401:
250:
676:
681:
671:
856:
41:
1317:
852:
31:
855:
57:
1842:
1078:
857:
1386:
Chlorobenzene can persist in soil for several months, in air for about 3.5 days, and in water for less than one day. Humans may be exposed to this agent via breathing contaminated air (primarily via occupational exposure), consuming contaminated food or water, or by coming into contact with
1591:
Rossberg, Manfred; Lendle, Wilhelm; Pfleiderer, Gerhard; Tögel, Adolf; Dreher, Eberhard-Ludwig; Langer, Ernst; Rassaerts, Heinz; Kleinschmidt, Peter; Strack, Heinz; Cook, Richard; Beck, Uwe; Lipper, Karl-August; Torkelson, Theodore R.; Löser, Eckhard; Beutel, Klaus K.; Mann, Trevor (2006).
1408:. It was speculated that the chlorobenzene might have been produced when the sample was heated in the instrument sampling chamber. The heating would have triggered a reaction of organics in the Martian soil, which is known to contain perchlorate.
689:
651:
1387:
contaminated soil (typically near hazardous waste sites). However, because it has only been found at 97 out of 1,177 NPL hazardous waste sites, it is not considered a widespread environmental contaminant. The bacterium
1740:
sp. nov., a novel bioprocessor isolated actinomycete with the ability to degrade chlorobenzene, dichlorobenzene and phenol as sole carbon sources" [Systematic and
Applied Microbiology 28 (2005) 695–701]"
873:
1275:, with the reaction carried out at 350 °C using fused sodium hydroxide without solvent. Labeling experiments show that the reaction proceeds via elimination/addition, through
1508:
1465:
965:
791:
1887:
1091:
1563:
858:
1243:(trichloroacetaldehyde), but this application has declined with the diminished use of DDT. At one time, chlorobenzene was the main precursor for the manufacture of
866:
1371:
450:
799:
1706:
sp. nov., a novel bioprocessor isolated actinomycete with the ability to degrade chlorobenzene, dichlorobenzene and phenol as sole carbon sources".
880:
743:
1542:
1203:, which are separated and used as intermediates in production of other chemicals. These mononitrochlorobenzenes are converted to related
1642:
1572:
1867:
415:
1675:
1609:
1396:
Upon entering the body, typically via contaminated air, chlorobenzene is excreted both via the lungs and the urinary system.
795:
632:
1862:
783:
737:
348:
680:
379:
675:
1877:
1375:
1288:
1098:
989:
976:
1882:
1348:
1060:
257:
1846:
755:
1525:
Beck, Uwe; Löser, Eckhard (2011). "Chlorinated
Benzenes and Other Nucleus-Chlorinated Aromatic Hydrocarbons".
811:
670:
1872:
1561:
Uwe Beck; Eckhard Löser (2012). "Chlorinated
Benzenes and other Nucleus-Chlorinated Aromatic Hydrocarbons".
803:
584:
1425:
245:
1389:
40:
763:
1181:, which are used in the production of herbicides, dyestuffs, chemicals for rubber, and pharmaceuticals.
1154:
703:
663:
207:
1378:
at 75 ppm (350 mg/m) over an eight-hour time-weighted average for workers handling chlorobenzene.
1784:
1272:
1200:
1196:
69:
1772:
1188:
in industrial and laboratory applications, for materials such as oils, waxes, resins, and rubber.
1048:
396:
95:
751:
30:
1364:
1352:
1308:
827:
767:
167:
147:
775:
729:
1820:
1802:
1723:
1671:
1638:
1605:
1568:
1538:
1304:
717:
129:
1810:
1792:
1751:
1715:
1663:
1630:
1597:
1530:
1323:
Industrially the reaction is conducted as a continuous process to minimize the formation of
1216:
1212:
1178:
1134:
819:
597:
551:
473:
807:
357:
1324:
1300:
1174:
902:
787:
276:
227:
105:
1788:
400:
249:
187:
1815:
1208:
1170:
1122:
1069:
573:
1856:
1204:
1118:
1024:
540:
530:
238:
1316:
831:
1165:
The major use of chlorobenzene is as a precursor for further intermediates such as
1028:
771:
1667:
1601:
779:
321:
1756:
1735:
1719:
1166:
1032:
953:
926:
892:
815:
1690:
1461:
1296:
747:
562:
492:
218:
1806:
1634:
1534:
1500:
1335:
Cl exhibits somewhat decreased susceptibility toward further chlorination.
1232:
1192:
759:
616:
368:
1824:
1727:
1393:
degrades chlorobenzene, dichlorobenzene and phenol as sole carbon sources.
709:
56:
1841:
1797:
1658:
Weber, Manfred; Weber, Markus; Kleine-Boymann, Michael (2004). "Phenol".
1130:
879:
872:
865:
838:
1344:
1292:
1240:
1220:
1185:
1150:
1126:
1044:
520:
308:
258:
1363:
Chlorobenzene exhibits "low to moderate" toxicity as indicated by its
721:
1244:
1146:
198:
1068:
Except where otherwise noted, data are given for materials in their
713:
296:
1287:
It was first described in 1851. Chlorobenzene is manufactured by
1276:
725:
332:
178:
128:
118:
823:
1405:
1211:
by nucleophilic displacement of the chloride, with respectively
1002:
510:
287:
1773:"Organic molecules in the Sheepbed Mudstone, Gale Crater, Mars"
1556:
1554:
1236:
1505:
Immediately
Dangerous to Life or Health Concentrations (IDLH)
384:
39:
29:
1223:. The conversions of the 4-nitro derivative are similar.
1404:
Chlorobenzene has been detected in a sedimentary rock on
850:
1086:
1509:
National
Institute for Occupational Safety and Health
1466:
National
Institute for Occupational Safety and Health
1207:, 2-nitroanisole, bis(2-nitrophenyl)disulfide, and
1625:Gerald Booth (2007). "Nitro Compounds, Aromatic".
1231:Chlorobenzene once was used in the manufacture of
146:
320:
854:
545:131.70 °C (269.06 °F; 404.85 K)
535:−45.58 °C (−50.04 °F; 227.57 K)
104:
1660:Ullmann's Encyclopedia of Industrial Chemistry
1627:Ullmann's Encyclopedia of Industrial Chemistry
1594:Ullmann's Encyclopedia of Industrial Chemistry
1564:Ullmann's Encyclopedia of Industrial Chemistry
1527:Ullmann's Encyclopedia of Industrial Chemistry
1372:Occupational Safety and Health Administration
8:
1691:CDC - NIOSH Pocket Guide to Chemical Hazards
399:
248:
226:
18:
1888:Substances discovered in the 19th century
1814:
1796:
1755:
1586:
1584:
1327:. Because chlorine is electronegative, C
1295:in the presence of a catalytic amount of
356:
1777:Journal of Geophysical Research: Planets
1460:NIOSH Pocket Guide to Chemical Hazards.
1416:
1157:in the manufacture of other chemicals.
455:
420:
395:
1520:
1518:
1495:
1493:
1455:
1453:
1451:
1449:
1447:
1445:
1195:on a large scale to give a mixture of
424:InChI=1S/C6H5Cl/c7-6-4-2-1-3-5-6/h1-5H
239:
1343:Chlorobenzene could be produced from
434:InChI=1/C6H5Cl/c7-6-4-2-1-3-5-6/h1-5H
427:Key: MVPPADPHJFYWMZ-UHFFFAOYSA-N
206:
186:
7:
1771:Freissinet, C.; et al. (2015).
897:29 °C (84 °F; 302 K)
1744:Systematic and Applied Microbiology
1708:Systematic and Applied Microbiology
437:Key: MVPPADPHJFYWMZ-UHFFFAOYAG
311:
295:
1184:It is also used as a high-boiling
83:Phenyl chloride, monochlorobenzene
14:
568:soluble in most organic solvents
1840:
1702:Rehfuss, M.; Urban, J. (2005). "
1315:
1076:
679:
674:
669:
55:
16:Aromatic organochlorine compound
1072:(at 25 °C , 100 kPa).
1145:Cl. This colorless, flammable
941:2250 mg/kg (guinea pig, oral)
633:Occupational safety and health
1:
1668:10.1002/14356007.a19_299.pub2
1602:10.1002/14356007.a06_233.pub2
1382:Toxicology and biodegradation
1271:The reaction is known as the
970:(US health exposure limits):
1592:"Chlorinated Hydrocarbons".
1757:10.1016/j.syapm.2005.11.005
1720:10.1016/j.syapm.2005.05.011
915:or concentration (LD, LC):
25:
1904:
1376:permissible exposure limit
1129:ring substituted with one
557:0.5 g l in water at 20 °C
497:112.56 g/mol
1351:, otherwise known as the
1349:benzenediazonium chloride
1066:
1061:Chlorobenzene (data page)
1059:
1054:
1013:
964:
937:2250 mg/kg (rabbit, oral)
935:590 mg/kg (mouse, orally)
911:
650:
630:
625:
466:
446:
411:
88:
80:
68:
63:
54:
24:
1868:Hazardous air pollutants
1635:10.1002/14356007.a17_411
1535:10.1002/14356007.o06_o03
1430:pubchem.ncbi.nlm.nih.gov
1219:, sodium disulfide, and
1121:and the simplest of the
1055:Supplementary data page
939:2300 mg/kg (mouse, oral)
738:Precautionary statements
1629:. Weinheim: Wiley-VCH.
1567:. Weinheim: Wiley-VCH.
933:2290 mg/kg (rat, oral)
646:Low to moderate hazard
585:Magnetic susceptibility
1738:Rhodococcus phenolicus
1704:Rhodococcus phenolicus
1478:Chlorobenzene toxicity
1390:Rhodococcus phenolicus
983:TWA 75 ppm (350 mg/m)
861:
45:
35:
1279:as the intermediate.
1227:Niche and former uses
960:8000 ppm (cat, 3 hr)
860:
43:
33:
1863:Halogenated solvents
1849:at Wikimedia Commons
1798:10.1002/2014JE004737
1734:Rehfuss, M. (2006).
1201:4-nitrochlorobenzene
1197:2-nitrochlorobenzene
1020:Related Halobenzenes
843:(fire diamond)
70:Preferred IUPAC name
1789:2015JGRE..120..495F
1487:Chlorobenzene: LD50
1239:, by reaction with
1049:1,4-dichlorobenzene
552:Solubility in water
168:Beilstein Reference
21:
1353:Sandmeyer reaction
1309:aluminium chloride
1259:Cl + NaOH → C
1153:and a widely used
1125:, consisting of a
1099:Infobox references
1014:Related compounds
1005:(Immediate danger)
862:
565:in other solvents
525:1.11 g/cm, liquid
46:
36:
19:
1878:Aromatic solvents
1845:Media related to
1544:978-3-527-30385-4
1370:of 2.9 g/kg. The
1339:Laboratory routes
1305:sulfur dichloride
1191:Chlorobenzene is
1179:phenylenediamines
1107:Chemical compound
1105:
1104:
1040:Related compounds
704:Hazard statements
591:−69.97·10 cm/mol
505:colorless liquid
380:CompTox Dashboard
130:Interactive image
50:
49:
1895:
1883:Phenyl compounds
1844:
1829:
1828:
1818:
1800:
1768:
1762:
1761:
1759:
1731:
1699:
1693:
1688:
1682:
1681:
1655:
1649:
1648:
1622:
1616:
1615:
1588:
1579:
1578:
1558:
1549:
1548:
1522:
1513:
1512:
1497:
1488:
1485:
1479:
1476:
1470:
1469:
1457:
1440:
1439:
1437:
1436:
1421:
1400:On other planets
1325:dichlorobenzenes
1319:
1217:sodium methoxide
1213:sodium hydroxide
1135:chemical formula
1089:
1083:
1080:
1079:
954:lowest published
903:Explosive limits
882:
875:
868:
853:
833:
829:
825:
821:
817:
813:
809:
805:
801:
797:
793:
789:
785:
781:
777:
773:
769:
765:
761:
757:
753:
749:
745:
731:
727:
723:
719:
715:
711:
683:
678:
673:
598:Refractive index
474:Chemical formula
404:
403:
388:
386:
360:
324:
313:
299:
277:Gmelin Reference
260:
252:
241:
230:
210:
190:
150:
132:
108:
59:
26:
22:
1903:
1902:
1898:
1897:
1896:
1894:
1893:
1892:
1853:
1852:
1837:
1832:
1770:
1769:
1765:
1733:
1701:
1700:
1696:
1689:
1685:
1678:
1657:
1656:
1652:
1645:
1624:
1623:
1619:
1612:
1590:
1589:
1582:
1575:
1560:
1559:
1552:
1545:
1524:
1523:
1516:
1501:"Chlorobenzene"
1499:
1498:
1491:
1486:
1482:
1477:
1473:
1459:
1458:
1443:
1434:
1432:
1426:"Chlorobenzene"
1423:
1422:
1418:
1414:
1402:
1384:
1368:
1361:
1341:
1334:
1330:
1301:ferric chloride
1285:
1266:
1262:
1258:
1254:
1235:, most notably
1229:
1163:
1144:
1140:
1108:
1101:
1096:
1095:
1094: ?)
1085:
1081:
1077:
1073:
1047:
1041:
1031:
1027:
1021:
1006:
993:
980:
957:
951:
940:
938:
936:
930:
924:
887:
886:
885:
884:
877:
870:
863:
859:
851:
740:
706:
692:
666:
643:
608:
606:
588:
554:
486:
482:
476:
462:
459:
454:
453:
442:
439:
438:
435:
429:
428:
425:
419:
418:
407:
389:
382:
363:
343:
327:
314:
302:
279:
270:
233:
213:
193:
170:
153:
135:
122:
111:
98:
84:
76:
75:
17:
12:
11:
5:
1901:
1899:
1891:
1890:
1885:
1880:
1875:
1873:Chlorobenzenes
1870:
1865:
1855:
1854:
1851:
1850:
1836:
1835:External links
1833:
1831:
1830:
1783:(3): 495–514.
1763:
1714:(8): 695–701.
1694:
1683:
1676:
1650:
1644:978-3527306732
1643:
1617:
1610:
1580:
1574:978-3527306732
1573:
1550:
1543:
1514:
1489:
1480:
1471:
1441:
1415:
1413:
1410:
1401:
1398:
1383:
1380:
1366:
1360:
1357:
1340:
1337:
1332:
1328:
1321:
1320:
1284:
1281:
1269:
1268:
1264:
1260:
1256:
1252:
1228:
1225:
1209:2-nitroaniline
1162:
1159:
1142:
1138:
1123:chlorobenzenes
1106:
1103:
1102:
1097:
1075:
1074:
1070:standard state
1067:
1064:
1063:
1057:
1056:
1052:
1051:
1042:
1039:
1036:
1035:
1022:
1019:
1016:
1015:
1011:
1010:
1007:
1001:
998:
997:
994:
988:
985:
984:
981:
975:
972:
971:
962:
961:
958:
949:
947:
944:
943:
931:
922:
920:
917:
916:
909:
908:
905:
899:
898:
895:
889:
888:
878:
871:
864:
849:
848:
847:
846:
844:
835:
834:
792:P303+P361+P353
741:
736:
733:
732:
707:
702:
699:
698:
693:
688:
685:
684:
667:
662:
659:
658:
648:
647:
644:
641:
638:
637:
628:
627:
623:
622:
619:
613:
612:
609:
604:
596:
593:
592:
589:
583:
580:
579:
576:
574:Vapor pressure
570:
569:
566:
559:
558:
555:
550:
547:
546:
543:
537:
536:
533:
527:
526:
523:
517:
516:
513:
507:
506:
503:
499:
498:
495:
489:
488:
484:
480:
477:
472:
469:
468:
464:
463:
461:
460:
457:
449:
448:
447:
444:
443:
441:
440:
436:
433:
432:
430:
426:
423:
422:
414:
413:
412:
409:
408:
406:
405:
392:
390:
378:
375:
374:
371:
365:
364:
362:
361:
353:
351:
345:
344:
342:
341:
337:
335:
329:
328:
326:
325:
317:
315:
307:
304:
303:
301:
300:
292:
290:
284:
283:
280:
275:
272:
271:
269:
268:
264:
262:
254:
253:
243:
235:
234:
232:
231:
223:
221:
215:
214:
212:
211:
203:
201:
195:
194:
192:
191:
183:
181:
175:
174:
171:
166:
163:
162:
159:
158:Abbreviations
155:
154:
152:
151:
143:
141:
137:
136:
134:
133:
125:
123:
116:
113:
112:
110:
109:
101:
99:
94:
91:
90:
86:
85:
82:
78:
77:
73:
72:
66:
65:
61:
60:
52:
51:
48:
47:
37:
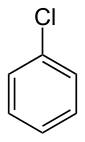
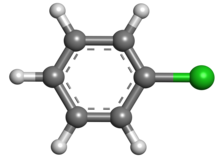
20:Chlorobenzene
15:
13:
10:
9:
6:
4:
3:
2:
1900:
1889:
1886:
1884:
1881:
1879:
1876:
1874:
1871:
1869:
1866:
1864:
1861:
1860:
1858:
1848:
1847:Chlorobenzene
1843:
1839:
1838:
1834:
1826:
1822:
1817:
1812:
1808:
1804:
1799:
1794:
1790:
1786:
1782:
1778:
1774:
1767:
1764:
1758:
1753:
1749:
1745:
1741:
1739:
1736:"Erratum to "
1729:
1725:
1721:
1717:
1713:
1709:
1705:
1698:
1695:
1692:
1687:
1684:
1679:
1673:
1669:
1665:
1661:
1654:
1651:
1646:
1640:
1636:
1632:
1628:
1621:
1618:
1613:
1607:
1603:
1599:
1595:
1587:
1585:
1581:
1576:
1570:
1566:
1565:
1557:
1555:
1551:
1546:
1540:
1536:
1532:
1528:
1521:
1519:
1515:
1510:
1506:
1502:
1496:
1494:
1490:
1484:
1481:
1475:
1472:
1467:
1463:
1456:
1454:
1452:
1450:
1448:
1446:
1442:
1431:
1427:
1420:
1417:
1411:
1409:
1407:
1399:
1397:
1394:
1392:
1391:
1381:
1379:
1377:
1373:
1369:
1358:
1356:
1354:
1350:
1346:
1338:
1336:
1326:
1318:
1314:
1313:
1312:
1310:
1306:
1302:
1298:
1294:
1290:
1282:
1280:
1278:
1274:
1250:
1249:
1248:
1246:
1242:
1238:
1234:
1226:
1224:
1222:
1218:
1214:
1210:
1206:
1205:2-nitrophenol
1202:
1198:
1194:
1189:
1187:
1182:
1180:
1176:
1175:chloroaniline
1172:
1168:
1160:
1158:
1156:
1152:
1148:
1136:
1132:
1128:
1124:
1120:
1119:aryl chloride
1116:
1113:(abbreviated
1112:
1111:Chlorobenzene
1100:
1093:
1088:
1071:
1065:
1062:
1058:
1053:
1050:
1046:
1043:
1038:
1037:
1034:
1030:
1026:
1025:Fluorobenzene
1023:
1018:
1017:
1012:
1008:
1004:
1000:
999:
995:
992:(Recommended)
991:
987:
986:
982:
979:(Permissible)
978:
974:
973:
969:
968:
963:
959:
955:
946:
945:
942:
932:
928:
919:
918:
914:
910:
906:
904:
901:
900:
896:
894:
891:
890:
883:
876:
869:
845:
842:
841:
837:
836:
742:
739:
735:
734:
708:
705:
701:
700:
697:
694:
691:
687:
686:
682:
677:
672:
668:
665:
661:
660:
656:
654:
649:
645:
640:
639:
635:
634:
629:
624:
620:
618:
615:
614:
610:
603:
599:
595:
594:
590:
586:
582:
581:
577:
575:
572:
571:
567:
564:
561:
560:
556:
553:
549:
548:
544:
542:
541:Boiling point
539:
538:
534:
532:
531:Melting point
529:
528:
524:
522:
519:
518:
514:
512:
509:
508:
504:
501:
500:
496:
494:
491:
490:
478:
475:
471:
470:
465:
456:
452:
445:
431:
421:
417:
410:
402:
398:
397:DTXSID4020298
394:
393:
391:
381:
377:
376:
372:
370:
367:
366:
359:
355:
354:
352:
350:
347:
346:
339:
338:
336:
334:
331:
330:
323:
319:
318:
316:
310:
306:
305:
298:
294:
293:
291:
289:
286:
285:
281:
278:
274:
273:
266:
265:
263:
261:
256:
255:
251:
247:
244:
242:
240:ECHA InfoCard
237:
236:
229:
225:
224:
222:
220:
217:
216:
209:
205:
204:
202:
200:
197:
196:
189:
185:
184:
182:
180:
177:
176:
172:
169:
165:
164:
160:
157:
156:
149:
145:
144:
142:
139:
138:
131:
127:
126:
124:
120:
115:
114:
107:
103:
102:
100:
97:
93:
92:
87:
79:
74:Chlorobenzene
71:
67:
62:
58:
53:
44:Chlorobenzene
42:
38:
34:Chlorobenzene
32:
28:
27:
23:
1780:
1776:
1766:
1747:
1743:
1737:
1711:
1707:
1703:
1697:
1686:
1659:
1653:
1626:
1620:
1593:
1562:
1526:
1504:
1483:
1474:
1433:. Retrieved
1429:
1419:
1403:
1395:
1388:
1385:
1362:
1342:
1322:
1289:chlorination
1286:
1270:
1230:
1190:
1183:
1171:nitroanisole
1167:nitrophenols
1164:
1155:intermediate
1149:is a common
1114:
1110:
1109:
1029:Bromobenzene
966:
934:
912:
839:
695:
652:
642:Main hazards
631:
601:
515:almond-like
333:RTECS number
89:Identifiers
81:Other names
1273:Dow process
1267:OH + NaCl
1033:Iodobenzene
927:median dose
913:Lethal dose
893:Flash point
690:Signal word
636:(OHS/OSH):
502:Appearance
467:Properties
246:100.003.299
208:ChEMBL16200
188:CHEBI:28097
1857:Categories
1750:(2): 182.
1677:3527306730
1611:3527306730
1435:2022-08-21
1412:References
1374:has set a
1297:Lewis acid
1283:Production
1233:pesticides
1133:atom. Its
907:1.3%-9.6%
664:Pictograms
563:Solubility
493:Molar mass
458:Clc1ccccc1
358:K18102WN1G
219:ChemSpider
117:3D model (
96:CAS Number
1807:2169-9097
1732:Erratum:
1424:Pubchem.
1009:1000 ppm
828:P403+P235
820:P370+P378
812:P332+P313
800:P304+P340
796:P304+P312
788:P302+P352
655:labelling
617:Viscosity
487:Cl
369:UN number
340:CZ0175000
267:203-628-5
259:EC Number
1825:26690960
1728:16261859
1511:(NIOSH).
1468:(NIOSH).
1299:such as
1193:nitrated
1131:chlorine
1117:) is an
840:NFPA 704
626:Hazards
611:1.52138
587:(χ)
106:108-90-7
1816:4672966
1785:Bibcode
1462:"#0121"
1345:aniline
1293:benzene
1277:benzyne
1241:chloral
1221:ammonia
1186:solvent
1151:solvent
1127:benzene
1092:what is
1090: (
1045:benzene
696:Warning
621:0.7232
578:9 mmHg
521:Density
309:PubChem
173:605632
1823:
1813:
1805:
1726:
1674:
1641:
1608:
1571:
1541:
1359:Safety
1307:, and
1245:phenol
1177:, and
1147:liquid
1087:verify
1084:
451:SMILES
297:C06990
282:26704
199:ChEMBL
148:B02152
140:3DMet
64:Names
996:none
967:NIOSH
416:InChI
373:1134
179:ChEBI
161:PhCl
119:JSmol
1821:PMID
1803:ISSN
1724:PMID
1672:ISBN
1639:ISBN
1606:ISBN
1569:ISBN
1539:ISBN
1406:Mars
1347:via
1199:and
1161:Uses
1137:is C
1115:PhCl
1003:IDLH
832:P501
824:P391
816:P362
808:P321
804:P312
784:P280
780:P273
776:P271
772:P264
768:P261
764:P243
760:P242
756:P241
752:P240
748:P233
744:P210
730:H411
726:H332
722:H315
718:H305
714:H302
710:H226
511:Odor
349:UNII
322:7964
288:KEGG
228:7676
1811:PMC
1793:doi
1781:120
1752:doi
1716:doi
1664:doi
1631:doi
1598:doi
1531:doi
1291:of
1237:DDT
990:REL
977:PEL
653:GHS
385:EPA
312:CID
1859::
1819:.
1809:.
1801:.
1791:.
1779:.
1775:.
1748:29
1746:.
1742:.
1722:.
1712:28
1710:.
1670:.
1662:.
1637:.
1604:.
1596:.
1583:^
1553:^
1537:.
1529:.
1517:^
1507:.
1503:.
1492:^
1464:.
1444:^
1428:.
1367:50
1365:LD
1355:.
1311::
1303:,
1247::
1215:,
1173:,
1169:,
950:Lo
948:LC
923:50
921:LD
830:,
826:,
822:,
818:,
814:,
810:,
806:,
802:,
798:,
794:,
790:,
786:,
782:,
778:,
774:,
770:,
766:,
762:,
758:,
754:,
750:,
746:,
728:,
724:,
720:,
716:,
712:,
657::
1827:.
1795::
1787::
1760:.
1754::
1730:.
1718::
1680:.
1666::
1647:.
1633::
1614:.
1600::
1577:.
1547:.
1533::
1438:.
1333:5
1331:H
1329:6
1265:5
1263:H
1261:6
1257:5
1255:H
1253:6
1251:C
1143:5
1141:H
1139:6
1082:Y
956:)
952:(
929:)
925:(
881:0
874:3
867:2
607:)
605:D
602:n
600:(
485:5
483:H
481:6
479:C
387:)
383:(
121:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.