210:
363:
and a bond energy of 498 kJ/mol. It is a colourless gas with a boiling point of −183 °C (90 K; −297 °F). It can be condensed from air by cooling with liquid nitrogen, which has a boiling point of −196 °C (77 K; −321 °F). Liquid oxygen is pale blue in colour, and is
404:. Because of the differences in their electron shells, singlet oxygen has different chemical and physical properties than triplet oxygen, including absorbing and emitting light at different wavelengths. It can be generated in a photosensitized process by energy transfer from dye molecules such as
618:
Tetraoxygen had been suspected to exist since the early 1900s, when it was known as oxozone. It was identified in 2001 by a team led by Fulvio Cacace at the
University of Rome. The molecule
749:
941:
Paul
Wentworth Jr.; Jonathan E. McDunn; Anita D. Wentworth; Cindy Takeuchi; Jorge Nieva; Teresa Jones; Cristina Bautista; Julie M. Ruedi; Abel Gutierrez; Kim D. Janda;
186:
1183:
874:
1116:
Gadzhiev, Oleg B.; Ignatov, Stanislav K.; Kulikov, Mikhail Yu.; Feigin, Alexander M.; Razuvaev, Alexey G.; Sennikov, Peter G.; Schrems, Otto (2013).
721:
772:
150:, is very reactive, as the individual atoms of oxygen tend to quickly bond with nearby molecules. Its lowest-energy electronic state is a
739:
1176:
1104:
844:
450:
1015:
916:
492:
204:
317:
state requires an odd number of electrons, and so cannot occur in dioxygen without gaining or losing electrons, such as in the
1050:
1329:
1169:
803:
368:
due to the unpaired electrons; liquid oxygen contained in a flask suspended by a string is attracted to a magnet.
567:
that is especially harmful for senior citizens, children, and people with heart and lung conditions such as
507:
1077:
163:
699:, both of which show significant metallic character. At very low temperatures, this phase also becomes
491:, as it is formed whenever air is subjected to an electrical discharge. It was named "ozon" in 1840 by
604:
molecule in which its three atoms of oxygen bond in an equilateral triangle instead of an open angle.
958:
718:
274:
266:
218:
82:
52:
532:. Ozone absorbs strongly in the ultraviolet and in the stratosphere functions as a shield for the
984:
776:
560:
551:). Tropospheric ozone is formed near the Earth's surface by the photochemical disintegration of
467:, and they are unstable and explosive. In its gas phase, ozone is destructive to materials like
1150:
1100:
1054:
1019:
976:
840:
421:
1142:
1046:
1011:
966:
869:
552:
522:
510:
and tends to react toward the more common dioxygen form. It is formed by reaction of intact
386:
270:
104:
1128:≤ 6) in the Covalently Bound and van der Waals Forms: Ab Initio Study at the CCSD(T) Level"
1324:
920:
878:
807:
725:
700:
480:
278:
178:
167:
962:
1235:
1229:
947:"Evidence for Antibody-Catalyzed Ozone Formation in Bacterial Killing and Inflammation"
942:
409:
401:
382:
377:
298:
286:
182:
88:
56:
1318:
580:
564:
484:
365:
314:
151:
1093:
988:
479:
tissue. Traces of it can be detected as a pungent, chlorine-like smell, coming from
1259:
1117:
659:
626:
592:
544:
518:
425:
282:
213:
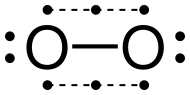
The most commonly encountered allotrope of elemental oxygen is triplet dioxygen, a
209:
123:
1276:
613:
548:
488:
405:
159:
155:
110:
839:. (3rd Edition). New York, London, Sydney, Toronto: Interscience Publications.
668:
There are six known distinct phases of solid oxygen. One of them is a dark-red
1292:
684:
663:
572:
556:
499:, commonly used at the time to designate a derived compound and anglicized as
417:
413:
334:
318:
310:
127:
800:
971:
946:
692:
568:
533:
360:
214:
32:
1154:
1058:
1023:
980:
675:
cluster. When oxygen is subjected to a pressure of 96 GPa, it becomes
252:
to distinguish it from the element itself and from the triatomic allotrope
1016:
10.1002/1521-3773(20011105)40:21<4062::AID-ANIE4062>3.0.CO;2-X
696:
688:
680:
17:
537:
1146:
1118:"Structure, Energy, and Vibrational Frequencies of Oxygen Allotropes O
269:, elemental oxygen is most commonly encountered in the diatomic form.
1223:
650:
molecules loosely held together by induced dipole dispersion forces.
576:
472:
468:
390:
198:
158:
P. On Earth's surface, it exists naturally for a very short time. In
41:
36:
1161:
1051:
10.1002/1439-7641(20020118)3:1<53::AID-CPHC53>3.0.CO;2-2
1037:
Peter P. Edwards; Friedrich Hensel (2002-01-14). "Metallic Oxygen".
449:) is a very reactive allotrope of oxygen that is a pale blue gas at
1254:
676:
437:
297:, because it has two unpaired electrons. The first excited state,
254:
60:
1002:
Cacace, Fulvio (2001). "Experimental
Detection of Tetraoxygen".
744:
740:"Flying observatory detects atomic oxygen in Martian Atmosphere"
476:
416:, or by chemical processes such as spontaneous decomposition of
174:
1165:
495:, from ancient Greek ὄζειν (ozein: "to smell") plus the suffix
541:
170:
atmosphere in which 96% of the oxygen occurs in atomic form.
126:, existing in six variously colored phases, of which one is
265:. As a major component (about 21% by volume) of Earth's
1114:
Theoretical analysis of some and lead-ref for others:
945:; Albert Eschenmoser; Richard A. Lerner (2002-12-13).
691:(exhibiting a pink-red color in its elemental state),
881:, p. 3, Basel: Schweighauser'sche Buchhandlung, 1844.
273:
use atmospheric dioxygen as the terminal oxidant in
27:
Different forms of the 8th element of
Periodic Table
225:The common allotrope of elemental oxygen on Earth,
1092:
837:Advanced Inorganic Chemistry: A comprehensive Text
835:Cotton, F. Albert and Wilkinson, Geoffrey (1972).
232:, is generally known as oxygen, but may be called
137:, red oxygen) and another one metallic (ζ-oxygen).
583:produces ozone as an antimicrobial (see below).
871:Über die Erzeugung des Ozons auf chemischen Wege
1084:(6th ed.). London: Longmans, Green and Co.
1177:
1099:(Revised ed.). Oxford University Press.
903:
856:
8:
824:
400:) with higher energy than the ground state
1201:
1184:
1170:
1162:
796:
794:
683:, and becomes more similar to the heavier
625:was thought to be in one of the phases of
597:Cyclic ozone is a theoretically predicted
970:
1298:
1282:
1266:
1242:
820:
818:
816:
775:. University of Waterloo. Archived from
671:
646:
639:
632:
621:
600:
528:
513:
463:
456:
445:
396:
355:
304:
292:
261:
228:
217:. The unpaired electrons participate in
208:
133:
116:
77:
66:
47:
1004:Angewandte Chemie International Edition
711:
643:probably consists of two dumbbell-like
460:have a deeper blue color than ordinary
7:
801:Chemistry Tutorial : Allotropes
752:from the original on 8 November 2020
385:is the common name used for the two
51:), present at significant levels in
1082:Mellor's Modern Inorganic Chemistry
309:, has no unpaired electrons and is
173:Atomic oxygen has been detected on
1210:
25:
917:"Who is most at risk from ozone?"
517:with atomic oxygen produced when
451:standard temperature and pressure
59:. Another is the highly reactive
221:, shown here using dashed lines.
205:Dioxygen in biological reactions
894:online, retrieved 29 June 2020.
868:Christian Friedrich Schönbein,
636:. Cacace's team suggested that
738:Bell, Kassandra (6 May 2016).
540:and other damaging effects of
359:has a bond length of 121
55:and also known as dioxygen or
1:
728:.NASA.gov. February 17, 2011.
493:Christian Friedrich Schönbein
146:Atomic oxygen, denoted O or O
919:. airnow.gov. Archived from
420:in water or the reaction of
773:"Bond Lengths and Energies"
120:), another metastable form.
1346:
657:
611:
590:
508:thermodynamically unstable
435:
375:
202:
196:
1199:
1091:Stwertka, Albert (1998).
892:Oxford English Dictionary
679:, in a similar manner to
442:Triatomic oxygen (ozone,
285:of dioxygen is known as
162:, the presence of ample
30:There are several known
972:10.1126/science.1077642
40:. The most familiar is
1135:J. Chem. Theory Comput
810:from AUS-e-TUTE.com.au
654:Phases of solid oxygen
222:
219:three-electron bonding
1095:Guide to the Elements
212:
197:Further information:
164:ultraviolet radiation
1330:Allotropes of oxygen
629:later identified as
352:The ground state of
275:cellular respiration
154:, designated by the
107:of molecular oxygen.
963:2002Sci...298.2195W
957:(5601): 2195–2199.
779:on 14 December 2007
475:and is damaging to
453:. Liquid and solid
277:in order to obtain
1205:
923:on 17 January 2008
877:2020-06-30 at the
806:2021-11-17 at the
724:2017-06-23 at the
561:Ground-level ozone
555:in the exhaust of
223:
53:Earth's atmosphere
1312:
1311:
1307:
1306:
1193:Oxygen allotropes
1147:10.1021/ct3006584
1010:(21): 4062–4065.
943:Bernard M. Babior
719:"Out of Thin Air"
422:hydrogen peroxide
418:hydrogen trioxide
387:metastable states
271:Aerobic organisms
105:metastable states
16:(Redirected from
1337:
1301:
1285:
1269:
1245:
1216:
1202:
1186:
1179:
1172:
1163:
1158:
1132:
1111:
1110:
1098:
1086:
1085:
1063:
1062:
1034:
1028:
1027:
999:
993:
992:
974:
938:
932:
931:
929:
928:
913:
907:
901:
895:
888:
882:
866:
860:
854:
848:
833:
827:
822:
811:
798:
789:
788:
786:
784:
768:
762:
761:
759:
757:
735:
729:
716:
674:
649:
642:
635:
624:
603:
553:nitrogen dioxide
531:
523:upper atmosphere
516:
466:
459:
448:
399:
358:
348:
347:
346:
343:
332:
331:
330:
327:
308:
296:
264:
242:molecular oxygen
231:
136:
119:
102:
101:
100:
80:
69:
50:
42:molecular oxygen
21:
1345:
1344:
1340:
1339:
1338:
1336:
1335:
1334:
1315:
1314:
1313:
1308:
1303:
1300:
1296:
1295:
1287:
1284:
1280:
1279:
1271:
1268:
1264:
1263:
1258: and
1247:
1244:
1240:
1239:
1234: and
1218:
1214:
1213:
1195:
1190:
1130:
1123:
1115:
1107:
1090:
1089:
1075:
1074:
1071:
1069:Further reading
1066:
1036:
1035:
1031:
1001:
1000:
996:
940:
939:
935:
926:
924:
915:
914:
910:
902:
898:
889:
885:
879:Wayback Machine
867:
863:
855:
851:
834:
830:
823:
814:
808:Wayback Machine
799:
792:
782:
780:
770:
769:
765:
755:
753:
737:
736:
732:
726:Wayback Machine
717:
713:
709:
701:superconducting
673:
669:
666:
658:Main articles:
656:
648:
644:
641:
637:
634:
630:
623:
619:
616:
610:
602:
598:
595:
589:
530:
526:
515:
511:
481:electric motors
465:
461:
458:
454:
447:
443:
440:
434:
398:
394:
380:
374:
364:quite markedly
357:
353:
344:
341:
340:
338:
328:
325:
324:
322:
306:
302:
294:
290:
279:chemical energy
263:
259:
238:diatomic oxygen
230:
226:
207:
201:
195:
168:low Earth orbit
149:
144:
135:
131:
118:
114:
99:
96:
95:
94:
92:
79:
75:
74:Atomic oxygen (
70:). Others are:
68:
64:
49:
45:
28:
23:
22:
15:
12:
11:
5:
1343:
1341:
1333:
1332:
1327:
1317:
1316:
1310:
1309:
1305:
1304:
1290:
1288:
1274:
1272:
1250:
1248:
1221:
1219:
1211:Nascent oxygen
1208:
1206:
1200:
1197:
1196:
1191:
1189:
1188:
1181:
1174:
1166:
1160:
1159:
1141:(1): 247–262.
1119:
1112:
1105:
1087:
1076:Parks, G. D.;
1070:
1067:
1065:
1064:
1029:
994:
933:
908:
896:
883:
861:
849:
828:
812:
790:
771:Chieh, Chung.
763:
730:
710:
708:
705:
655:
652:
612:Main article:
609:
606:
591:Main article:
588:
585:
485:laser printers
436:Main article:
433:
430:
410:methylene blue
402:triplet oxygen
383:Singlet oxygen
378:Singlet oxygen
376:Main article:
373:
372:Singlet oxygen
370:
299:singlet oxygen
287:triplet oxygen
194:
191:
147:
143:
140:
139:
138:
121:
108:
103:), one of two
97:
89:Singlet oxygen
86:
57:triplet oxygen
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1342:
1331:
1328:
1326:
1323:
1322:
1320:
1302:
1294:
1289:
1286:
1278:
1273:
1270:
1261:
1257:
1256:
1249:
1246:
1237:
1233:
1232:
1227:
1226:
1220:
1217:
1212:
1207:
1204:
1203:
1198:
1194:
1187:
1182:
1180:
1175:
1173:
1168:
1167:
1164:
1156:
1152:
1148:
1144:
1140:
1136:
1129:
1127:
1122:
1113:
1108:
1106:0-19-508083-1
1102:
1097:
1096:
1088:
1083:
1079:
1078:Mellor, J. W.
1073:
1072:
1068:
1060:
1056:
1052:
1048:
1044:
1040:
1033:
1030:
1025:
1021:
1017:
1013:
1009:
1005:
998:
995:
990:
986:
982:
978:
973:
968:
964:
960:
956:
952:
948:
944:
937:
934:
922:
918:
912:
909:
905:
904:Stwertka 1998
900:
897:
893:
887:
884:
880:
876:
873:
872:
865:
862:
858:
857:Stwertka 1998
853:
850:
846:
845:0-471-17560-9
842:
838:
832:
829:
826:
821:
819:
817:
813:
809:
805:
802:
797:
795:
791:
778:
774:
767:
764:
751:
747:
746:
741:
734:
731:
727:
723:
720:
715:
712:
706:
704:
702:
698:
694:
690:
686:
682:
678:
665:
661:
653:
651:
628:
615:
607:
605:
594:
586:
584:
582:
581:immune system
578:
574:
570:
566:
565:air pollutant
562:
558:
554:
550:
546:
543:
539:
535:
524:
520:
509:
504:
502:
498:
494:
490:
486:
482:
478:
474:
470:
452:
439:
431:
429:
427:
423:
419:
415:
411:
407:
403:
392:
389:of molecular
388:
384:
379:
371:
369:
367:
362:
350:
336:
320:
316:
312:
300:
288:
284:
280:
276:
272:
268:
258:
256:
251:
247:
243:
239:
235:
220:
216:
211:
206:
200:
192:
190:
189:observatory.
188:
184:
180:
176:
171:
169:
166:results in a
165:
161:
157:
153:
142:Atomic oxygen
141:
129:
125:
122:
112:
109:
106:
90:
87:
84:
73:
72:
71:
62:
58:
54:
43:
39:
38:
34:
19:
1291:
1275:
1260:cyclic ozone
1253:
1251:
1230:
1224:
1222:
1209:
1192:
1138:
1134:
1125:
1120:
1094:
1081:
1045:(1): 53–56.
1042:
1039:ChemPhysChem
1038:
1032:
1007:
1003:
997:
954:
950:
936:
925:. Retrieved
921:the original
911:
899:
891:
886:
870:
864:
852:
836:
831:
781:. Retrieved
777:the original
766:
756:30 September
754:. Retrieved
743:
733:
714:
667:
660:Solid oxygen
627:solid oxygen
617:
596:
593:Cyclic ozone
587:Cyclic ozone
545:UV radiation
519:UV radiation
505:
500:
496:
489:photocopiers
441:
426:hypochlorite
381:
366:paramagnetic
351:
283:ground state
253:
249:
245:
241:
237:
233:
224:
172:
152:spin triplet
145:
124:Solid oxygen
83:free radical
31:
29:
1277:Tetraoxygen
1252:Trioxygen (
825:Mellor 1939
783:16 December
614:Tetraoxygen
608:Tetraoxygen
557:automobiles
549:ozone layer
406:rose bengal
160:outer space
156:term symbol
111:Tetraoxygen
1319:Categories
1293:Octaoxygen
927:2008-01-06
707:References
687:, such as
685:chalcogens
664:Octaoxygen
573:bronchitis
414:porphyrins
335:dioxygenyl
319:superoxide
311:metastable
267:atmosphere
250:oxygen gas
203:See also:
185:, and the
128:octaoxygen
33:allotropes
890:"Ozone",
693:tellurium
569:emphysema
538:mutagenic
534:biosphere
506:Ozone is
333:) or the
246:dioxidene
215:diradical
1225:Dioxygen
1155:26589027
1080:(1939).
1059:12465476
1024:12404493
989:36537588
981:12434011
875:Archived
804:Archived
750:Archived
722:Archived
697:polonium
689:selenium
681:hydrogen
677:metallic
536:against
234:dioxygen
193:Dioxygen
18:Dioxygen
1236:triplet
1231:singlet
959:Bibcode
951:Science
525:splits
521:in the
315:doublet
179:Mariner
1325:Oxygen
1153:
1103:
1057:
1022:
987:
979:
906:, p.49
859:, p.48
843:
579:. The
577:asthma
575:, and
563:is an
487:, and
473:fabric
469:rubber
391:oxygen
313:. The
303:[O
291:[O
281:. The
199:Oxygen
183:Viking
37:oxygen
1255:ozone
1131:(PDF)
985:S2CID
547:(see
542:solar
438:Ozone
432:Ozone
424:with
337:ion (
321:ion (
255:ozone
187:SOFIA
81:), a
61:ozone
1151:PMID
1101:ISBN
1055:PMID
1020:PMID
977:PMID
841:ISBN
785:2007
758:2021
745:NASA
695:and
662:and
501:-one
477:lung
471:and
175:Mars
1143:doi
1047:doi
1012:doi
967:doi
955:298
497:-on
412:or
349:).
248:or
177:by
35:of
1321::
1262:)
1149:.
1137:.
1133:.
1053:.
1041:.
1018:.
1008:40
1006:.
983:.
975:.
965:.
953:.
949:.
815:^
793:^
748:.
742:.
703:.
571:,
559:.
503:.
483:,
428:.
408:,
361:pm
301:,
289:,
244:,
240:,
236:,
181:,
1299:8
1297:O
1283:4
1281:O
1267:3
1265:O
1243:2
1241:O
1238:)
1228:(
1215:O
1185:e
1178:t
1171:v
1157:.
1145::
1139:9
1126:n
1124:(
1121:n
1109:.
1061:.
1049::
1043:3
1026:.
1014::
991:.
969::
961::
930:.
847:.
787:.
760:.
672:8
670:O
647:2
645:O
640:4
638:O
633:8
631:O
622:4
620:O
601:3
599:O
529:2
527:O
514:2
512:O
464:2
462:O
457:3
455:O
446:3
444:O
397:2
395:O
393:(
356:2
354:O
345:2
342:+
339:O
329:2
326:−
323:O
307:]
305:2
295:]
293:2
262:3
260:O
257:,
229:2
227:O
148:1
134:8
132:O
130:(
117:4
115:O
113:(
98:2
93:O
91:(
85:.
78:1
76:O
67:3
65:O
63:(
48:2
46:O
44:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.