5655:
5650:
5645:
5640:
5634:
5632:
5626:
5625:
5623:
5622:
5617:
5612:
5607:
5602:
5597:
5592:
5587:
5582:
5577:
5572:
5567:
5562:
5557:
5552:
5547:
5545:Esterification
5542:
5537:
5532:
5527:
5522:
5516:
5514:
5506:
5505:
5502:
5501:
5499:
5498:
5493:
5488:
5483:
5478:
5473:
5468:
5463:
5458:
5453:
5448:
5443:
5438:
5433:
5428:
5423:
5418:
5413:
5408:
5403:
5398:
5392:
5390:
5386:
5385:
5383:
5382:
5377:
5372:
5367:
5362:
5357:
5352:
5346:
5344:
5335:
5334:
5329:
5324:
5322:Wurtz reaction
5319:
5314:
5309:
5304:
5299:
5294:
5289:
5284:
5279:
5274:
5269:
5264:
5259:
5254:
5249:
5244:
5239:
5234:
5229:
5224:
5219:
5214:
5209:
5204:
5199:
5194:
5192:Prins reaction
5189:
5184:
5179:
5174:
5169:
5164:
5159:
5154:
5149:
5144:
5139:
5134:
5129:
5124:
5119:
5114:
5109:
5104:
5099:
5094:
5089:
5084:
5079:
5074:
5069:
5064:
5059:
5054:
5049:
5044:
5039:
5034:
5029:
5024:
5019:
5014:
5009:
5007:Hydrocyanation
5004:
4999:
4994:
4989:
4984:
4979:
4977:Henry reaction
4974:
4969:
4964:
4959:
4954:
4949:
4944:
4939:
4934:
4929:
4924:
4919:
4914:
4909:
4904:
4899:
4894:
4889:
4884:
4879:
4874:
4869:
4864:
4859:
4854:
4849:
4844:
4839:
4834:
4829:
4824:
4819:
4814:
4809:
4804:
4799:
4794:
4789:
4784:
4779:
4774:
4769:
4764:
4759:
4754:
4749:
4744:
4739:
4734:
4729:
4724:
4719:
4714:
4709:
4704:
4699:
4694:
4689:
4684:
4679:
4677:Aldol reaction
4674:
4669:
4664:
4658:
4656:
4651:Carbon-carbon
4648:
4647:
4642:
4636:
4635:
4630:
4628:Zaitsev's rule
4625:
4620:
4615:
4610:
4605:
4600:
4595:
4590:
4585:
4580:
4575:
4573:Steric effects
4570:
4565:
4560:
4555:
4550:
4545:
4540:
4535:
4530:
4525:
4520:
4515:
4510:
4505:
4500:
4495:
4490:
4485:
4480:
4475:
4470:
4465:
4460:
4455:
4450:
4445:
4440:
4435:
4430:
4425:
4420:
4415:
4410:
4405:
4400:
4395:
4390:
4385:
4380:
4375:
4370:
4365:
4360:
4355:
4350:
4345:
4340:
4335:
4330:
4325:
4320:
4315:
4310:
4305:
4300:
4295:
4290:
4285:
4280:
4275:
4270:
4265:
4260:
4254:
4251:
4250:
4248:
4247:
4242:
4237:
4232:
4227:
4225:Redox reaction
4222:
4217:
4212:
4210:Polymerization
4207:
4202:
4196:
4193:
4192:
4186:
4184:
4183:
4176:
4169:
4161:
4152:
4151:
4149:
4148:
4147:
4146:
4141:
4129:
4122:
4116:
4113:
4112:
4110:
4109:
4107:Sulfinylamines
4104:
4099:
4094:
4089:
4087:Phosphoramides
4084:
4082:Isothiocyanate
4078:
4076:
4072:
4071:
4068:
4067:
4065:
4064:
4059:
4058:
4057:
4047:
4046:
4045:
4035:
4034:
4033:
4028:
4023:
4018:
4013:
4002:
4000:
3992:
3991:
3989:
3988:
3983:
3977:
3975:
3969:
3968:
3966:
3965:
3960:
3958:Selenenic acid
3955:
3953:Seleninic acid
3950:
3948:Selenonic acid
3945:
3939:
3937:
3931:
3930:
3928:
3927:
3922:
3916:
3914:
3908:
3907:
3905:
3904:
3899:
3894:
3889:
3884:
3879:
3874:
3869:
3864:
3859:
3854:
3849:
3844:
3839:
3834:
3829:
3828:
3827:
3817:
3811:
3809:
3803:
3802:
3800:
3799:
3794:
3789:
3784:
3783:
3782:
3772:
3767:
3762:
3757:
3756:
3755:
3745:
3744:
3743:
3741:Phosphodiester
3732:
3730:
3724:
3723:
3721:
3720:
3715:
3710:
3705:
3700:
3695:
3690:
3685:
3680:
3675:
3670:
3665:
3660:
3655:
3650:
3645:
3640:
3635:
3630:
3625:
3620:
3619:
3618:
3613:
3602:
3600:
3591:
3587:(one element,
3571:
3570:
3567:
3566:
3564:
3563:
3562:
3561:
3551:
3550:
3549:
3544:
3533:
3531:
3527:
3526:
3524:
3523:
3518:
3513:
3512:
3511:
3501:
3500:
3499:
3494:
3489:
3478:
3476:
3470:
3469:
3467:
3466:
3464:Methylenedioxy
3461:
3456:
3455:
3454:
3449:
3439:
3438:
3437:
3432:
3422:
3421:
3420:
3410:
3405:
3399:
3397:
3390:
3368:
3367:
3365:
3364:
3359:
3354:
3353:
3352:
3347:
3337:
3336:
3335:
3330:
3325:
3320:
3315:
3310:
3300:
3299:
3298:
3293:
3283:
3282:
3281:
3276:
3271:
3266:
3261:
3256:
3245:
3243:
3241:(only C and H)
3233:
3232:
3227:
3225:
3224:
3217:
3210:
3202:
3196:
3195:
3190:
3182:
3181:External links
3179:
3176:
3175:
3153:
3144:
3140:The value is p
3133:
3114:(3): 927–942.
3098:
3089:
3049:
3037:
3010:
3002:
2976:
2958:Smith, Brian.
2950:
2932:
2926:978-1439855119
2925:
2901:
2894:
2873:
2861:
2831:
2830:
2828:
2825:
2824:
2823:
2819:
2816:Carbon dioxide
2813:
2808:
2803:
2798:
2793:
2788:
2783:
2778:
2773:
2771:Acid anhydride
2766:
2763:
2742:
2739:
2738:
2737:
2730:
2723:
2716:
2693:
2690:decarboxylases
2670:
2651:
2644:
2637:
2626:
2609:
2606:
2591:
2588:
2583:
2575:
2567:
2563:
2559:
2555:
2547:
2519:sulfur dioxide
2488:acyl chlorides
2479:
2476:
2458:[ClHC=N(CH
2414:
2411:
2350:acid chlorides
2333:
2330:
2329:
2328:
2299:carbon dioxide
2295:sodium acetate
2262:
2259:
2246:
2243:
2242:
2241:
2226:
2220:
2205:
2202:
2201:
2200:
2182:
2181:
2174:
2168:methyl ketones
2160:
2159:
2134:
2112:
2111:
2100:
2087:Hydrolysis of
2085:
2066:
2031:
2028:
2027:
2026:
2019:salicylic acid
2011:
2001:
1992:Hydrolysis of
1989:
1988:
1964:
1963:
1928:
1925:
1918:
1874:
1863:
1860:Cativa process
1851:
1848:
1846:
1843:
1835:propionic acid
1791:
1788:
1775:
1768:
1757:
1754:
1741:
1738:
1733:
1721:
1720:
1717:
1698:
1697:
1694:
1683:
1682:
1679:
1648:
1647:
1644:
1621:
1620:
1617:
1598:
1597:
1594:
1575:
1574:
1571:
1552:
1551:
1548:
1529:
1528:
1525:
1506:
1505:
1502:
1483:
1482:
1479:
1456:
1455:
1452:
1433:
1432:
1429:
1414:
1413:
1410:
1395:
1394:
1390:
1383:
1373:
1362:
1355:
1344:
1335:
1268:
1265:
1264:
1263:
1242:hydrogen bonds
1237:
1236:Boiling points
1234:
1233:
1232:
1194:
1191:
1189:
1186:
1183:
1182:
1179:
1173:
1172:
1169:
1163:
1162:
1159:
1153:
1152:
1133:
1127:
1126:
1124:isocitric acid
1112:
1106:
1105:
1090:
1084:
1083:
1076:salicylic acid
1068:
1061:
1060:
1046:
1040:
1039:
1033:
1027:
1026:
1015:
1009:
1008:
994:
990:
989:
986:
975:
974:
969:
952:
951:Icosanoic acid
949:
947:Arachidic acid
944:
940:
939:
933:
916:
913:
908:
904:
903:
897:
880:
877:
872:
868:
867:
864:
847:
844:
839:
835:
834:
829:
812:
809:
804:
800:
799:
796:
779:
776:
771:
767:
766:
761:
744:
741:
736:
732:
731:
728:
711:
708:
703:
699:
698:
692:
675:
672:
667:
663:
662:
659:
642:
639:
637:Undecylic acid
634:
630:
629:
620:
603:
600:
595:
591:
590:
584:
567:
564:
559:
555:
554:
549:
532:
529:
524:
520:
519:
516:
499:
498:Heptanoic acid
496:
491:
487:
486:
480:
463:
460:
455:
451:
450:
444:
427:
426:Pentanoic acid
424:
419:
415:
414:
409:
392:
389:
384:
380:
379:
372:
359:
358:Propanoic acid
356:
354:Propionic acid
351:
347:
346:
341:
332:
329:
324:
320:
319:
314:
311:
310:Methanoic acid
308:
303:
299:
298:
295:
292:
289:
286:
250:conjugate acid
215:2-carboxyfuran
158:
155:
84:carboxyl group
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
7493:
7482:
7479:
7477:
7474:
7473:
7471:
7448:
7445:
7443:
7440:
7438:
7435:
7433:
7430:
7428:
7425:
7423:
7420:
7418:
7415:
7413:
7410:
7408:
7405:
7403:
7400:
7398:
7395:
7393:
7390:
7388:
7385:
7383:
7380:
7378:
7375:
7373:
7370:
7368:
7367:Herz reaction
7365:
7363:
7360:
7358:
7355:
7353:
7350:
7348:
7345:
7343:
7340:
7338:
7335:
7333:
7330:
7328:
7325:
7323:
7320:
7318:
7315:
7313:
7310:
7308:
7305:
7303:
7300:
7298:
7295:
7293:
7290:
7288:
7285:
7283:
7280:
7278:
7275:
7273:
7270:
7268:
7265:
7263:
7260:
7258:
7255:
7253:
7250:
7248:
7245:
7244:
7242:
7238:
7232:
7229:
7227:
7224:
7222:
7219:
7217:
7214:
7212:
7209:
7207:
7204:
7202:
7199:
7197:
7194:
7192:
7189:
7187:
7184:
7182:
7179:
7177:
7174:
7172:
7169:
7167:
7164:
7162:
7159:
7157:
7154:
7152:
7149:
7147:
7144:
7142:
7139:
7137:
7134:
7132:
7129:
7127:
7124:
7122:
7119:
7117:
7114:
7112:
7109:
7107:
7104:
7102:
7099:
7097:
7094:
7092:
7089:
7087:
7084:
7082:
7079:
7077:
7074:
7073:
7071:
7069:
7068:Cycloaddition
7065:
7059:
7056:
7054:
7051:
7049:
7046:
7044:
7041:
7039:
7036:
7034:
7031:
7029:
7026:
7024:
7021:
7019:
7016:
7014:
7011:
7009:
7006:
7004:
7001:
6999:
6996:
6994:
6991:
6989:
6986:
6984:
6981:
6979:
6976:
6974:
6971:
6969:
6966:
6964:
6961:
6959:
6956:
6954:
6951:
6949:
6946:
6944:
6941:
6939:
6936:
6934:
6931:
6929:
6926:
6924:
6921:
6919:
6916:
6914:
6913:Isay reaction
6911:
6909:
6906:
6904:
6901:
6899:
6896:
6894:
6891:
6889:
6886:
6884:
6881:
6879:
6876:
6874:
6871:
6869:
6866:
6864:
6861:
6859:
6856:
6854:
6851:
6849:
6846:
6844:
6841:
6839:
6836:
6834:
6831:
6829:
6826:
6824:
6821:
6819:
6816:
6814:
6811:
6809:
6808:Cycloaddition
6806:
6804:
6801:
6799:
6796:
6794:
6791:
6789:
6786:
6784:
6781:
6779:
6776:
6774:
6771:
6769:
6766:
6764:
6761:
6759:
6756:
6754:
6751:
6749:
6746:
6744:
6741:
6739:
6736:
6734:
6731:
6729:
6726:
6724:
6721:
6719:
6716:
6714:
6711:
6710:
6708:
6706:
6703:Ring forming
6700:
6694:
6691:
6689:
6686:
6684:
6681:
6679:
6676:
6674:
6671:
6669:
6666:
6664:
6661:
6659:
6656:
6654:
6651:
6649:
6646:
6644:
6641:
6639:
6636:
6634:
6631:
6629:
6626:
6624:
6621:
6619:
6616:
6614:
6611:
6609:
6606:
6604:
6603:Rupe reaction
6601:
6599:
6596:
6594:
6591:
6589:
6586:
6584:
6581:
6579:
6576:
6574:
6571:
6569:
6566:
6564:
6561:
6559:
6556:
6554:
6551:
6549:
6546:
6544:
6541:
6539:
6536:
6534:
6531:
6529:
6526:
6524:
6521:
6519:
6516:
6514:
6511:
6509:
6506:
6504:
6501:
6499:
6496:
6494:
6491:
6489:
6486:
6484:
6481:
6479:
6476:
6474:
6471:
6469:
6466:
6464:
6461:
6459:
6456:
6454:
6451:
6449:
6446:
6444:
6441:
6439:
6436:
6434:
6431:
6429:
6426:
6424:
6421:
6419:
6416:
6414:
6411:
6409:
6406:
6404:
6401:
6399:
6396:
6394:
6391:
6389:
6386:
6384:
6381:
6379:
6376:
6374:
6371:
6369:
6366:
6364:
6361:
6359:
6356:
6354:
6351:
6349:
6346:
6344:
6341:
6339:
6336:
6334:
6331:
6329:
6326:
6324:
6321:
6319:
6316:
6314:
6311:
6309:
6306:
6304:
6301:
6299:
6296:
6294:
6291:
6289:
6286:
6284:
6281:
6279:
6276:
6274:
6271:
6269:
6266:
6264:
6261:
6259:
6256:
6254:
6251:
6249:
6246:
6244:
6241:
6239:
6236:
6234:
6231:
6229:
6226:
6225:
6223:
6221:
6215:
6209:
6206:
6204:
6201:
6199:
6196:
6194:
6191:
6189:
6186:
6184:
6181:
6179:
6176:
6174:
6171:
6169:
6166:
6164:
6161:
6159:
6156:
6154:
6151:
6149:
6146:
6144:
6141:
6139:
6136:
6134:
6131:
6129:
6126:
6124:
6121:
6119:
6116:
6114:
6111:
6109:
6106:
6104:
6101:
6099:
6096:
6094:
6091:
6089:
6086:
6084:
6081:
6079:
6076:
6074:
6071:
6069:
6066:
6064:
6061:
6059:
6056:
6054:
6051:
6049:
6046:
6044:
6041:
6039:
6036:
6034:
6031:
6029:
6026:
6024:
6021:
6019:
6016:
6014:
6011:
6009:
6006:
6004:
6001:
5999:
5996:
5994:
5993:Ley oxidation
5991:
5989:
5986:
5984:
5981:
5979:
5976:
5974:
5971:
5969:
5966:
5964:
5961:
5959:
5958:Hydroxylation
5956:
5954:
5951:
5949:
5948:Hydrogenation
5946:
5944:
5941:
5939:
5936:
5934:
5931:
5929:
5926:
5924:
5921:
5919:
5916:
5914:
5911:
5909:
5906:
5904:
5901:
5899:
5896:
5894:
5891:
5889:
5886:
5884:
5883:DNA oxidation
5881:
5879:
5876:
5874:
5873:Deoxygenation
5871:
5869:
5866:
5864:
5861:
5859:
5856:
5854:
5851:
5849:
5846:
5844:
5841:
5839:
5836:
5834:
5831:
5829:
5826:
5824:
5821:
5819:
5816:
5814:
5811:
5809:
5806:
5804:
5801:
5799:
5796:
5794:
5791:
5789:
5786:
5784:
5781:
5779:
5776:
5774:
5771:
5769:
5766:
5764:
5763:Aromatization
5761:
5759:
5756:
5754:
5751:
5749:
5746:
5744:
5741:
5739:
5736:
5734:
5731:
5729:
5726:
5724:
5721:
5720:
5718:
5716:
5710:
5704:
5701:
5699:
5696:
5694:
5691:
5689:
5686:
5684:
5681:
5679:
5676:
5674:
5671:
5669:
5666:
5664:
5661:
5659:
5656:
5654:
5651:
5649:
5646:
5644:
5641:
5639:
5636:
5635:
5633:
5627:
5621:
5618:
5616:
5613:
5611:
5608:
5606:
5603:
5601:
5600:Reed reaction
5598:
5596:
5593:
5591:
5588:
5586:
5583:
5581:
5578:
5576:
5573:
5571:
5568:
5566:
5563:
5561:
5558:
5556:
5553:
5551:
5548:
5546:
5543:
5541:
5538:
5536:
5533:
5531:
5528:
5526:
5523:
5521:
5518:
5517:
5515:
5511:bond forming
5507:
5497:
5494:
5492:
5489:
5487:
5484:
5482:
5479:
5477:
5474:
5472:
5469:
5467:
5464:
5462:
5459:
5457:
5454:
5452:
5449:
5447:
5444:
5442:
5439:
5437:
5434:
5432:
5429:
5427:
5424:
5422:
5419:
5417:
5416:Cope reaction
5414:
5412:
5409:
5407:
5404:
5402:
5399:
5397:
5394:
5393:
5391:
5387:
5381:
5378:
5376:
5373:
5371:
5368:
5366:
5363:
5361:
5358:
5356:
5353:
5351:
5348:
5347:
5345:
5343:
5339:
5333:
5330:
5328:
5325:
5323:
5320:
5318:
5315:
5313:
5310:
5308:
5305:
5303:
5300:
5298:
5295:
5293:
5290:
5288:
5285:
5283:
5280:
5278:
5275:
5273:
5270:
5268:
5265:
5263:
5260:
5258:
5255:
5253:
5250:
5248:
5245:
5243:
5240:
5238:
5235:
5233:
5230:
5228:
5225:
5223:
5220:
5218:
5215:
5213:
5210:
5208:
5205:
5203:
5200:
5198:
5195:
5193:
5190:
5188:
5185:
5183:
5180:
5178:
5175:
5173:
5170:
5168:
5165:
5163:
5160:
5158:
5155:
5153:
5150:
5148:
5145:
5143:
5140:
5138:
5135:
5133:
5130:
5128:
5127:Nef synthesis
5125:
5123:
5120:
5118:
5115:
5113:
5110:
5108:
5105:
5103:
5102:Methylenation
5100:
5098:
5095:
5093:
5090:
5088:
5085:
5083:
5080:
5078:
5075:
5073:
5070:
5068:
5065:
5063:
5060:
5058:
5055:
5053:
5050:
5048:
5045:
5043:
5040:
5038:
5035:
5033:
5030:
5028:
5025:
5023:
5020:
5018:
5015:
5013:
5010:
5008:
5005:
5003:
5000:
4998:
4995:
4993:
4990:
4988:
4985:
4983:
4980:
4978:
4975:
4973:
4972:Heck reaction
4970:
4968:
4965:
4963:
4960:
4958:
4955:
4953:
4950:
4948:
4945:
4943:
4940:
4938:
4935:
4933:
4930:
4928:
4925:
4923:
4920:
4918:
4915:
4913:
4910:
4908:
4905:
4903:
4900:
4898:
4895:
4893:
4890:
4888:
4885:
4883:
4880:
4878:
4875:
4873:
4870:
4868:
4865:
4863:
4860:
4858:
4855:
4853:
4850:
4848:
4845:
4843:
4840:
4838:
4835:
4833:
4830:
4828:
4825:
4823:
4820:
4818:
4815:
4813:
4810:
4808:
4805:
4803:
4800:
4798:
4795:
4793:
4790:
4788:
4785:
4783:
4780:
4778:
4775:
4773:
4770:
4768:
4765:
4763:
4760:
4758:
4755:
4753:
4750:
4748:
4745:
4743:
4740:
4738:
4735:
4733:
4730:
4728:
4725:
4723:
4720:
4718:
4715:
4713:
4710:
4708:
4705:
4703:
4700:
4698:
4695:
4693:
4690:
4688:
4685:
4683:
4680:
4678:
4675:
4673:
4670:
4668:
4665:
4663:
4660:
4659:
4657:
4653:bond forming
4649:
4645:
4640:
4634:
4631:
4629:
4626:
4624:
4621:
4619:
4618:Y-aromaticity
4616:
4614:
4611:
4609:
4606:
4604:
4603:Walsh diagram
4601:
4599:
4596:
4594:
4591:
4589:
4588:Taft equation
4586:
4584:
4581:
4579:
4576:
4574:
4571:
4569:
4566:
4564:
4561:
4559:
4558:Σ-aromaticity
4556:
4554:
4551:
4549:
4546:
4544:
4541:
4539:
4536:
4534:
4531:
4529:
4526:
4524:
4521:
4519:
4516:
4514:
4511:
4509:
4506:
4504:
4501:
4499:
4496:
4494:
4491:
4489:
4486:
4484:
4483:Marcus theory
4481:
4479:
4476:
4474:
4471:
4469:
4466:
4464:
4461:
4459:
4458:Hückel's rule
4456:
4454:
4451:
4449:
4446:
4444:
4441:
4439:
4436:
4434:
4431:
4429:
4426:
4424:
4421:
4419:
4416:
4414:
4413:Evelyn effect
4411:
4409:
4406:
4404:
4401:
4399:
4396:
4394:
4393:Electron-rich
4391:
4389:
4386:
4384:
4381:
4379:
4376:
4374:
4371:
4369:
4366:
4364:
4361:
4359:
4356:
4354:
4351:
4349:
4346:
4344:
4341:
4339:
4336:
4334:
4331:
4329:
4326:
4324:
4321:
4319:
4316:
4314:
4311:
4309:
4308:Bema Hapothle
4306:
4304:
4301:
4299:
4296:
4294:
4291:
4289:
4286:
4284:
4281:
4279:
4276:
4274:
4271:
4269:
4266:
4264:
4261:
4259:
4256:
4255:
4252:
4246:
4243:
4241:
4238:
4236:
4233:
4231:
4228:
4226:
4223:
4221:
4218:
4216:
4213:
4211:
4208:
4206:
4203:
4201:
4198:
4197:
4194:
4190:
4182:
4177:
4175:
4170:
4168:
4163:
4162:
4159:
4145:
4142:
4140:
4137:
4136:
4135:
4134:
4130:
4128:
4127:
4123:
4118:
4117:
4114:
4108:
4105:
4103:
4100:
4098:
4095:
4093:
4090:
4088:
4085:
4083:
4080:
4079:
4077:
4073:
4063:
4060:
4056:
4053:
4052:
4051:
4048:
4044:
4041:
4040:
4039:
4036:
4032:
4029:
4027:
4024:
4022:
4019:
4017:
4014:
4012:
4009:
4008:
4007:
4004:
4003:
4001:
3999:
3998:
3993:
3987:
3986:Telluroketone
3984:
3982:
3979:
3978:
3976:
3974:
3970:
3964:
3961:
3959:
3956:
3954:
3951:
3949:
3946:
3944:
3941:
3940:
3938:
3936:
3932:
3926:
3923:
3921:
3918:
3917:
3915:
3913:
3909:
3903:
3900:
3898:
3895:
3893:
3890:
3888:
3885:
3883:
3880:
3878:
3875:
3873:
3872:Sulfonic acid
3870:
3868:
3865:
3863:
3862:Sulfinic acid
3860:
3858:
3857:Thiosulfonate
3855:
3853:
3850:
3848:
3847:Thiosulfinate
3845:
3843:
3842:Sulfenic acid
3840:
3838:
3835:
3833:
3830:
3826:
3823:
3822:
3821:
3818:
3816:
3813:
3812:
3810:
3808:
3804:
3798:
3797:Phosphaallene
3795:
3793:
3792:Phosphaalkyne
3790:
3788:
3787:Phosphaalkene
3785:
3781:
3778:
3777:
3776:
3773:
3771:
3768:
3766:
3763:
3761:
3758:
3754:
3751:
3750:
3749:
3746:
3742:
3739:
3738:
3737:
3734:
3733:
3731:
3729:
3725:
3719:
3716:
3714:
3711:
3709:
3706:
3704:
3701:
3699:
3696:
3694:
3691:
3689:
3686:
3684:
3681:
3679:
3676:
3674:
3671:
3669:
3666:
3664:
3661:
3659:
3656:
3654:
3651:
3649:
3646:
3644:
3641:
3639:
3636:
3634:
3631:
3629:
3626:
3624:
3621:
3617:
3614:
3612:
3609:
3608:
3607:
3604:
3603:
3601:
3599:
3595:
3592:
3572:
3560:
3557:
3556:
3555:
3552:
3548:
3545:
3543:
3540:
3539:
3538:
3535:
3534:
3532:
3528:
3522:
3519:
3517:
3514:
3510:
3507:
3506:
3505:
3502:
3498:
3495:
3493:
3490:
3488:
3485:
3484:
3483:
3480:
3479:
3477:
3475:
3471:
3465:
3462:
3460:
3459:Ethylenedioxy
3457:
3453:
3450:
3448:
3445:
3444:
3443:
3440:
3436:
3433:
3431:
3428:
3427:
3426:
3423:
3419:
3416:
3415:
3414:
3411:
3409:
3406:
3404:
3401:
3400:
3398:
3394:
3391:
3385:
3379:
3374:
3369:
3363:
3360:
3358:
3355:
3351:
3348:
3346:
3343:
3342:
3341:
3338:
3334:
3331:
3329:
3326:
3324:
3321:
3319:
3316:
3314:
3311:
3309:
3306:
3305:
3304:
3301:
3297:
3294:
3292:
3289:
3288:
3287:
3284:
3280:
3277:
3275:
3272:
3270:
3267:
3265:
3262:
3260:
3257:
3255:
3252:
3251:
3250:
3247:
3246:
3244:
3238:
3234:
3230:
3223:
3218:
3216:
3211:
3209:
3204:
3203:
3200:
3194:
3191:
3189:
3185:
3184:
3180:
3171:
3167:
3163:
3159:
3152:
3143:
3137:
3134:
3129:
3125:
3121:
3117:
3113:
3109:
3102:
3099:
3093:
3090:
3084:
3078:
3074:
3070:
3066:
3065:
3060:
3053:
3050:
3044:
3042:
3038:
3033:
3029:
3025:
3021:
3014:
3011:
3005:
2999:
2995:
2991:
2987:
2980:
2977:
2965:
2961:
2954:
2951:
2946:
2942:
2936:
2933:
2928:
2922:
2918:
2914:
2913:
2905:
2902:
2897:
2895:0-13-643669-2
2891:
2887:
2880:
2878:
2874:
2870:
2865:
2862:
2859:
2855:
2851:
2847:
2846:
2841:
2836:
2833:
2826:
2817:
2814:
2812:
2809:
2807:
2804:
2802:
2799:
2797:
2794:
2792:
2789:
2787:
2784:
2782:
2779:
2777:
2776:Acid chloride
2774:
2772:
2769:
2768:
2764:
2762:
2760:
2756:
2752:
2748:
2745:The carboxyl
2740:
2735:
2731:
2728:
2724:
2721:
2717:
2714:
2710:
2706:
2702:
2698:
2694:
2691:
2687:
2683:
2679:
2675:
2671:
2668:
2664:
2660:
2656:
2652:
2649:
2645:
2642:
2638:
2635:
2631:
2627:
2624:
2620:
2616:
2612:
2611:
2607:
2605:
2603:
2598:
2589:
2587:
2581:
2573:
2553:
2545:
2541:
2539:
2534:
2532:
2528:
2524:
2520:
2516:
2512:
2508:
2505:
2501:
2497:
2493:
2489:
2485:
2477:
2475:
2473:
2471:
2455:
2451:
2447:
2442:
2440:
2436:
2435:organolithium
2432:
2428:
2427:hydrogenation
2424:
2420:
2412:
2410:
2408:
2404:
2400:
2395:
2392:
2387:
2381:
2379:
2375:
2371:
2367:
2363:
2359:
2355:
2351:
2347:
2343:
2339:
2331:
2304:
2303:
2302:
2301:, and water:
2300:
2296:
2292:
2288:
2284:
2280:
2276:
2272:
2268:
2260:
2256:
2251:
2244:
2239:
2235:
2231:
2227:
2225:
2221:
2219:
2215:
2211:
2210:
2209:
2203:
2189:
2184:
2183:
2179:
2175:
2173:
2169:
2165:
2162:
2161:
2157:
2149:
2135:
2122:
2114:
2113:
2109:
2108:organolithium
2105:
2101:
2098:
2094:
2090:
2086:
2083:
2079:
2075:
2071:
2067:
2064:
2060:
2056:
2055:Jones reagent
2052:
2048:
2044:
2040:
2037:
2036:
2035:
2029:
2024:
2020:
2016:
2012:
2009:
2005:
2002:
1999:
1995:
1994:triglycerides
1991:
1990:
1986:
1970:
1966:
1965:
1961:
1957:
1953:
1949:
1945:
1944:Koch reaction
1941:
1937:
1933:
1929:
1926:
1923:
1919:
1916:
1912:
1908:
1904:
1900:
1899:phthalic acid
1896:
1892:
1888:
1884:
1880:
1875:
1872:
1868:
1865:Oxidation of
1864:
1861:
1857:
1856:
1855:
1849:
1844:
1842:
1840:
1836:
1832:
1828:
1824:
1820:
1816:
1812:
1808:
1803:
1801:
1797:
1789:
1787:
1785:
1781:
1774:
1767:
1763:
1755:
1753:
1751:
1747:
1739:
1737:
1731:
1727:
1726:Deprotonation
1718:
1703:
1700:
1699:
1695:
1688:
1685:
1684:
1680:
1657:
1653:
1650:
1649:
1645:
1626:
1623:
1622:
1618:
1603:
1600:
1599:
1595:
1580:
1577:
1576:
1572:
1557:
1554:
1553:
1549:
1534:
1531:
1530:
1526:
1511:
1508:
1507:
1503:
1488:
1485:
1484:
1480:
1461:
1458:
1457:
1453:
1438:
1435:
1434:
1430:
1419:
1416:
1415:
1411:
1400:
1397:
1396:
1393:
1389:
1384:
1381:
1380:
1377:
1372:
1368:
1361:
1354:
1350:
1343:
1339:
1331:
1327:
1323:
1319:
1316:
1302:
1299:
1289:
1285:
1280:
1278:
1274:
1266:
1260:
1255:
1251:
1250:
1249:
1247:
1243:
1235:
1230:
1226:
1225:
1224:
1222:
1221:enanthic acid
1218:
1211:
1204:
1200:
1192:
1187:
1180:
1178:
1175:
1174:
1170:
1168:
1165:
1164:
1160:
1158:
1155:
1154:
1150:
1149:tartaric acid
1146:
1142:
1141:glycolic acid
1138:
1137:glyceric acid
1134:
1132:
1129:
1128:
1125:
1121:
1120:citrus fruits
1117:
1113:
1111:
1108:
1107:
1103:
1099:
1095:
1091:
1089:
1086:
1085:
1081:
1077:
1073:
1069:
1066:
1063:
1062:
1059:
1055:
1051:
1047:
1045:
1042:
1041:
1038:
1034:
1032:
1029:
1028:
1024:
1020:
1016:
1014:
1011:
1010:
998:
995:
992:
991:
987:
984:
983:
973:
970:
953:
950:
948:
945:
942:
941:
938:
934:
917:
914:
912:
909:
906:
905:
901:
898:
881:
878:
876:
873:
870:
869:
865:
848:
845:
843:
842:Margaric acid
840:
837:
836:
833:
830:
813:
810:
808:
807:Palmitic acid
805:
802:
801:
797:
780:
777:
775:
772:
769:
768:
765:
762:
745:
742:
740:
739:Myristic acid
737:
734:
733:
729:
712:
709:
707:
704:
701:
700:
696:
693:
676:
673:
671:
668:
665:
664:
660:
643:
640:
638:
635:
632:
631:
628:
624:
621:
604:
602:Decanoic acid
601:
599:
596:
593:
592:
588:
585:
568:
566:Nonanoic acid
565:
563:
560:
557:
556:
553:
550:
533:
531:Octanoic acid
530:
528:
527:Caprylic acid
525:
522:
521:
517:
500:
497:
495:
494:Enanthic acid
492:
489:
488:
484:
481:
464:
462:Hexanoic acid
461:
459:
456:
453:
452:
448:
445:
428:
425:
423:
420:
417:
416:
413:
410:
393:
391:Butanoic acid
390:
388:
385:
382:
381:
377:
373:
360:
357:
355:
352:
349:
348:
345:
342:
333:
331:Ethanoic acid
330:
328:
325:
322:
321:
318:
317:Insect stings
315:
312:
309:
307:
304:
301:
300:
296:
293:
290:
287:
282:
281:
275:
273:
269:
265:
264:Carbonic acid
261:
259:
255:
251:
247:
243:
239:
218:
216:
212:
208:
204:
180:
176:
172:
168:
164:
163:trivial names
156:
154:
152:
149:
145:
144:Deprotonation
141:
137:
133:
129:
125:
121:
117:
116:organyl group
109:
99:
93:
85:
81:
77:
73:
64:
56:
48:
44:
40:
39:Carbolic acid
33:
19:
6408:Ene reaction
5768:Autoxidation
5629:Degradation
5520:Azo coupling
5297:Ugi reaction
4897:Ene reaction
4697:Alkynylation
4548:Polyfluorene
4543:Polar effect
4408:Electrophile
4323:Bredt's rule
4293:Baird's rule
4263:Alpha effect
4131:
4124:
4038:Vinyl halide
3995:
3925:Borinic acid
3920:Boronic acid
3897:Thioxanthate
3536:
3237:Hydrocarbons
3161:
3157:
3150:
3141:
3136:
3111:
3107:
3101:
3092:
3082:
3068:
3062:
3052:
3023:
3019:
3013:
2985:
2979:
2967:. Retrieved
2964:Spectroscopy
2963:
2953:
2944:
2935:
2911:
2904:
2885:
2864:
2843:
2835:
2744:
2682:carboxylases
2667:diazomethane
2593:
2542:
2535:
2526:
2522:
2514:
2510:
2506:
2499:
2481:
2474:
2469:
2453:
2449:
2443:
2416:
2407:diazomethane
2396:
2382:
2335:
2310:COOH + NaHCO
2289:reacts with
2264:
2207:
2164:Halogenation
2045:with strong
2033:
2004:Fermentation
1940:pivalic acid
1932:carbocations
1911:Acrylic acid
1902:
1890:
1879:Benzoic acid
1853:
1833:(polymers),
1829:(coatings),
1817:(polymers),
1804:
1793:
1772:
1765:
1759:
1743:
1724:
1655:
1625:Benzoic acid
1387:
1370:
1359:
1352:
1341:
1328:solution of
1281:
1277:organic acid
1270:
1239:
1196:
1102:aldaric acid
1072:benzoic acid
1058:pyruvic acid
997:acrylic acid
875:Stearic acid
458:Caproic acid
422:Valeric acid
387:Butyric acid
288:Common Name
262:
245:
241:
237:
219:
207:substituents
203:parent chain
179:butyric acid
174:
166:
160:
101:
95:
83:
80:organic acid
75:
69:
43:
4907:Ethenolysis
4553:Ring strain
4523:Nucleophile
4348:Clar's rule
4288:Aromaticity
4102:Thiocyanate
4097:Sulfonamide
4062:Perchlorate
4050:Acyl halide
4011:Fluoroethyl
3892:Thionoester
3780:Phosphonium
3765:Phosphinate
3760:Phosphonous
3748:Phosphonate
3447:Hydroperoxy
3269:Cyclopropyl
2969:12 February
2811:Thiocarboxy
2759:oxalic acid
2504:oxonium ion
2386:carboxylate
2370:amino acids
2283:acetic acid
1998:soap making
1936:isobutylene
1831:maleic acid
1827:fatty acids
1819:citric acid
1815:adipic acid
1807:acetic acid
1706:HO−C(=O)−CO
1687:Oxalic acid
1437:Acetic acid
1399:Formic acid
1330:acetic acid
1320:in neutral
1145:lactic acid
1118:– found in
1116:citric acid
1094:adipic acid
1031:Amino acids
1013:Fatty acids
695:Coconut oil
670:Lauric acid
598:Capric acid
587:Pelargonium
327:Acetic acid
306:Formic acid
291:IUPAC Name
254:acetic acid
148:carboxylate
140:fatty acids
136:amino acids
7470:Categories
7191:Ozonolysis
6718:Annulation
6068:Ozonolysis
4187:Topics in
4006:Haloalkane
3877:Thioketone
3832:Persulfide
3728:Phosphorus
3693:Isocyanate
3683:Isonitrile
3584:or oxygen
3582:hydrogen,
3578:not being
3559:Orthoester
3452:Dioxiranes
3430:Enol ether
3318:1-Propenyl
3020:Org. Synth
3003:3527306730
2827:References
2806:Pseudoacid
2786:Amino acid
2492:thioesters
2362:polyesters
2318:COONa + CO
2074:ozonolysis
1288:dissociate
1284:weak acids
1193:Solubility
1044:Keto acids
972:Peanut oil
518:Fragrance
376:body odour
209:, such as
18:Carboxylic
6705:reactions
6220:reactions
5715:reactions
5631:reactions
5513:reactions
4655:reactions
4139:inorganic
3973:Tellurium
3887:Thioester
3852:Sulfoxide
3837:Disulfide
3825:Sulfonium
3775:Phosphine
3753:Phosphite
3736:Phosphate
3668:Carbamate
3643:Hydrazone
3576:element,
3574:Only one
3547:Anhydride
3286:Methylene
2917:CRC Press
2697:aldehydes
2413:Reduction
2285:found in
2245:Reactions
2110:reagents:
2043:aldehydes
1942:. In the
1924:catalyst.
1867:aldehydes
1845:Synthesis
1376:of 4.76)
1369:, has a p
1351:, has a p
937:pheromone
900:Chocolate
798:Milk fat
175:-oic acid
88:−C(=O)−OH
4598:Vinylogy
4268:Annulene
4215:Reagents
4120:See also
4055:Chloride
3981:Tellurol
3935:Selenium
3902:Xanthate
3616:Ammonium
3598:Nitrogen
3580:carbon,
3537:Carboxyl
3504:Aldehyde
3492:Acryloyl
3474:carbonyl
3378:hydrogen
3333:Cumulene
2765:See also
2699:via the
2615:α-carbon
2486:to give
2401:via the
2391:condense
2374:peptides
2354:alcohols
2275:hydroxyl
2271:hydrogen
2214:aldehyde
2194:O → R−CO
2186:R−C(=O)−
2180:ketones:
2089:nitriles
2049:such as
2047:oxidants
1971:+ CO + H
1800:proteins
1784:hydroxyl
1210:hydroxyl
1203:carbonyl
1065:Aromatic
1037:proteins
1005:=CH−COOH
988:Members
832:Palm oil
552:Coconuts
447:Valerian
242:-ic acid
167:-ic acid
132:hydrogen
112:R−C(O)OH
4258:A value
4144:organic
3943:Selenol
3867:Sulfone
3820:Sulfide
3718:NONOate
3713:Nitroso
3703:Nitrite
3698:Nitrate
3688:Cyanate
3678:Nitrile
3663:Amidine
3658:Imidate
3628:Nitrene
3623:Hydrazo
3611:Enamine
3542:Acetoxy
3530:carboxy
3497:Benzoyl
3435:Epoxide
3418:Methoxy
3408:Alcohol
3362:Carbene
3296:Methine
3116:Bibcode
3071:: 121.
2747:radical
2678:Enzymes
2653:In the
2602:acetone
2597:geminal
2423:reduced
2287:vinegar
2273:of the
2238:phenols
2216:in the
2198:H + ArH
2170:in the
2070:olefins
2023:aspirin
2008:vinegar
1952:alkynes
1948:alkenes
1934:, e.g.
1915:propene
1883:toluene
1750:perfume
1460:Glycine
1322:aqueous
1301:cations
1267:Acidity
1206:−C(=O)−
623:Coconut
344:Vinegar
258:acetate
124:alkenyl
118:(e.g.,
92:R-group
4043:Iodide
3963:Selone
3807:Sulfur
3516:Ketone
3509:Ketene
3487:Acetyl
3442:Peroxy
3413:Alkoxy
3403:Acetal
3384:oxygen
3373:carbon
3357:Alkyne
3350:Benzyl
3345:Phenyl
3328:Allene
3323:Crotyl
3303:Alkene
3291:Bridge
3279:Pentyl
3264:Propyl
3254:Methyl
3026:: 28.
3000:
2923:
2892:
2634:amines
2550:) and
2419:esters
2399:esters
2366:amides
2358:esters
2352:, and
2342:amides
2338:esters
2279:cation
2116:RLi +
2097:amides
2093:esters
1981:=CH−CO
1907:xylene
1897:, and
1895:xylene
1746:Esters
1504:2.586
1318:anions
1293:[H
1259:dimers
1050:ketone
764:Nutmeg
589:plant
449:plant
412:Butter
285:atoms
283:Carbon
272:moiety
248:for a
130:), or
97:R−COOH
78:is an
4075:Other
3912:Boron
3882:Thial
3815:Thiol
3708:Nitro
3673:Imide
3653:Amide
3638:Oxime
3633:Imine
3606:Amine
3554:Ester
3521:Ynone
3425:Ether
3396:R-O-R
3371:Only
3313:Allyl
3308:Vinyl
3274:Butyl
3259:Ethyl
3249:Alkyl
2840:IUPAC
2791:Ester
2781:Amide
2705:DIBAL
2701:ester
2582:(POCl
2570:, or
2417:Like
2372:into
2267:bases
2236:from
2150:→ RCO
2146:Li +
2123:→ RCO
2095:, or
2080:, or
2061:, or
1969:HC≡CH
1903:ortho
1901:from
1889:from
1881:from
1740:Odour
1719:4.14
1696:1.27
1681:2.16
1656:ortho
1619:0.65
1596:1.29
1573:2.86
1550:0.23
1527:1.33
1481:2.34
1454:4.76
1431:0.27
1412:3.75
1338:group
1326:molar
1290:into
1199:polar
1098:nylon
313:HCOOH
222:R−COO
171:IUPAC
151:anion
120:alkyl
3997:Halo
3482:Acyl
3382:and
3340:Aryl
3193:PHC.
2998:ISBN
2971:2024
2921:ISBN
2890:ISBN
2732:The
2703:and
2646:The
2628:The
2554:(PCl
2546:(PCl
2444:The
2314:→ CH
2178:aryl
2156:LiCl
2154:H +
2106:and
2013:The
1975:O →
1891:para
1646:4.2
1587:CHCO
1560:ClCH
1518:CHCO
1422:ClCO
1306:R−CO
1303:and
1143:and
1122:and
1100:and
1056:and
1021:and
967:COOH
931:COOH
895:COOH
862:COOH
827:COOH
794:COOH
759:COOH
726:COOH
690:COOH
657:COOH
625:and
618:COOH
582:COOH
547:COOH
514:COOH
485:fat
483:Goat
478:COOH
442:COOH
407:COOH
370:COOH
339:COOH
246:-ate
244:and
238:-ate
226:R−CO
138:and
128:aryl
103:R−CO
74:, a
3648:Azo
3166:doi
3124:doi
3073:doi
3028:doi
2990:doi
2854:doi
2852:".
2818:(CO
2718:In
2531:HCl
2466:]Cl
2378:ATP
2322:+ H
2190:+ H
2148:HCl
2137:RCO
2072:by
2041:or
1950:or
1938:to
1780:NMR
1776:O–H
1769:C=O
1672:)CO
1668:(NO
1637:−CO
1606:CCl
1491:FCH
1403:HCO
1334:-CF
1213:−OH
959:(CH
923:(CH
887:(CH
854:(CH
819:(CH
786:(CH
751:(CH
718:(CH
682:(CH
649:(CH
610:(CH
574:(CH
539:(CH
506:(CH
470:(CH
434:(CH
399:(CH
256:is
224:or
100:or
70:In
7472::
3380:,
3375:,
3162:94
3160:.
3122:.
3112:54
3110:.
3080:;
3069:66
3067:.
3061:.
3040:^
3024:56
3022:.
2996:.
2962:.
2943:.
2876:^
2842:,
2761:.
2686:EC
2676:.
2566:PO
2533:.
2494:.
2380:.
2348:,
2344:,
2340:,
2306:CH
2297:,
2188:Ar
2132:Li
2118:CO
2091:,
2076:,
2057:,
2053:,
1977:CH
1962:."
1956:CO
1885:,
1802:.
1752:.
1610:CO
1583:Cl
1564:CO
1541:CO
1537:CF
1495:CO
1472:CO
1468:CH
1464:NH
1445:CO
1441:CH
1297:O]
1279:.
1139:,
1001:CH
965:18
955:CH
943:20
929:17
919:CH
907:19
893:16
883:CH
871:18
860:15
850:CH
838:17
825:14
815:CH
803:16
792:13
782:CH
770:15
757:12
747:CH
735:14
724:11
714:CH
702:13
688:10
678:CH
666:12
645:CH
633:11
606:CH
594:10
570:CH
535:CH
502:CH
466:CH
430:CH
395:CH
366:CH
362:CH
335:CH
260:.
217:.
195:CO
191:CH
187:CH
183:CH
169:.
153:.
142:.
126:,
122:,
4180:e
4173:t
4166:v
3221:e
3214:t
3207:v
3172:.
3168::
3154:a
3151:K
3145:a
3142:K
3130:.
3126::
3118::
3087:.
3075::
3034:.
3030::
3008:.
3006:.
2992::
2973:.
2947:.
2929:.
2898:.
2856::
2822:)
2820:2
2715:.
2684:(
2643:.
2636:.
2625:.
2584:3
2576:5
2568:3
2564:3
2560:3
2556:5
2548:3
2527:5
2523:4
2515:3
2511:2
2507:2
2500:1
2470:t
2464:2
2462:)
2460:3
2454:N
2452:,
2450:N
2448:(
2326:O
2324:2
2320:2
2316:3
2312:3
2308:3
2240:.
2196:2
2192:2
2152:2
2143:2
2140:−
2129:2
2126:−
2120:2
2084:.
2025:.
2010:.
2000:.
1985:H
1983:2
1979:2
1973:2
1917:.
1905:-
1893:-
1873:.
1773:ν
1766:ν
1734:2
1712:2
1709:−
1704:(
1689:(
1678:)
1676:H
1674:2
1670:2
1666:4
1664:H
1662:6
1660:C
1658:-
1654:(
1643:)
1641:H
1639:2
1635:5
1633:H
1631:6
1629:C
1627:(
1616:)
1614:H
1612:2
1608:3
1604:(
1593:)
1591:H
1589:2
1585:2
1581:(
1570:)
1568:H
1566:2
1562:2
1558:(
1547:)
1545:H
1543:2
1539:3
1535:(
1524:)
1522:H
1520:2
1516:2
1514:F
1512:(
1501:)
1499:H
1497:2
1493:2
1489:(
1478:)
1476:H
1474:2
1470:2
1466:2
1462:(
1451:)
1449:H
1447:2
1443:3
1439:(
1428:)
1426:H
1424:2
1420:(
1409:)
1407:H
1405:2
1401:(
1391:a
1388:K
1386:p
1374:a
1371:K
1363:a
1360:K
1356:a
1353:K
1345:a
1342:K
1336:3
1312:2
1309:−
1295:3
1003:2
963:)
961:2
957:3
927:)
925:2
921:3
891:)
889:2
885:3
858:)
856:2
852:3
823:)
821:2
817:3
790:)
788:2
784:3
755:)
753:2
749:3
722:)
720:2
716:3
686:)
684:2
680:3
655:9
653:)
651:2
647:3
616:8
614:)
612:2
608:3
580:7
578:)
576:2
572:3
558:9
545:6
543:)
541:2
537:3
523:8
512:5
510:)
508:2
504:3
490:7
476:4
474:)
472:2
468:3
454:6
440:3
438:)
436:2
432:3
418:5
405:2
403:)
401:2
397:3
383:4
368:2
364:3
350:3
337:3
323:2
302:1
232:2
229:−
199:H
197:2
193:2
189:2
185:3
181:(
107:H
105:2
86:(
41:.
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.