594:
if trauma to the head occurs, intracranial bleeding may also occur. Bleeding events may require massive blood transfusions and incur certain risks including infection, pulmonary insufficiency, increased costs, right heart failure, allosensitization, and viral transmission, which can prove fatal or preclude transplantation. When bleeding occurs, it impacts the one year Kaplan-Meier mortality. In addition to complexity of the patient population and the complexity of these procedures contributing to bleeding, the devices themselves may contribute to the severe coagulopathy that can ensue when these devices are implanted.
225:
deoxygenated blood from the patient's veins into an oxygenating device at the patient's bedside, after which a motor powered pump moves the oxygenated blood is back to the body (either into a vein or the arterial system, typically the aorta). There are different ECMO configurations (venoarterial ECMO, venovenous ECMO, etc.) the end goal remains the same; to oxygenate blood and return it to the body. In this sense, the ECMO circuit bypasses one or both ventricles and is therefore not in contact with the patient's native ventricle and is generally not considered a type of VAD.
265:. Both types have a central rotor containing permanent magnets. Controlled electric currents running through coils contained in the pump housing apply forces to the magnets, which in turn cause the rotors to spin. In the centrifugal pumps, the rotors are shaped to accelerate the blood circumferentially and thereby cause it to move toward the outer rim of the pump, whereas in the axial flow pumps the rotors are more or less cylindrical with blades that are helical, causing the blood to be accelerated in the direction of the rotor's axis.
286:
729:
374:
by
Anthony "Tony" Martin, a nurse practitioner (NP) and clinical manager of the mechanical circulatory support (MCS) program at Newark Beth Israel Medical Center, Newark, N.J. The ICCAC was developed as a 501c3 organization, dedicated to the development of best practices and education related to the care of individuals requiring MCS as a bridge to heart transplantation or as destination therapy in those individuals who don't meet the criteria for heart transplantation.
42:
234:
603:
fibriliation and high blood pressure may increase risk of stroke and high blood pressure can increase a patient's risk of stroke in the setting of VAD use. However, it is difficult to measure blood pressure in LVAD patients using standard blood pressure monitoring and the current practice is to measure by
593:
Due to the use of anticoagulation, bleeding is the most common postoperative early complication after implantation or explantation of VADs, necessitating reoperation in up to 60% of recipients. Most commonly bleeding occurs in the gastrointestinal tract resulting in dark or bright red stools, however
392:
In July 2009 in
England, surgeons removed a donor heart that had been implanted in a toddler next to her native heart, after her native heart had recovered. This technique suggests mechanical assist device, such as an LVAD, can take some or all the work away from the native heart and allow it time to
503:
began in 2005 and included the evaluation of HeartMate II for two indications: Bridge to transplantation (BTT) and destination therapy (DT), or long-term, permanent support. Thoratec Corp. announced that this was the first time the FDA had approved a clinical trial to include both indications in one
457:
technology based on technique called
Coplanar Energy Transfer (CET) which is capable of transferring energy from an external transmitting coil to a small receiving coil that is implanted in the human body. In the early postoperative phase, CET operation was accomplished as expected in both patients,
373:
in 2007 at the age of 69. Since then, patient Lidia Pluhar has exceeded
Houghton's longevity on a VAD, having received a HeartMate II in March 2011 at age 75, and currently continues to use the device. In August 2007 the International Consortium of Circulatory Assist Clinicians (ICCAC) was founded
627:
Infections in VAD patients occur because the artificial surfaces of the devices serve as a surface for bacterial and or fungal growth. Most infections are classified as driveline infections, which are infections that occur where the device's power cord enters the skin (usually in the upper abdomen)
602:
In patients with VADs, ischemic strokes and pump thrombosis occur when there is inadequate anticoagulation to counter act the blood's tendency to form blood clots when exposed to the foreign materials in a VAD. Stroke risk varies based on the type of VAD in place and other risk factors. Both atrial
408:
In
September 2009, a New Zealand news outlet, Stuff, reported that in another 18 months to two years, a new wireless device will be ready for clinical trial that will power VADs without direct contact. If successful, this may reduce the chance of infection as a result of the power cable through the
547:
The REMATCH (Randomized
Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure) clinical trial began in May 1998 and ran through July 2001 in 20 cardiac transplant centers around the USA. The trial was designed to compare long-term implantation of left ventricular assist
434:
announced in August 2009 that it had surpassed 50 implants of their HeartWare
Ventricular Assist System in their ADVANCE Clinical Trial, an FDA-approved IDE study. The study is to assess the system as bridge-to-transplantation for patients with end-stage heart failure. The study, Evaluation of the
404:
A phase 1 clinical trial is underway (as of August 2009), consisting of patients with coronary artery bypass grafting and patients in end-stage heart failure who have a left ventricular assist device. The trial involves testing a patch called
Anginera which contains cells that secrete hormone-like
165:
Temporary use of VADs may vary in scale (e.g. days to months) depending on a patient's condition. Certain types of VADS may be used in patients with signs of acute (sudden onset) heart failure or cardiogenic shock as a result of an infarction, valvular disease, among other causes. In patients with
1009:
Obtained CE Mark for distribution in Europe, January 2009. Obtained FDA approval in the U.S., November 2012. Initiated US BTT trial in
October 2008 (completed February 2010) and US DT trial in August 2010 (enrollment completed May 2012). FDA approval for BTT in 2012 and DT in 2017. Withdrawn from
714:
Considering the multitude of risks and lifestyle modifications associated with ventricular assist device implants, it is important for prospective patients to be informed prior to decision making. In addition to physician consult, various
Internet-based patient directed resources are available to
534:
The Harefield Recovery Protocol Study (HARPS) is a clinical trial to evaluate whether advanced heart failure patients requiring VAD support can recover sufficient myocardial function to allow device removal (known as explantation). HARPS combines an LVAD (the HeartMate XVE) with conventional oral
471:
The majority of VADs on the market today are somewhat bulky. The smallest device approved by the FDA, the HeartMate II, weighs about 1 pound (0.45 kg) and measures 3 inches (7.6 cm). This has proven particularly important for women and children, for whom alternatives would have been too
178:
Long-term use of VADs may also vary in its scale (i.e. months to permanently). VADs that are intended for long term use are also termed "durable" VADS, due to their design to function for longer periods of time compared to short term VADs (e.g. Impella, etc.). The long-term VADs can be used in a
551:
According to a retrospective cohort study comparing patients treated with a left ventricular assist device versus inotrope therapy while awaiting heart transplantation, the group treated with LVAD had improved clinical and metabolic function at the time of transplant with better blood pressure,
520:
of heart failure had significantly improved after six months of LVAD support compared to the pre-LVAD baseline. Although this trial involved bridge-to-transplant indication, the results provide early evidence that continuous flow LVADs have advantages in terms of durability and reliability for
142:
may require an LVAD). The LVAD is the most common device applied to a defective heart (it is sufficient in most cases; the right side of the heart is then often able to make use of the heavily increased blood flow), but when the pulmonary arterial resistance is high, then an (additional) right
524:
Following the FDA approval of HeartMate II LVAD for bridge-to-transplantation purposes, a post-approval ("registry") study was undertaken to assess the efficacy of the device in a commercial setting. The study found that the device improved outcomes, both compared to other LVAD treatments and
368:
was the longest surviving recipient of a VAD for permanent use. He received an experimental Jarvik 2000 LVAD in June 2000. Since then, he completed a 91-mile charity walk, published two books, lectured widely, hiked in the Swiss Alps and the American West, flew in an ultra-light aircraft, and
589:
and does not require long term anticoagulation (except aspirin); unfortunately, this biologic surface may also predispose the patient to infection through selective reduction of certain types of leukocytes, however this device was phased out of use starting in 2009 in favor of newer devices.
515:
Eighteen-month follow up data on 281 patients who had either reached the study end-point or completed 18 months of post-operative follow-up showed improved survival, less frequent adverse events and greater reliability with continuous flow LVADS compared to pulsatile flow devices. Of the 281
352:
A very different approach in the early stages of development was the use of an inflatable cuff around the aorta. Inflating the cuff contracts the aorta and deflating the cuff allows the aorta to expand – in effect the aorta becomes a second left ventricle. A proposed refinement is to use the
187:
to heart transplantation but is expected to improve with the VADs support) , bridge to decision (used to support a patient while their candidacy status is decided), and bridge to recovery (used until a patient’s native heart function improves after which the device would be removed). In some
224:
Extracorporeal Membrane Oxygenation (ECMO) – is a form of mechanical circulatory support typically used in critically ill patients in cardiogenic shock that is established by introducing cannula into the arteries and or veins of the neck, axilla or groin. Generally, a venous cannula pulls
318:, a program of the NIH. The early VADs emulated the heart by using a "pulsatile" action where blood is alternately sucked into the pump from the left ventricle then forced out into the aorta. Devices of this kind include the HeartMate IP LVAS, which was approved for use in the US by the
400:
Heidelberg University Hospital reported in July 2009 that the first HeartAssist5, known as the modern version of the DeBakey VAD, was implanted there. The HeartAssist5 weighs 92 grams, is made of titanium and plastic, and serves to pump blood from the left ventricle into the
475:
The HeartWare HVAD works similarly to the VentrAssist—albeit much smaller and not requiring an abdominal pocket to be implanted into. The device has obtained CE Mark in Europe, and FDA approval in the U.S. The HeartWare HVAD could be implanted through limited access without
548:
devices with optimal medical management for patients with end-stage heart failure who require, but do not qualify to receive cardiac transplantation. As a result of the clinical outcomes, the device received FDA approval for both indications, in 2001 and 2003, respectively.
438:
On 27 June 2014 Hannover Medical School in Hannover, Germany performed the first human implant of HeartMate III under the direction of Professor Axel Haverich M.D., chief of the Cardiothoracic, Transplantation and Vascular Surgery Department and surgeon Jan Schmitto, M.D.,
388:
In August 2007 The International Consortium of Circulatory Assist Clinicians (ICCAC) was founded by Anthony "Tony" Martin. A nurse practitioner (NP) and clinical manager of the mechanical circulatory support (MCS) program at Newark Beth Israel Medical Center, Newark,
918:
Currently used in the United States as a bridge to heart transplant under an FDA-approved clinical investigation. In Europe, the Jarvik 2000 has earned CE Mark certification for both bridge-to-transplant and lifetime use. Child version currently being developed.
396:
In July 2009, 18-month follow-up results from the HeartMate II Clinical Trial concluded that continuous-flow LVAD provides effective hemodynamic support for at least 18 months in patients awaiting transplantation, with improved functional status and quality of
2471:"Medical Aspects of End-Stage Heart Failure: Transplantation and Device Therapies I, Abstract 1762: An Emerging Option for Women with Advanced Heart Failure: Results of the HeartMate II Continuous Flow Left Ventricular Assist Device Bridge to Transplant Trial"
405:
growth factors stimulating other cells to grow. The patches are seeded with heart muscle cells and then implanted onto the heart with the goal of getting the muscle cells to start communicating with native tissues in a way that allows for regular contractions.
511:
Based on one-year follow up data from the first 194 patients enrolled in the trial, the FDA approved HeartMate II for bridge-to-transplantation. The trial provided clinical evidence of improved survival rates and quality of life for a broad range of
462:
FiVAD System with wireless, coplanar energy transfer technology which ameliorates infection risk by driveline elimination while providing successful energy transmission allowing for a substantial (approximately 6 hours) unholstered support of the
507:
A multicenter study in the United States from 2005 to 2007 with 113 patients (of which 100 reported principal outcomes) showed that significant improvements in function were prevalent after three months, and a survival rate of 68% after twelve
445:
Hall-of-Fame Baseball Player Rod Carew had congestive heart failure and was fitted with a HeartMate II. He struggled with wearing the equipment, so he joined efforts to help supply the most helpful wear to assist the HeartMate II and HeartMate
192:(DT) which indicates that the VAD will remain implanted indefinitely. VADs as destination therapy are used in circumstances where patients are not candidates for transplantation and will thus rely on the VAD for the remainder of their life.
428:. The grant was renewed for a second year of research in August 2009. The total artificial heart was created using two HeartAssist5 VADs, whereby one VAD pumps blood throughout the body and the other circulates blood to and from the lungs.
2581:
Potapov, EV; Politis, N; Karck, M; Weyand, M; Tandler, R; Walther, T; Emrich, F; Reichenspurrner, H; Bernhardt, A; Barten, MJ; Svenarud, P; Gummert, J; Sef, D; Doenst, T; Tsyganenko, D; Loforte, A; Schoenrath, F; Falk, V (31 March 2022).
516:
patients, 157 patients had undergone transplant, 58 patients were continuing with LVADs in their body and seven patients had the LVAD removed because their heart recovered; the remaining 56 had died. The results showed that the patients'
302:. The patient was a 37-year-old woman, and a paracorporeal (external) circuit was able to provide mechanical support for 10 days after the surgery. The first successful long-term implantation of an LVAD was conducted in 1988 by Dr.
487:). Several surgical approaches, including interventional decommissioning, off-pump explantation using a custom-made plug and complete LVAD removal through redo sternotomy, have been described with a 5-year survival of up to 80%.
525:
baseline patients. Specifically, HeartMate II patients showed lower creatinine levels, 30-day survival rates were considerably higher at 96%, and 93% reached successful outcomes (transplant, cardiac recovery, or long-term LVAD).
466:
On June 3, 2021, Medtronic issued an urgent medical device notice stating that their HVAD devices should no longer be implanted due to higher rates of neurological events and mortality with the HVAD vs. other available devices
254:. In some pulsatile pumps (that use compressed air as an energy source), the volume occupied by blood varies during the pumping cycle. If the pump is contained inside the body then a vent tube to the outside air is required.
1281:
Balthazar, Tim; Vandenbriele, Christophe; Verbrugge, Frederik H.; Den Uil, Corstiaan; Engström, Annemarie; Janssens, Stefan; Rex, Steffen; Meyns, Bart; Van Mieghem, Nicolas; Price, Susanna; Adriaenssens, Tom (9 March 2021).
174:
5.5, Impella RP, and others can be introduced to either the left or right ventricle (depending on the patient-specific needs) using a wire and that is introduced through the arteries or veins of the neck, axilla, or groin.
344:
driven pumps. These pumps have the advantage of greater simplicity resulting in smaller size and greater reliability. These devices are referred to as second-generation VADs. A side effect is that the user will not have a
1709:
Ruden, Serena A. S. Von; Murray, Margaret A.; Grice, Jennifer L.; Proebstle, Amy K.; Kopacek, Karen J. (2012). "The Pharmacotherapy Implications of Ventricular Assist Device in the Patient With End-Stage Heart Failure".
3668:
Iacovetto, MC; Matlock, DD; Mcillvennan, CK; et al. (2014). "Educational resources for patients considering a left ventricular assist device: a cross-sectional review of internet, print, and multimedia materials".
1863:
2830:
Starling, RC; Naka, Y; Boyle, AJ; et al. (August 2009). "Initial FDA Post-Approval Study INTERMACS Registry Results with a Continuous Flow Left Ventricular Assist Device as a Bridge to Heart Transplantation".
297:
at Baylor College of Medicine in Houston in 1962. The first LVAD was implanted in 1963 by Liotta and E. Stanley Crawford. The first successful implantation of an LVAD was completed in 1966 by Liotta along with Dr.
314:(NIH) research contract which developed HeartMate, an electronically controlled assist device. This was funded by a three-year $ 6.2 million contract to Thermedics and Children's Hospital, Boston, MA, from the
472:
large. As of 2017, HeartMate III has been approved by the FDA. It is smaller than its predecessor HeartMate II and uses a full maglev impeller instead of the cup-and-ball bearing system found in HeartMate II.
1237:
Sef, D; Mohite, P; De Robertis, F; Verzelloni Sef, A; Mahesh, B; Stock, U; Simon, A (September 2020). "Bridge to heart transplantation using the Levitronix CentriMag short-term ventricular assist device".
951:
Approved for use in European Union and Australia. Company declared bankrupt while clinical trials for FDA approval were underway in 2009. Company now dissolved and intellectual property sold to Thoratec.
3731:
Ventracor was put into liquidation on 3 July 2009, whereby the company's assets including its intellectual property, data from clinical trials, plant and equipment and residual assets will be put up for
556:
versus 16.6% in the LVAD group; 31.6% of the inotrope group had right heart failure versus 5.6% in the LVAD group; and event-free survival was 15.8% in the inotrope group versus 55.6% in the LVAD group.
268:
An important issue with continuous flow pumps is the method used to suspend the rotor. Early versions used solid bearings; however, newer pumps, some of which are approved for use in the EU, use either
143:
ventricular assist device (RVAD) might be necessary to resolve the problem of cardiac circulation. If both an LVAD and an RVAD are needed a BiVAD is normally used, rather than a separate LVAD and RVAD.
183:(BTT) – keeping the patient alive, and in reasonably good condition, and able to await heart transplant outside of the hospital. Other "bridges" include bridge to candidacy (used when a patient has a
3372:
Castagna, Francesco; Stöhr, Eric J.; Pinsino, Alberto; Cockcroft, John R.; Willey, Joshua; Reshad Garan, A.; Topkara, Veli K.; Colombo, Paolo C.; Yuzefpolskaya, Melana; McDonnell, Barry J. (2017).
2355:
Canseco, Diana C.; Kimura, Wataru; Garg, Sonia; Mukherjee, Shibani; Bhattacharya, Souparno; Abdisalaam, Salim; Das, Sandeep; Asaithamby, Aroumougame; Mammen, Pradeep P.A.; Sadek, Hesham A. (2015).
495:
A series of studies involving the use of the HeartMate II LVAD have proven useful in establishing the viability and risks of using LVADs for bridge-to-transplantation and destination therapy.
4434:
4065:
2498:
3065:
Samuels, LE; Kohout, J; Casanova-Ghosh, E; et al. (2008). "Argatroban as a Primary or Secondary Postoperative Anticoagulant in Patients Implanted with Ventricular Assist Devices".
573:
Because the VADs generally result in blood flowing over a non-biologic surface (e.g. metal, synthetic polymers, etc.) this can result in formation of blood clots, also referred to as
118:
VADs may be used to manage a variety of cardiac diseases and can be categorized based on which ventricle the device is assisting, and whether the VAD will be temporary or permanent.
2427:
844:
Approved for use in North America and EU. CE Mark Authorized. FDA approval for BTT in April 2008. Recently approved by FDA in the US for Destination Therapy (as at January 2010).
3761:
2927:
Rogers, JG; Butler, J; Lansman, SL; et al. (2007). "Chronic Mechanical Circulatory Support for Inotrope-Dependent Heart Failure Patients Who Are Not Transplant Candidates".
2301:
517:
483:
In a small number of cases left ventricular assist devices, combined with drug therapy, have enabled the heart to recover sufficiently for the device to be able to be removed (
1647:
250:
used in VADs can be divided into two main categories – pulsatile pumps, which mimic the natural pulsing action of the heart, and continuous-flow pumps. Pulsatile VADs use
3825:
812:
Was approved for use in North America, European Union and Japan. Now defunct and no longer supported by the manufacturer. (HeartWare completed acquisition August 2012)
2470:
2761:
151:
VADs can further be divided by the duration of their use (i.e. temporary versus permanent). Some VADs are for short-term use, typically for patients recovering from
3235:
Elder, Theresa; Raghavan, Alankrita; Smith, Arvin; Wright, Christina Huang; Wright, James; Burant, Christopher; Sajatovic, Martha; Hoffer, Alan (December 2019).
937:
Approved for use in the European Union. The child version is approved by the FDA for use in children in USA. Undergoing clinical trials in USA for FDA approval.
796:
Approved for use in the European Union. The child version is approved by the FDA for use in children in USA. Undergoing clinical trials in USA for FDA approval.
2929:
2793:
2361:
2150:
2337:
1801:
Slaughter, MS; Pagani, FD; Rogers, JG; et al. (2010). "Clinical management of continuous-flow left ventricular assist devices in advanced heart failure".
4103:
2445:
1076:
CE Mark Authorized. Received FDA approval for BTT in 2004. Authorized only for internal implant, not for paracorporeal implant due to reliability issues.
442:
On 21 January 2015 a study was published in Journal of American College of Cardiology suggesting that long-term use of LVAD may induce heart regeneration.
46:
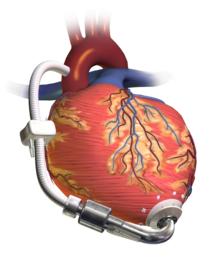
A left ventricular assist device (LVAD) pumping blood from the left ventricle to the aorta, connected to an externally worn control unit and battery pack.
2530:
2544:
Popov, AF; Hosseini, MT; Zych, B; et al. (2012). "HeartWare Left Ventricular Assist Device Implantation Through Bilateral Anterior Thoracotomy".
315:
2677:
2886:"The REMATCH trial: Rationale, design, and end points. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure"
1006:
Miniature "third generation" device with centrifugal blood path and hydromagnetically suspended rotor that may be placed in the pericardial space.
565:
There are a number of potential risks associated with VADs. The most common of these are bleeding events, stroke, pump thrombosis, and infections.
334:
began popularizing the concept that patients could live outside the hospital. Media coverage of outpatients with VADs underscored these arguments.
3931:
882:
Approved for use in European Union. Used on humanitarian approvals on a case-by-case basis in the US. Entered clinical trials in the US in 2009.
2281:
200:
Some devices are designed to support the heart and its various components/function but are not considered VADs, below are some common examples.
2505:
2319:
1952:
2743:
2069:. Columbia University Medical Center. quote: "A patient who is implanted with a HeartMate II usually has a dampened pulse." Viewed 2016-08-27.
3818:
1617:
1023:
HeartWare's MVAD Pump is a development-stage miniature ventricular assist device, approximately one-third the size of HeartWare's HVAD pump.
90:
device that provides support for cardiac pump function, which is used either to partially or to completely replace the function of a failing
2096:
1133:
Savarese, Gianluigi; Becher, Peter Moritz; Lund, Lars H.; Seferovic, Petar; Rosano, Giuseppe M. C.; Coats, Andrew J. S. (18 January 2023).
3619:"Decision making for destination therapy left ventricular assist devices: "there was no choice" versus "I thought about it an awful lot""
1073:
Pulsatile system includes three major components: Blood pump, cannulae and pneumatic driver (dual drive console or portable VAD driver).
1057:
Pulsatile system includes three major components: Blood pump, cannulae and pneumatic driver (dual drive console or portable VAD driver).
585:
medications are used to decrease the risk of thrombosis. One device, the HeartMate XVE, is designed with a biologic surface derived from
4026:
4011:
3951:
3735:
3323:
Varshney, Anubodh S.; DeFilippis, Ersilia M.; Cowger, Jennifer A.; Netuka, Ivan; Pinney, Sean P.; Givertz, Michael M. (22 March 2022).
2967:
Varshney, Anubodh S.; DeFilippis, Ersilia M.; Cowger, Jennifer A.; Netuka, Ivan; Pinney, Sean P.; Givertz, Michael M. (22 March 2022).
4523:
2681:
4424:
4121:
3716:"A decision aid for Left Ventricular Assist Device (LVAD) for Destination Therapy A device for patients with advanced heart failure"
3811:
1684:
3528:
Holman, WL; Rayburn, BK; McGiffin, DC; et al. (2003). "Infection in ventricular assist devices: Prevention and treatment".
4267:
4126:
4059:
2705:
2187:
1469:
1430:
991:
449:
In December 2018, two clinical cases were performed in Kazakhstan and a fully wireless LVAD system of Jarvik 2000 combine with
135:
130:(RVAD) or the left ventricle (LVAD) or to both ventricles (BiVAD). The type of VAD implanted depends on the type of underlying
2632:
4398:
3530:
3153:
3111:
3067:
2890:
2658:
2546:
3237:"Outcomes After Intracranial Hemorrhage in Patients with Left Ventricular Assist Devices: A Systematic Review of Literature"
2484:
2054:
337:
More recent work has concentrated on continuous-flow pumps, which can be roughly categorized as either centrifugal pumps or
1065:
1049:
3905:
3888:
3151:
Goldstein, Daniel J.; Robert B. Beauford (2003). "Left ventricular assist devices and bleeding: adding insult to injury".
2341:
413:
311:
307:
1349:
1060:
CE Mark Authorized. Received FDA approval for BTT in 1995 and for post-cardiotomy recovery (open heart surgery) in 1998.
4508:
3882:
3766:
2765:
2079:
1093:
905:
319:
207:
1917:
Hoshi, H; Shinshi, T; Takatani, S (2006). "Third-generation Blood Pumps with Mechanical Noncontact Magnetic Bearings".
1182:
Kirkpatrick, James N.; Wieselthaler, Georg; Strueber, Martin; St John Sutton, Martin G.; Rame, J. Eduardo (July 2015).
4308:
4242:
2428:"First human use of a wireless coplanar energy transfer coupled with a continuous-flow left ventricular assist device"
2210:
3434:
O'Horo, John C.; Abu Saleh, Omar M.; Stulak, John M.; Wilhelm, Mark P.; Baddour, Larry M.; Rizwan Sohail, M. (2018).
2261:
435:
HeartWare LVAD System for the Treatment of Advanced Heart Failure, is a multi-center study that started in May 2009.
257:
Continuous-flow VADs are smaller and have proven to be more durable than pulsatile VADs. They normally use either a
3900:
3845:
1643:"Comparisons of infection complications between continuous flow and pulsatile flow left ventricular assist devices"
251:
828:
FDA approval for BTT in 2001 and DT in 2003. CE Mark Authorized. Rarely used anymore due to reliability concerns.
4429:
4220:
4171:
1836:
Fukamachi, Kiyo; Smedira, Nicholas (August 2005). "Smaller, Safer, Totally Implantable LVADs: Fact or Fantasy?".
1108:
804:
180:
170:(introduced to the heart through the skin into a blood vessel rather than through an incision) VADs such as the
4528:
2678:"FDA Approves HeartMate II Mechanical Heart Pump for Heart-Failure Patients Waiting for Organ Transplantation"
2124:
431:
4323:
4166:
4151:
3834:
612:
604:
454:
322:(FDA) in October 1994. These devices began to gain acceptance in the late 1990s as heart surgeons including
285:
99:
4403:
1031:
221:, which are designed to assume cardiac function, and generally require the removal of the patient's heart.
4205:
3374:"The Unique Blood Pressures and Pulsatility of LVAD Patients: Current Challenges and Future Opportunities"
2305:
887:
658:
274:
4408:
4329:
3671:
3623:
3567:"Modification of self-concept in patients with a left-ventricular assist device: an initial exploration"
3566:
2475:
1978:
1600:
Fajdek, B; Krzysztof, J (2–5 September 2014). "Automatic control system for ventricular assist device".
643:
539:. This opens the possibility that some advanced heart failure patients may forgo heart transplantation.
152:
139:
3107:"Bleeding Complications and Blood Product Utilization With Left Ventricular Assist Device Implantation"
960:
900:
Approved for use in European Union. FDA granted Humanitarian Device Exemption for US in December 2011.
458:
which powered the pump and maintained the battery charged to allow medical and nursing procedures. The
416:(NIH) awarded a $ 2.8 million grant to develop a "pulse-less" total artificial heart using two VADs by
2685:
2146:"Extended Mechanical Circulatory Support with a Continuous-Flow Rotary Left Ventricular Assist Device"
459:
450:
4518:
4260:
4080:
1889:
4513:
4303:
4210:
4021:
4016:
4006:
3959:
3876:
3860:
3762:"FDA Alerts Health Care Providers to Stop New Implants of Certain Ventricular Assist Device System"
2747:
2584:"Results from a multicentre evaluation of plug use for left ventricular assist device explantation"
1015:
370:
303:
270:
189:
159:; some are for long-term use (months to years to perpetuity), typically for patients with advanced
103:
4352:
4183:
4116:
4033:
3994:
3969:
3894:
3696:
2215:
1623:
1582:
1331:
1284:"Managing Patients With Short-Term Mechanical Circulatory Support: JACC Review Topic of the Week"
1263:
1219:
425:
357:, to power this device – which would make it truly self-contained. However, a similar operation (
299:
3190:"Gastrointestinal bleeding in recipients of left ventricular assist devices-a systematic review"
869:
552:
sodium, blood urea nitrogen, and creatinine. After transplant, 57.7% of the inotrope group had
4455:
4201:
4001:
3979:
3964:
3855:
3688:
3650:
3599:
3547:
3510:
3491:
Gordon, RJ; Quagliarello, B; Lowy, FD (2006). "Ventricular assist device-related infections".
3473:
3455:
3411:
3393:
3354:
3346:
3305:
3264:
3256:
3217:
3209:
3170:
3130:
3084:
3047:
3039:
2998:
2990:
2946:
2909:
2860:"Harefield Recovery Protocol Study for Patients With Refractory Chronic Heart Failure (HARPS)"
2812:
2724:
2613:
2563:
2524:
2388:
2266:
2169:
2129:
2005:
1957:
1934:
1818:
1727:
1666:
1613:
1574:
1566:
1527:
1509:
1449:
1403:
1395:
1323:
1315:
1255:
1211:
1203:
1164:
1156:
688:
417:
127:
4313:
3984:
3680:
3640:
3632:
3589:
3581:
3539:
3502:
3493:
3463:
3447:
3401:
3385:
3336:
3295:
3248:
3201:
3162:
3120:
3076:
3029:
2980:
2938:
2899:
2840:
2802:
2714:
2603:
2595:
2555:
2378:
2370:
2159:
1995:
1987:
1926:
1897:
1845:
1810:
1719:
1656:
1605:
1558:
1517:
1499:
1439:
1387:
1305:
1295:
1247:
1195:
1146:
696:
679:
258:
218:
206:
and Internal Cardiac Defibrillators (ICDs) – the function of a VAD differs from that of an
184:
107:
87:
2038:
1184:"Ventricular assist devices for treatment of acute heart failure and chronic heart failure"
41:
4339:
4237:
4111:
3715:
3284:"Activation of coagulation and fibrinolytic pathways with left ventricular assist devices"
1602:
2014 19th International Conference on Methods and Models in Automation and Robotics (MMAR)
1113:
973:
582:
421:
358:
338:
262:
156:
1773:
892:
2235:"A Study of Anginera in Patients Undergoing Coronary Artery Bypass Graft (CABG) Surgery"
1991:
1893:
1486:
Chung, Joshua S.; Emerson, Dominic; Megna, Dominick; Arabia, Francisco A. (March 2020).
742:
Please help update this article to reflect recent events or newly available information.
4450:
4293:
4070:
3645:
3618:
3468:
3435:
3406:
3373:
2608:
2583:
2383:
2356:
2000:
1973:
1878:"Development of a Compact Maglev Centrifugal Blood Pump Enclosed in a Titanium Housing"
1522:
1488:"Total artificial heart: surgical technique in the patient with normal cardiac anatomy"
1487:
553:
365:
294:
3543:
3506:
3300:
3283:
3166:
2904:
2885:
126:
First, VADs can be categorized based on whether they are designed to assist the right
4502:
4178:
4161:
3617:
Mcillvennan, CK; Allen, LA; Nowels, C; Brieke, A; Cleveland, JC; Matlock, DD (2014).
3585:
3125:
3106:
3080:
2559:
2302:"Evaluation of the HeartWare LVAD System for the Treatment of Advanced Heart Failure"
1930:
1586:
1335:
1267:
637:
500:
327:
160:
131:
95:
3684:
3636:
2282:"$ 2.8 Million Grant Renewed for Development of "Pulse-Less" Total Artificial Heart"
2195:
1849:
1627:
1135:"Global burden of heart failure: a comprehensive and updated review of epidemiology"
94:. VADs can be used in patients with acute (sudden onset) or chronic (long standing)
4487:
4388:
4255:
4247:
4193:
4131:
4095:
4085:
3989:
3946:
3700:
3325:"Trends and Outcomes of Left Ventricular Assist Device Therapy: JACC Focus Seminar"
2969:"Trends and Outcomes of Left Ventricular Assist Device Therapy: JACC Focus Seminar"
2844:
2286:
2188:"Heidelberg Cardiac Surgeons implant world's first new DeBakey Heart Assist Device"
2023:"Heart Pump Progress Announced - A promising step in artificial heart technology".
1547:"Mechanical circulatory support devices in advanced heart failure: 2020 and beyond"
1426:"Left Ventricular Assist Device and Drug Therapy for the Reversal of Heart Failure"
1223:
692:
664:
167:
2859:
2662:
2234:
453:
FiVAD (Fully Implantable Ventricular Assist Device) were implanted in humans. The
58:
3451:
2446:"Urgent Medical Device Communication: Notification Letter Medtronic HVAD™ System"
2338:"Thoratec Announces First HeartMate III Human Implant And Start of CE Mark Trial"
1814:
1199:
4470:
4393:
3974:
3188:
Naveed, Ali; Naveed, Bazigh; Khan, Muhammad Atif; Asif, Talal (September 2023).
3034:
3017:
1745:
1661:
1642:
968:
Currently in animal testing, recently completed successful 60-day calf implant.
874:
708:
649:
608:
578:
536:
233:
52:
3341:
3324:
3252:
3205:
2985:
2968:
2942:
2807:
2788:
2374:
2164:
2145:
1562:
1300:
1283:
924:
4477:
4460:
4372:
4288:
4232:
4038:
3389:
1953:"Dr. Denton Cooley and Dr. Michael E. DeBakey: Rock stars of Houston medicine"
1609:
1391:
670:
631:
VAD-related infection can be caused by a large number of different organisms:
616:
574:
477:
203:
3459:
3397:
3350:
3260:
3213:
3043:
2994:
2083:
1731:
1723:
1570:
1513:
1399:
1319:
1207:
1160:
4465:
4347:
4283:
2701:"Use of a Continuous-Flow Device in Patients Awaiting Heart Transplantation"
2599:
1504:
1089:
Versatile wireless system for LVAD. Allow 6-hour of freedom to the patients
354:
331:
323:
3692:
3654:
3603:
3551:
3514:
3477:
3415:
3358:
3268:
3236:
3221:
3189:
3174:
3134:
3088:
3051:
3002:
2950:
2913:
2816:
2728:
2661:. Cardiopulmonary Research Science and Technology Institute. Archived from
2617:
2567:
2392:
2220:
2173:
2066:
2009:
1938:
1822:
1670:
1578:
1546:
1531:
1453:
1407:
1375:
1327:
1259:
1215:
1183:
1168:
3803:
3309:
1151:
1134:
948:
Continuous flow driven by a hydrodynamically suspended centrifugal rotor.
4367:
4318:
4298:
4156:
4075:
3850:
3594:
2719:
2700:
1902:
1877:
1444:
1425:
341:
17:
788:
214:, whereas a pacemaker delivers electrical impulses to the heart muscle.
68:
4482:
4362:
3797:
699:
when blood comes in contact with them. This predisposes the patient to
171:
138:
may require an RVAD, versus those with left ventricular failure from a
1774:"The HeartMate XVE too has a vent line, despite being battery-powered"
1310:
1251:
965:
Continuous flow driven by a magnetically suspended centrifugal rotor.
293:
The first left ventricular assist device (LVAD) system was created by
237:
Close-up illustration of typical left ventricular assist device (LVAD)
3941:
3936:
3714:
Matlock, DD; Allen, LA; Thompson, JS; Mcilvennan, CK (31 July 2014).
1036:
934:
Continuous flow driven by axial rotor supported by ceramic bearings.
879:
Continuous flow driven by a magnetically suspended axial flow rotor.
857:
Continuous flow driven by a magnetically suspended axial flow rotor.
700:
586:
1974:"Mechanical circulatory support: registering a therapy in evolution"
1545:
Vieira, Jefferson L.; Ventura, Hector O.; Mehra, Mandeep R. (2020).
1376:"Left Ventricular Assist Device: Indication, Timing, and Management"
1350:"Impella 5.5® with SmartAssist®| Product | Healthcare Professionals"
860:
Pivotal trials for HeartMate III started in 2014 and supported with
2406:
1685:"Panel A shows a first-generation pulsatile flow left ve - Open-i"
704:
346:
232:
211:
91:
2357:"Human Ventricular Unloading Induces Cardiomyocyte Proliferation"
1882:
Journal of Advanced Mechanical Design, Systems, and Manufacturing
978:
695:
components used in the devices cause the deletion of a subset of
4357:
4043:
3436:"Left Ventricular Assist Device Infections: A Systematic Review"
1864:"Magnetic levitation heart pump implanted in first U.S. patient"
1092:
Investigation device, 2 patients trial conduct in Dec 2018 with
1084:
861:
247:
3807:
3800:- Non-branded site with information on decision making for LVAD
3105:
Schaffer, JM; Arnaoutakis, GJ; Allen, JG; et al. (2011).
722:
521:
patients receiving mechanical support for destination therapy.
2097:
Patient Sets World Record for Living with Heart Assist Device
1641:
Schulman, AR; Martens, TP; Christos, PJ; et al. (2007).
929:
910:
1000:
915:
Continuous flow, axial rotor supported by ceramic bearings.
535:
heart failure medications, followed by the novel β2 agonist
996:
852:
841:
Rotor driven continuous axial flow, ball and cup bearings.
820:
310:
Medical Center and Thermedics, Inc. of Woburn, MA, under a
4435:
European Society of Paediatric and Neonatal Intensive Care
3429:
3427:
3425:
1470:"First VentrAssist Heart Recovery Featured on National TV"
983:
Pulsatile, driven by an inflatable cuff around the aorta.
833:
2699:
Miller, LW; Pagani, FD; Russell, SD; et al. (2007).
2320:"HeartWare International Surpasses 50 Implants in the US"
2144:
Pagani, FD; Miller, LW; Russell, SD; et al. (2009).
349:, or that the pulse intensity will be seriously reduced.
2884:
Rose, EA; Moskowitz, AJ; Packer, M; et al. (1999).
2109:
1050:
Thoratec PVAD (Paracorporeal Ventricular Assist Device)
1044:
CE approved, US FDA trials underway as at January 2010.
2789:"Continuous Flow Rotary Left Ventricular Assist Device"
2746:. University of Michigan Health System. Archived from
2340:. Thoratec Corporation. 20 August 2009. Archived from
1424:
Birks, EJ; Tansley, PD; Hardy, J; et al. (2006).
4066:
Critical illness–related corticosteroid insufficiency
3282:
Spanier, Talia; Oz, M; Levin, H; et al. (1996).
3016:
Stansfield, William E.; Rao, Vivek (September 2016).
2962:
2960:
2469:
Bogaev, R; Chen, L; Russell, SD; et al. (2007).
897:
External membrane pump device designed for children.
480:, however in 2021 Medtronic discontinued the device.
361:) was tried in the 1990s with disappointing results.
3791:
4443:
4417:
4381:
4338:
4276:
4192:
4144:
4094:
4052:
3921:
3914:
3869:
3794:—Non-branded site with information on various LVADs
3018:"HeartMate 3: Facing the challenge of past success"
2744:"Exciting times for heart-assisting devices at U-M"
2241:. U.S. National Institutes of Health. 27 March 2009
1876:Pai, CN; Shinshi, T; Asama, J; et al. (2008).
134:(e.g. patients with right ventricular failure from
51:
34:
3022:The Journal of Thoracic and Cardiovascular Surgery
2430:. Heart and Lung Transplantation. 4 February 2019.
2125:"Transplant shows heart's reparative capabilities"
2055:"No Pulse: How Doctors Reinvented the Human Heart"
1648:The Journal of Thoracic and Cardiovascular Surgery
1419:
1417:
1026:HeartWare Completed GLP Studies (September 2011).
369:traveled extensively around the world. He died of
2483:. American Heart Association: 372. Archived from
217:Total Artificial Heart – VADs are distinct from
179:variety of scenarios. First, VADs may be used as
3672:Circulation: Cardiovascular Quality and Outcomes
3624:Circulation: Cardiovascular Quality and Outcomes
2633:"HeartMate II Pivotal Clinical Trial Fact Sheet"
760:This is a partial list and may never be complete
155:(heart attack) and for patients recovering from
2588:Interactive Cardiovascular and Thoracic Surgery
1465:
1463:
793:Continuous flow driven by an axial flow rotor.
3565:Marcuccilli, L; Casida, J; Peters, RM (2013).
3288:Journal of Thoracic and Cardiovascular Surgery
3819:
3329:Journal of the American College of Cardiology
2973:Journal of the American College of Cardiology
2930:Journal of the American College of Cardiology
2794:Journal of the American College of Cardiology
2362:Journal of the American College of Cardiology
2151:Journal of the American College of Cardiology
1803:The Journal of Heart and Lung Transplantation
1288:Journal of the American College of Cardiology
687:Other immune system related problems include
8:
3721:. University of Colorado School of Medicine.
3146:
3144:
2858:Miller, Leslie; Aaronson and Pagani (2008).
3100:
3098:
27:Medical device to assist or replace a heart
3918:
3826:
3812:
3804:
1972:Kirklin, JK; Naftel, DC (September 2008).
1066:IVAD—Implantable Ventricular Assist Device
40:
3644:
3593:
3467:
3405:
3340:
3299:
3124:
3033:
2984:
2903:
2806:
2718:
2607:
2382:
2163:
1999:
1901:
1746:"SynCardia TAH pulsatile pump components"
1660:
1521:
1503:
1443:
1309:
1299:
1150:
1041:Magnetically levitated centrifugal pump.
316:National Heart, Lung, and Blood Institute
767:
284:
1125:
353:patient's skeletal muscle, driven by a
289:1966 DeBakey ventricular assist device.
2762:"Thoratec HeartMate II LVAS – P060040"
2529:: CS1 maint: archived copy as title (
2522:
31:
2659:"The HeartMate II LVAS Pivotal Trial"
2439:
2437:
2422:
2420:
2099:. Texas Heart Institute. 6 July 2007.
707:infections necessitating appropriate
7:
4314:Recombinant activated protein C
979:Sunshine Heart (Now "CHF Solutions")
166:acute signs of heart failure, small
4027:Multiple organ dysfunction syndrome
4012:Acute respiratory distress syndrome
3952:Multiple organ dysfunction syndrome
1992:10.1161/circheartfailure.108.782599
1866:. "Cardiology Today". October 2008.
1551:Progress in Cardiovascular Diseases
598:Ischemic Stroke and Pump Thrombosis
64:
25:
4425:Society of Critical Care Medicine
4122:Ventilator-associated lung injury
2260:Hunter, Tim (13 September 2009).
986:Currently available commercially
188:instances, VADs are also used as
3798:DECIDE-LVAD Patient Decision Aid
3586:10.1111/j.1365-2702.2012.04332.x
3126:10.1016/j.athoracsur.2010.11.007
3081:10.1016/j.athoracsur.2008.01.100
2676:Mager, Belinda (25 April 2008).
2631:Benton, Susan (19 August 2008).
2560:10.1016/j.athoracsur.2011.09.055
2194:. 17 August 2009. Archived from
2080:"The First Lifetime-Use Patient"
1931:10.1111/j.1525-1594.2006.00222.x
1492:Annals of Cardiothoracic Surgery
1374:Frigerio, Maria (October 2021).
780:Approval Status as of July 2010
764:Referenced additions are welcome
727:
4127:Ventilator-associated pneumonia
4060:Critical illness polyneuropathy
3685:10.1161/CIRCOUTCOMES.114.000892
3637:10.1161/CIRCOUTCOMES.113.000729
2787:Eisen, HJ; Hankins, SR (2009).
2706:New England Journal of Medicine
2211:"VA study: heart-healing patch"
1850:10.1016/j.accreview.2005.06.001
1431:New England Journal of Medicine
1188:Heart (British Cardiac Society)
864:. FDA approval for BTT in 2017
719:List of implantable VAD devices
491:HeartMate II LVAD pivotal study
136:pulmonary arterial hypertension
3531:The Annals of Thoracic Surgery
3494:The Lancet Infectious Diseases
3154:The Annals of Thoracic Surgery
3112:The Annals of Thoracic Surgery
3068:The Annals of Thoracic Surgery
2891:The Annals of Thoracic Surgery
2845:10.1016/j.cardfail.2009.06.252
2657:Dewey, Todd (19 August 2008).
2547:The Annals of Thoracic Surgery
2123:Maugh, Thomas (14 July 2009).
2082:. Jarvik Heart. Archived from
561:Complications and side effects
1:
3906:Geriatric intensive-care unit
3889:Pediatric intensive care unit
3544:10.1016/S0003-4975(03)00479-X
3507:10.1016/S1473-3099(06)70522-9
3301:10.1016/s0022-5223(96)70111-3
3167:10.1016/s0003-4975(03)00478-8
2905:10.1016/S0003-4975(99)00042-9
2742:Gavin, Kara (23 April 2008).
2682:NewYork–Presbyterian Hospital
2444:Njoku, Nnamdi (3 June 2021).
2209:Quinn, Dale (4 August 2009).
715:assist in patient education.
414:National Institutes of Health
312:National Institutes of Health
196:Other Cardiac Support Devices
4309:Neuromuscular-blocking drugs
4252:Nutritional supplementation
3883:Neonatal intensive care unit
3767:Food and Drug Administration
3452:10.1097/MAT.0000000000000684
3378:Current Hypertension Reports
2766:Food and Drug Administration
2027:(March): 1,5. 19 March 1988.
1815:10.1016/j.healun.2010.01.011
1712:Journal of Pharmacy Practice
1200:10.1136/heartjnl-2014-306789
1096:LVAD in Astana by prof Pya.
320:Food and Drug Administration
208:artificial cardiac pacemaker
4409:Water-electrolyte imbalance
4243:Early goal-directed therapy
3734:Boyd, Tony (13 July 2009).
3574:Journal of Clinical Nursing
3035:10.1016/j.jtcvs.2016.04.048
1662:10.1016/j.jtcvs.2006.09.083
252:positive displacement pumps
4545:
4261:Total parenteral nutrition
4194:Life-supporting treatments
3901:Critical illness insurance
3342:10.1016/j.jacc.2022.01.017
3253:10.1016/j.wneu.2019.08.211
3206:10.1007/s10741-023-10313-6
2986:10.1016/j.jacc.2022.01.017
2943:10.1016/j.jacc.2007.03.063
2833:Journal of Cardiac Failure
2808:10.1016/j.jacc.2009.04.028
2375:10.1016/j.jacc.2014.12.027
2262:"Meet the Kiwi bionic man"
2165:10.1016/j.jacc.2009.03.055
1979:Circulation: Heart Failure
1838:ACC Current Journal Review
1563:10.1016/j.pcad.2020.09.003
1301:10.1016/j.jacc.2020.12.054
308:Boston Children's Hospital
4524:Interventional cardiology
4430:Surviving Sepsis Campaign
4226:Ventricular assist device
4221:Intra-aortic balloon pump
4172:Pulmonary artery catheter
3841:
3390:10.1007/s11906-017-0782-6
1610:10.1109/MMAR.2014.6957472
1392:10.1016/j.hfc.2021.05.007
1109:Intra-aortic balloon pump
736:This section needs to be
181:bridge to transplantation
98:, which can occur due to
80:ventricular assist device
65:
39:
35:Ventricular assist device
2057:. Popular Science. 2012.
1724:10.1177/0897190011431635
656:Gram negative bacteria (
635:Gram positive bacteria (
110:, and other conditions.
4324:Stress ulcer prevention
4268:Therapeutic hypothermia
4167:Central venous catheter
3835:Intensive care medicine
1505:10.21037/acs.2020.02.09
1139:Cardiovascular Research
974:C-Pulse (Now "Aquadex")
613:arterial blood pressure
605:Doppler ultrasonography
455:Wireless power transfer
432:HeartWare International
420:, initially created by
275:hydrodynamic suspension
100:coronary artery disease
4399:Level of consciousness
4206:mechanical ventilation
4104:Methicillin-resistant
2638:. Thoratec Corporation
2306:Johns Hopkins Hospital
659:Pseudomonas aeruginosa
290:
238:
122:Ventricular Assistance
114:Categorization of VADs
4106:Staphylococcus aureus
3194:Heart Failure Reviews
2600:10.1093/icvts/ivab344
1380:Heart Failure Clinics
288:
236:
153:myocardial infarction
140:myocardial infarction
4081:Stress hyperglycemia
3922:Organ system failure
3856:Medical specialities
3580:(2456–64): 2456–64.
3538:(6 Suppl): S48–S57.
2720:10.1056/NEJMoa067758
2411:carewmedicalwear.com
2086:on 21 November 2010.
1903:10.1299/jamdsm.2.343
1604:. pp. 874–879.
1445:10.1056/NEJMoa053063
1354:www.heartrecovery.eu
1010:market in June 2021
925:MicroMed DeBakey VAD
709:prophylactic therapy
378:Studies and outcomes
210:in that a VAD pumps
4509:Implants (medicine)
4444:Related specialties
4404:Acid–base imbalance
4340:ICU scoring systems
4211:Tracheal intubation
4022:Respiratory failure
4017:Acute liver failure
4007:Acute renal failure
3877:Intensive care unit
3861:Respiratory therapy
3760:FDA (3 June 2021).
1894:2008JAMDS...2..343P
1152:10.1093/cvr/cvac013
383:Recent developments
371:acute kidney injury
304:William F. Bernhard
271:magnetic levitation
190:destination therapy
104:atrial fibrillation
4353:Glasgow Coma Scale
4304:Intravenous fluids
4184:Screening cultures
4152:Arterial blood gas
4117:Refeeding syndrome
4034:Neonatal infection
3995:Vasodilatory shock
3970:Distributive shock
3895:Coronary care unit
3740:Business Spectator
3241:World Neurosurgery
2864:ClinicalTrials.gov
2511:on 10 January 2018
2344:on 7 October 2014.
2239:ClinicalTrials.gov
2216:Arizona Daily Star
1476:. 19 October 2006.
677:Fungi, especially
300:Michael E. DeBakey
291:
239:
4496:
4495:
4456:Internal medicine
4202:Airway management
4140:
4139:
3980:Obstructive shock
3965:Cardiogenic shock
3335:(11): 1092–1107.
2979:(11): 1092–1107.
2326:. 20 August 2009.
2267:Manawatu Standard
2223:on 7 August 2009.
2130:Los Angeles Times
1958:Houston Chronicle
1919:Artificial Organs
1689:openi.nlm.nih.gov
1619:978-1-4799-5081-2
1438:(18): 1873–1884.
1252:10.1111/aor.13709
1240:Artificial Organs
1194:(14): 1091–1096.
1145:(17): 3272–3287.
1100:
1099:
961:www.mitiheart.com
757:
756:
689:immunosuppression
499:The HeartMate II
219:artificial hearts
88:electromechanical
76:
75:
16:(Redirected from
4536:
4217:Cardiac devices
4071:Decubitus ulcers
3985:Neurogenic shock
3919:
3828:
3821:
3814:
3805:
3779:
3778:
3776:
3774:
3757:
3751:
3750:
3748:
3746:
3729:
3723:
3722:
3720:
3711:
3705:
3704:
3665:
3659:
3658:
3648:
3614:
3608:
3607:
3597:
3571:
3562:
3556:
3555:
3525:
3519:
3518:
3488:
3482:
3481:
3471:
3431:
3420:
3419:
3409:
3369:
3363:
3362:
3344:
3320:
3314:
3313:
3303:
3294:(4): 1090–1097.
3279:
3273:
3272:
3232:
3226:
3225:
3200:(5): 1163–1175.
3185:
3179:
3178:
3148:
3139:
3138:
3128:
3102:
3093:
3092:
3075:(5): 1651–1655.
3062:
3056:
3055:
3037:
3013:
3007:
3006:
2988:
2964:
2955:
2954:
2924:
2918:
2917:
2907:
2881:
2875:
2874:
2872:
2870:
2855:
2849:
2848:
2827:
2821:
2820:
2810:
2784:
2778:
2777:
2775:
2773:
2758:
2752:
2751:
2739:
2733:
2732:
2722:
2696:
2690:
2689:
2684:. Archived from
2673:
2667:
2666:
2665:on 13 July 2007.
2654:
2648:
2647:
2645:
2643:
2637:
2628:
2622:
2621:
2611:
2578:
2572:
2571:
2541:
2535:
2534:
2528:
2520:
2518:
2516:
2510:
2504:. Archived from
2503:
2495:
2489:
2488:
2466:
2460:
2459:
2457:
2455:
2450:
2441:
2432:
2431:
2424:
2415:
2414:
2403:
2397:
2396:
2386:
2352:
2346:
2345:
2334:
2328:
2327:
2316:
2310:
2309:
2298:
2292:
2291:
2290:. 6 August 2009.
2278:
2272:
2271:
2257:
2251:
2250:
2248:
2246:
2231:
2225:
2224:
2219:. Archived from
2206:
2200:
2199:
2198:on 18 July 2011.
2184:
2178:
2177:
2167:
2141:
2135:
2134:
2120:
2114:
2113:
2106:
2100:
2094:
2088:
2087:
2076:
2070:
2064:
2058:
2051:
2045:
2044:
2035:
2029:
2028:
2025:Children's Today
2020:
2014:
2013:
2003:
1969:
1963:
1962:
1949:
1943:
1942:
1914:
1908:
1907:
1905:
1873:
1867:
1860:
1854:
1853:
1833:
1827:
1826:
1798:
1792:
1791:
1789:
1787:
1778:
1770:
1764:
1763:
1761:
1759:
1750:
1742:
1736:
1735:
1706:
1700:
1699:
1697:
1695:
1681:
1675:
1674:
1664:
1638:
1632:
1631:
1597:
1591:
1590:
1542:
1536:
1535:
1525:
1507:
1483:
1477:
1467:
1458:
1457:
1447:
1421:
1412:
1411:
1371:
1365:
1364:
1362:
1360:
1346:
1340:
1339:
1313:
1303:
1294:(9): 1243–1256.
1278:
1272:
1271:
1246:(9): 1006–1008.
1234:
1228:
1227:
1179:
1173:
1172:
1154:
1130:
1085:Leviticus Cardio
862:CarewMedicalWear
768:
752:
749:
743:
731:
730:
723:
460:Leviticus Cardio
451:Leviticus Cardio
259:centrifugal pump
185:contraindication
108:valvular disease
69:edit on Wikidata
61:
44:
32:
21:
4544:
4543:
4539:
4538:
4537:
4535:
4534:
4533:
4529:Medical devices
4499:
4498:
4497:
4492:
4439:
4413:
4377:
4334:
4294:Antithrombotics
4272:
4256:Enteral feeding
4238:Kidney dialysis
4188:
4136:
4112:Oxygen toxicity
4090:
4048:
3910:
3865:
3837:
3832:
3788:
3783:
3782:
3772:
3770:
3759:
3758:
3754:
3744:
3742:
3733:
3730:
3726:
3718:
3713:
3712:
3708:
3667:
3666:
3662:
3616:
3615:
3611:
3569:
3564:
3563:
3559:
3527:
3526:
3522:
3490:
3489:
3485:
3433:
3432:
3423:
3371:
3370:
3366:
3322:
3321:
3317:
3281:
3280:
3276:
3234:
3233:
3229:
3187:
3186:
3182:
3150:
3149:
3142:
3104:
3103:
3096:
3064:
3063:
3059:
3015:
3014:
3010:
2966:
2965:
2958:
2926:
2925:
2921:
2883:
2882:
2878:
2868:
2866:
2857:
2856:
2852:
2829:
2828:
2824:
2786:
2785:
2781:
2771:
2769:
2768:. 23 April 2008
2760:
2759:
2755:
2750:on 10 May 2008.
2741:
2740:
2736:
2698:
2697:
2693:
2675:
2674:
2670:
2656:
2655:
2651:
2641:
2639:
2635:
2630:
2629:
2625:
2580:
2579:
2575:
2543:
2542:
2538:
2521:
2514:
2512:
2508:
2501:
2499:"Archived copy"
2497:
2496:
2492:
2487:on 8 June 2011.
2468:
2467:
2463:
2453:
2451:
2448:
2443:
2442:
2435:
2426:
2425:
2418:
2405:
2404:
2400:
2354:
2353:
2349:
2336:
2335:
2331:
2318:
2317:
2313:
2300:
2299:
2295:
2280:
2279:
2275:
2259:
2258:
2254:
2244:
2242:
2233:
2232:
2228:
2208:
2207:
2203:
2186:
2185:
2181:
2143:
2142:
2138:
2122:
2121:
2117:
2108:
2107:
2103:
2095:
2091:
2078:
2077:
2073:
2065:
2061:
2052:
2048:
2037:
2036:
2032:
2022:
2021:
2017:
1971:
1970:
1966:
1961:. 3 April 2014.
1951:
1950:
1946:
1916:
1915:
1911:
1875:
1874:
1870:
1861:
1857:
1835:
1834:
1830:
1800:
1799:
1795:
1785:
1783:
1776:
1772:
1771:
1767:
1757:
1755:
1748:
1744:
1743:
1739:
1708:
1707:
1703:
1693:
1691:
1683:
1682:
1678:
1640:
1639:
1635:
1620:
1599:
1598:
1594:
1544:
1543:
1539:
1485:
1484:
1480:
1468:
1461:
1423:
1422:
1415:
1373:
1372:
1368:
1358:
1356:
1348:
1347:
1343:
1280:
1279:
1275:
1236:
1235:
1231:
1181:
1180:
1176:
1132:
1131:
1127:
1122:
1114:Pump thrombosis
1105:
888:Excor Pediatric
762:
753:
747:
744:
741:
732:
728:
721:
583:anticoagulation
581:abnormalities,
577:. Due to these
563:
545:
532:
493:
422:Michael DeBakey
385:
380:
359:cardiomyoplasty
283:
263:axial flow pump
244:
231:
157:cardiac surgery
116:
72:
57:
47:
28:
23:
22:
15:
12:
11:
5:
4542:
4540:
4532:
4531:
4526:
4521:
4516:
4511:
4501:
4500:
4494:
4493:
4491:
4490:
4485:
4480:
4475:
4474:
4473:
4468:
4463:
4453:
4451:Anesthesiology
4447:
4445:
4441:
4440:
4438:
4437:
4432:
4427:
4421:
4419:
4415:
4414:
4412:
4411:
4406:
4401:
4396:
4391:
4385:
4383:
4379:
4378:
4376:
4375:
4370:
4365:
4360:
4355:
4350:
4348:APACHE II
4344:
4342:
4336:
4335:
4333:
4332:
4327:
4321:
4316:
4311:
4306:
4301:
4296:
4291:
4286:
4280:
4278:
4274:
4273:
4271:
4270:
4265:
4264:
4263:
4258:
4250:
4245:
4240:
4235:
4230:
4229:
4228:
4223:
4215:
4214:
4213:
4198:
4196:
4190:
4189:
4187:
4186:
4181:
4179:Blood cultures
4176:
4175:
4174:
4169:
4164:
4154:
4148:
4146:
4142:
4141:
4138:
4137:
4135:
4134:
4129:
4124:
4119:
4114:
4109:
4100:
4098:
4092:
4091:
4089:
4088:
4083:
4078:
4073:
4068:
4063:
4056:
4054:
4050:
4049:
4047:
4046:
4041:
4036:
4030:
4029:
4024:
4019:
4014:
4009:
4004:
3998:
3997:
3992:
3987:
3982:
3977:
3972:
3967:
3962:
3955:
3954:
3949:
3944:
3939:
3934:
3929:
3928:Shock sequence
3925:
3923:
3916:
3912:
3911:
3909:
3908:
3903:
3898:
3892:
3886:
3880:
3873:
3871:
3867:
3866:
3864:
3863:
3858:
3853:
3848:
3846:Health science
3842:
3839:
3838:
3833:
3831:
3830:
3823:
3816:
3808:
3802:
3801:
3795:
3787:
3786:External links
3784:
3781:
3780:
3752:
3724:
3706:
3660:
3609:
3557:
3520:
3483:
3446:(3): 287–294.
3421:
3364:
3315:
3274:
3227:
3180:
3140:
3119:(3): 740–749.
3094:
3057:
3028:(3): 683–685.
3008:
2956:
2937:(8): 741–747.
2919:
2898:(3): 723–730.
2876:
2850:
2822:
2801:(4): 322–324.
2779:
2753:
2734:
2713:(9): 885–896.
2691:
2688:on 8 May 2008.
2668:
2649:
2623:
2594:(4): 683–690.
2573:
2554:(2): 674–676.
2536:
2490:
2461:
2433:
2416:
2398:
2369:(9): 892–900.
2347:
2329:
2311:
2293:
2273:
2252:
2226:
2201:
2179:
2158:(4): 312–321.
2136:
2115:
2110:"Home | ICCAC"
2101:
2089:
2071:
2059:
2046:
2040:Electric Heart
2030:
2015:
1964:
1944:
1925:(5): 324–338.
1909:
1888:(3): 343–355.
1868:
1862:Smart, Frank.
1855:
1828:
1793:
1765:
1737:
1718:(2): 232–249.
1701:
1676:
1655:(3): 841–842.
1633:
1618:
1592:
1557:(5): 630–639.
1537:
1478:
1459:
1413:
1386:(4): 619–634.
1366:
1341:
1273:
1229:
1174:
1124:
1123:
1121:
1118:
1117:
1116:
1111:
1104:
1101:
1098:
1097:
1090:
1087:
1082:
1078:
1077:
1074:
1071:
1068:
1062:
1061:
1058:
1055:
1052:
1046:
1045:
1042:
1039:
1034:
1028:
1027:
1024:
1021:
1018:
1012:
1011:
1007:
1004:
994:
988:
987:
984:
981:
976:
970:
969:
966:
963:
958:
954:
953:
949:
946:
943:
939:
938:
935:
932:
927:
921:
920:
916:
913:
908:
902:
901:
898:
895:
890:
884:
883:
880:
877:
872:
866:
865:
858:
855:
850:
849:HeartMate III
846:
845:
842:
839:
836:
830:
829:
826:
823:
818:
817:HeartMate XVE
814:
813:
810:
807:
802:
798:
797:
794:
791:
786:
782:
781:
778:
775:
772:
755:
754:
735:
733:
726:
720:
717:
691:. Some of the
685:
684:
675:
654:
615:monitoring in
562:
559:
554:kidney failure
544:
541:
531:
528:
527:
526:
522:
513:
509:
505:
492:
489:
469:
468:
464:
447:
443:
440:
436:
429:
410:
406:
402:
398:
394:
390:
384:
381:
379:
376:
366:Peter Houghton
295:Domingo Liotta
282:
279:
273:("maglev") or
243:
240:
230:
227:
115:
112:
74:
73:
66:
63:
62:
55:
49:
48:
45:
37:
36:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
4541:
4530:
4527:
4525:
4522:
4520:
4517:
4515:
4512:
4510:
4507:
4506:
4504:
4489:
4486:
4484:
4481:
4479:
4476:
4472:
4469:
4467:
4464:
4462:
4459:
4458:
4457:
4454:
4452:
4449:
4448:
4446:
4442:
4436:
4433:
4431:
4428:
4426:
4423:
4422:
4420:
4418:Organisations
4416:
4410:
4407:
4405:
4402:
4400:
4397:
4395:
4392:
4390:
4387:
4386:
4384:
4380:
4374:
4371:
4369:
4368:SAPS III
4366:
4364:
4361:
4359:
4356:
4354:
4351:
4349:
4346:
4345:
4343:
4341:
4337:
4331:
4328:
4325:
4322:
4320:
4317:
4315:
4312:
4310:
4307:
4305:
4302:
4300:
4297:
4295:
4292:
4290:
4287:
4285:
4282:
4281:
4279:
4275:
4269:
4266:
4262:
4259:
4257:
4254:
4253:
4251:
4249:
4246:
4244:
4241:
4239:
4236:
4234:
4231:
4227:
4224:
4222:
4219:
4218:
4216:
4212:
4209:
4208:
4207:
4203:
4200:
4199:
4197:
4195:
4191:
4185:
4182:
4180:
4177:
4173:
4170:
4168:
4165:
4163:
4162:Arterial line
4160:
4159:
4158:
4155:
4153:
4150:
4149:
4147:
4143:
4133:
4130:
4128:
4125:
4123:
4120:
4118:
4115:
4113:
4110:
4108:
4107:
4102:
4101:
4099:
4097:
4093:
4087:
4084:
4082:
4079:
4077:
4074:
4072:
4069:
4067:
4064:
4061:
4058:
4057:
4055:
4053:Complications
4051:
4045:
4042:
4040:
4037:
4035:
4032:
4031:
4028:
4025:
4023:
4020:
4018:
4015:
4013:
4010:
4008:
4005:
4003:
4002:Organ failure
4000:
3999:
3996:
3993:
3991:
3988:
3986:
3983:
3981:
3978:
3976:
3973:
3971:
3968:
3966:
3963:
3961:
3957:
3956:
3953:
3950:
3948:
3945:
3943:
3942:Severe sepsis
3940:
3938:
3935:
3933:
3930:
3927:
3926:
3924:
3920:
3917:
3913:
3907:
3904:
3902:
3899:
3896:
3893:
3890:
3887:
3884:
3881:
3878:
3875:
3874:
3872:
3870:General terms
3868:
3862:
3859:
3857:
3854:
3852:
3849:
3847:
3844:
3843:
3840:
3836:
3829:
3824:
3822:
3817:
3815:
3810:
3809:
3806:
3799:
3796:
3793:
3790:
3789:
3785:
3769:
3768:
3763:
3756:
3753:
3741:
3737:
3728:
3725:
3717:
3710:
3707:
3702:
3698:
3694:
3690:
3686:
3682:
3679:(6): 905–11.
3678:
3674:
3673:
3664:
3661:
3656:
3652:
3647:
3642:
3638:
3634:
3631:(3): 374–80.
3630:
3626:
3625:
3620:
3613:
3610:
3605:
3601:
3596:
3595:2027.42/99069
3591:
3587:
3583:
3579:
3575:
3568:
3561:
3558:
3553:
3549:
3545:
3541:
3537:
3533:
3532:
3524:
3521:
3516:
3512:
3508:
3504:
3501:(7): 426–37.
3500:
3496:
3495:
3487:
3484:
3479:
3475:
3470:
3465:
3461:
3457:
3453:
3449:
3445:
3441:
3440:ASAIO Journal
3437:
3430:
3428:
3426:
3422:
3417:
3413:
3408:
3403:
3399:
3395:
3391:
3387:
3383:
3379:
3375:
3368:
3365:
3360:
3356:
3352:
3348:
3343:
3338:
3334:
3330:
3326:
3319:
3316:
3311:
3307:
3302:
3297:
3293:
3289:
3285:
3278:
3275:
3270:
3266:
3262:
3258:
3254:
3250:
3246:
3242:
3238:
3231:
3228:
3223:
3219:
3215:
3211:
3207:
3203:
3199:
3195:
3191:
3184:
3181:
3176:
3172:
3168:
3164:
3160:
3156:
3155:
3147:
3145:
3141:
3136:
3132:
3127:
3122:
3118:
3114:
3113:
3108:
3101:
3099:
3095:
3090:
3086:
3082:
3078:
3074:
3070:
3069:
3061:
3058:
3053:
3049:
3045:
3041:
3036:
3031:
3027:
3023:
3019:
3012:
3009:
3004:
3000:
2996:
2992:
2987:
2982:
2978:
2974:
2970:
2963:
2961:
2957:
2952:
2948:
2944:
2940:
2936:
2932:
2931:
2923:
2920:
2915:
2911:
2906:
2901:
2897:
2893:
2892:
2887:
2880:
2877:
2865:
2861:
2854:
2851:
2846:
2842:
2838:
2834:
2826:
2823:
2818:
2814:
2809:
2804:
2800:
2796:
2795:
2790:
2783:
2780:
2767:
2763:
2757:
2754:
2749:
2745:
2738:
2735:
2730:
2726:
2721:
2716:
2712:
2708:
2707:
2702:
2695:
2692:
2687:
2683:
2679:
2672:
2669:
2664:
2660:
2653:
2650:
2634:
2627:
2624:
2619:
2615:
2610:
2605:
2601:
2597:
2593:
2589:
2585:
2577:
2574:
2569:
2565:
2561:
2557:
2553:
2549:
2548:
2540:
2537:
2532:
2526:
2507:
2500:
2494:
2491:
2486:
2482:
2478:
2477:
2472:
2465:
2462:
2447:
2440:
2438:
2434:
2429:
2423:
2421:
2417:
2412:
2408:
2402:
2399:
2394:
2390:
2385:
2380:
2376:
2372:
2368:
2364:
2363:
2358:
2351:
2348:
2343:
2339:
2333:
2330:
2325:
2321:
2315:
2312:
2307:
2303:
2297:
2294:
2289:
2288:
2283:
2277:
2274:
2269:
2268:
2263:
2256:
2253:
2240:
2236:
2230:
2227:
2222:
2218:
2217:
2212:
2205:
2202:
2197:
2193:
2189:
2183:
2180:
2175:
2171:
2166:
2161:
2157:
2153:
2152:
2147:
2140:
2137:
2132:
2131:
2126:
2119:
2116:
2111:
2105:
2102:
2098:
2093:
2090:
2085:
2081:
2075:
2072:
2068:
2063:
2060:
2056:
2050:
2047:
2042:
2041:
2034:
2031:
2026:
2019:
2016:
2011:
2007:
2002:
1997:
1993:
1989:
1985:
1981:
1980:
1975:
1968:
1965:
1960:
1959:
1954:
1948:
1945:
1940:
1936:
1932:
1928:
1924:
1920:
1913:
1910:
1904:
1899:
1895:
1891:
1887:
1883:
1879:
1872:
1869:
1865:
1859:
1856:
1851:
1847:
1843:
1839:
1832:
1829:
1824:
1820:
1816:
1812:
1808:
1804:
1797:
1794:
1782:
1775:
1769:
1766:
1754:
1753:syncardia.com
1747:
1741:
1738:
1733:
1729:
1725:
1721:
1717:
1713:
1705:
1702:
1690:
1686:
1680:
1677:
1672:
1668:
1663:
1658:
1654:
1650:
1649:
1644:
1637:
1634:
1629:
1625:
1621:
1615:
1611:
1607:
1603:
1596:
1593:
1588:
1584:
1580:
1576:
1572:
1568:
1564:
1560:
1556:
1552:
1548:
1541:
1538:
1533:
1529:
1524:
1519:
1515:
1511:
1506:
1501:
1497:
1493:
1489:
1482:
1479:
1475:
1474:Ventracor.com
1471:
1466:
1464:
1460:
1455:
1451:
1446:
1441:
1437:
1433:
1432:
1427:
1420:
1418:
1414:
1409:
1405:
1401:
1397:
1393:
1389:
1385:
1381:
1377:
1370:
1367:
1355:
1351:
1345:
1342:
1337:
1333:
1329:
1325:
1321:
1317:
1312:
1307:
1302:
1297:
1293:
1289:
1285:
1277:
1274:
1269:
1265:
1261:
1257:
1253:
1249:
1245:
1241:
1233:
1230:
1225:
1221:
1217:
1213:
1209:
1205:
1201:
1197:
1193:
1189:
1185:
1178:
1175:
1170:
1166:
1162:
1158:
1153:
1148:
1144:
1140:
1136:
1129:
1126:
1119:
1115:
1112:
1110:
1107:
1106:
1102:
1095:
1091:
1088:
1086:
1083:
1080:
1079:
1075:
1072:
1069:
1067:
1064:
1063:
1059:
1056:
1053:
1051:
1048:
1047:
1043:
1040:
1038:
1035:
1033:
1030:
1029:
1025:
1022:
1019:
1017:
1014:
1013:
1008:
1005:
1002:
998:
995:
993:
990:
989:
985:
982:
980:
977:
975:
972:
971:
967:
964:
962:
959:
957:MTIHeartLVAD
956:
955:
950:
947:
944:
941:
940:
936:
933:
931:
928:
926:
923:
922:
917:
914:
912:
909:
907:
904:
903:
899:
896:
894:
891:
889:
886:
885:
881:
878:
876:
873:
871:
868:
867:
863:
859:
856:
854:
851:
848:
847:
843:
840:
837:
835:
832:
831:
827:
824:
822:
819:
816:
815:
811:
808:
806:
803:
800:
799:
795:
792:
790:
787:
785:HeartAssist5
784:
783:
779:
776:
774:Manufacturer
773:
770:
769:
766:
765:
761:
751:
739:
734:
725:
724:
718:
716:
712:
710:
706:
702:
698:
694:
690:
682:
681:
676:
673:
672:
667:
666:
661:
660:
655:
652:
651:
646:
645:
644:Staph. aureus
641:, especially
640:
639:
638:Staphylococci
634:
633:
632:
629:
625:
624:
620:
618:
614:
611:and invasive
610:
606:
600:
599:
595:
591:
588:
584:
580:
576:
571:
570:
566:
560:
558:
555:
549:
542:
540:
538:
529:
523:
519:
514:
510:
506:
502:
501:pivotal trial
498:
497:
496:
490:
488:
486:
481:
479:
473:
465:
461:
456:
452:
448:
444:
441:
437:
433:
430:
427:
423:
419:
415:
411:
407:
403:
399:
395:
391:
387:
386:
382:
377:
375:
372:
367:
362:
360:
356:
350:
348:
343:
340:
335:
333:
329:
328:O. H. Frazier
325:
321:
317:
313:
309:
305:
301:
296:
287:
280:
278:
276:
272:
266:
264:
260:
255:
253:
249:
241:
235:
228:
226:
222:
220:
215:
213:
209:
205:
201:
198:
197:
193:
191:
186:
182:
176:
173:
169:
163:
162:
161:heart failure
158:
154:
149:
148:
144:
141:
137:
133:
132:heart disease
129:
124:
123:
119:
113:
111:
109:
105:
101:
97:
96:heart failure
93:
89:
85:
81:
70:
60:
56:
54:
50:
43:
38:
33:
30:
19:
4488:Traumatology
4389:Hemodynamics
4363:SAPS II
4330:Vasopressors
4248:Induced coma
4225:
4105:
4096:Iatrogenesis
4086:Stress ulcer
3990:Spinal shock
3947:Septic shock
3771:. Retrieved
3765:
3755:
3745:15 September
3743:. Retrieved
3739:
3727:
3709:
3676:
3670:
3663:
3628:
3622:
3612:
3577:
3573:
3560:
3535:
3529:
3523:
3498:
3492:
3486:
3443:
3439:
3381:
3377:
3367:
3332:
3328:
3318:
3291:
3287:
3277:
3244:
3240:
3230:
3197:
3193:
3183:
3161:(6): S42–7.
3158:
3152:
3116:
3110:
3072:
3066:
3060:
3025:
3021:
3011:
2976:
2972:
2934:
2928:
2922:
2895:
2889:
2879:
2867:. Retrieved
2863:
2853:
2836:
2832:
2825:
2798:
2792:
2782:
2770:. Retrieved
2756:
2748:the original
2737:
2710:
2704:
2694:
2686:the original
2671:
2663:the original
2652:
2642:10 September
2640:. Retrieved
2626:
2591:
2587:
2576:
2551:
2545:
2539:
2513:. Retrieved
2506:the original
2493:
2485:the original
2480:
2474:
2464:
2454:30 September
2452:. Retrieved
2410:
2401:
2366:
2360:
2350:
2342:the original
2332:
2324:Bio-Medicine
2323:
2314:
2296:
2287:BusinessWire
2285:
2276:
2265:
2255:
2245:15 September
2243:. Retrieved
2238:
2229:
2221:the original
2214:
2204:
2196:the original
2191:
2182:
2155:
2149:
2139:
2128:
2118:
2104:
2092:
2084:the original
2074:
2062:
2049:
2039:
2033:
2024:
2018:
1986:(3): 200–5.
1983:
1977:
1967:
1956:
1947:
1922:
1918:
1912:
1885:
1881:
1871:
1858:
1844:(8): 40–42.
1841:
1837:
1831:
1809:(4): S1–39.
1806:
1802:
1796:
1784:. Retrieved
1780:
1768:
1756:. Retrieved
1752:
1740:
1715:
1711:
1704:
1692:. Retrieved
1688:
1679:
1652:
1646:
1636:
1601:
1595:
1554:
1550:
1540:
1498:(2): 81–88.
1495:
1491:
1481:
1473:
1435:
1429:
1383:
1379:
1369:
1357:. Retrieved
1353:
1344:
1291:
1287:
1276:
1243:
1239:
1232:
1191:
1187:
1177:
1142:
1138:
1128:
942:VentrAssist
911:Jarvik Heart
893:Berlin Heart
875:Berlin Heart
834:HeartMate II
789:ReliantHeart
763:
759:
758:
745:
737:
713:
697:immune cells
693:polyurethane
686:
678:
669:
665:Enterobacter
663:
657:
648:
642:
636:
630:
626:
622:
621:
601:
597:
596:
592:
572:
568:
567:
564:
550:
546:
533:
494:
484:
482:
474:
470:
364:At one time
363:
351:
336:
292:
267:
256:
245:
223:
216:
202:
199:
195:
194:
177:
168:percutaneous
164:
150:
146:
145:
125:
121:
120:
117:
83:
79:
77:
29:
4519:Prosthetics
4471:Pulmonology
4394:Hypotension
4289:Antibiotics
4132:Dialytrauma
3975:Anaphylaxis
3247:: 265–272.
2476:Circulation
2407:"LVAD Wear"
2308:. May 2009.
1094:Jarvik 2000
906:Jarvik 2000
809:Pulsatile.
805:World Heart
650:Enterococci
609:outpatients
537:clenbuterol
426:George Noon
53:MedlinePlus
4514:Cardiology
4503:Categories
4478:Pediatrics
4461:Cardiology
4382:Physiology
4284:Analgesics
4233:Chest tube
4062:/ myopathy
4039:Polytrauma
3915:Conditions
3792:MyLVAD.com
3736:"No Heart"
3384:(10): 85.
2839:(6): S46.
2192:Insciences
2067:"VAD FAQs"
2053:Dan Baum.
1359:26 October
1311:1942/34157
1120:References
1020:HeartWare
945:Ventracor
825:Pulsatile
748:April 2015
671:Klebsiella
623:Infections
617:inpatients
575:thrombosis
518:NYHA Class
478:sternotomy
339:axial flow
204:Pacemakers
4466:Neurology
4319:Sedatives
4299:Inotropes
4145:Diagnosis
3460:1538-943X
3398:1522-6417
3351:0735-1097
3261:1878-8769
3214:1573-7322
3044:1097-685X
2995:1558-3597
2772:28 August
2515:9 January
1781:aldmd.com
1732:0897-1900
1587:221917384
1571:1873-1740
1514:2225-319X
1400:1551-7136
1336:232123771
1320:1558-3597
1268:218506853
1208:1468-201X
1161:1755-3245
1070:Thoratec
1054:Thoratec
1032:DuraHeart
1001:Medtronic
997:HeartWare
838:Thoratec
703:and some
668:species,
512:patients.
504:protocol.
485:explanted
355:pacemaker
332:Mehmet Oz
324:Eric Rose
128:ventricle
18:Heartmate
4157:Catheter
4076:Fungemia
3851:Medicine
3693:25316772
3655:24823949
3604:23506318
3552:12820735
3515:16790383
3478:29095732
3416:29043581
3359:35300822
3269:31493616
3222:37145271
3175:12820734
3135:21352991
3089:18442558
3052:27210469
3003:35300822
2951:17707178
2914:10215217
2869:3 August
2817:19608029
2729:17761592
2618:34888681
2568:22269746
2525:cite web
2393:25618530
2174:19608028
2010:19808290
1939:16683949
1823:20181499
1786:23 April
1758:23 April
1694:23 April
1671:17320612
1628:13070912
1579:32971112
1532:32309155
1454:17079761
1408:34511210
1328:33663742
1260:32367538
1216:25948420
1169:35150240
1103:See also
930:MicroMed
853:Thoratec
821:Thoratec
801:Novacor
674:species)
579:clotting
569:Bleeding
418:Micromed
342:impeller
147:Duration
86:) is an
4483:Surgery
3701:7560734
3646:4081474
3469:5920737
3407:5645430
3310:8873737
2609:9026212
2384:4488905
2001:3437761
1890:Bibcode
1523:7160624
1224:6958416
771:Device
738:updated
683:species
680:Candida
543:REMATCH
508:months.
281:History
172:Impella
3958:Other
3937:Sepsis
3891:(PICU)
3885:(NICU)
3773:8 July
3699:
3691:
3653:
3643:
3602:
3550:
3513:
3476:
3466:
3458:
3414:
3404:
3396:
3357:
3349:
3308:
3267:
3259:
3220:
3212:
3173:
3133:
3087:
3050:
3042:
3001:
2993:
2949:
2912:
2815:
2727:
2616:
2606:
2566:
2391:
2381:
2172:
2043:. PBS.
2008:
1998:
1937:
1821:
1730:
1669:
1626:
1616:
1585:
1577:
1569:
1530:
1520:
1512:
1452:
1406:
1398:
1334:
1326:
1318:
1266:
1258:
1222:
1214:
1206:
1167:
1159:
1081:FiVAD
1037:Terumo
701:fungal
587:fibrin
401:aorta.
261:or an
229:Design
59:007268
4326:drugs
4277:Drugs
3960:shock
3897:(CCU)
3879:(ICU)
3719:(PDF)
3697:S2CID
3570:(PDF)
2636:(PDF)
2509:(PDF)
2502:(PDF)
2449:(PDF)
1777:(PDF)
1749:(PDF)
1624:S2CID
1583:S2CID
1332:S2CID
1264:S2CID
1220:S2CID
999:(now
870:Incor
777:Type
705:viral
530:HARPS
463:LVAD.
409:skin.
397:life.
393:heal.
389:N.J..
347:pulse
248:pumps
242:Pumps
212:blood
92:heart
67:[
4373:SOFA
4358:PIM2
4204:and
4044:Coma
3932:SIRS
3775:2021
3747:2009
3732:sale
3689:PMID
3651:PMID
3600:PMID
3548:PMID
3511:PMID
3474:PMID
3456:ISSN
3412:PMID
3394:ISSN
3355:PMID
3347:ISSN
3306:PMID
3265:PMID
3257:ISSN
3218:PMID
3210:ISSN
3171:PMID
3131:PMID
3085:PMID
3048:PMID
3040:ISSN
2999:PMID
2991:ISSN
2947:PMID
2910:PMID
2871:2009
2813:PMID
2774:2009
2725:PMID
2644:2009
2614:PMID
2564:PMID
2531:link
2517:2018
2456:2021
2389:PMID
2247:2009
2170:PMID
2006:PMID
1935:PMID
1819:PMID
1788:2018
1760:2018
1728:ISSN
1696:2018
1667:PMID
1614:ISBN
1575:PMID
1567:ISSN
1528:PMID
1510:ISSN
1450:PMID
1404:PMID
1396:ISSN
1361:2023
1324:PMID
1316:ISSN
1256:PMID
1212:PMID
1204:ISSN
1165:PMID
1157:ISSN
1016:MVAD
992:HVAD
446:III.
424:and
412:The
330:and
246:The
3681:doi
3641:PMC
3633:doi
3590:hdl
3582:doi
3540:doi
3503:doi
3464:PMC
3448:doi
3402:PMC
3386:doi
3337:doi
3296:doi
3292:112
3249:doi
3245:132
3202:doi
3163:doi
3121:doi
3077:doi
3030:doi
3026:152
2981:doi
2939:doi
2900:doi
2841:doi
2803:doi
2715:doi
2711:357
2604:PMC
2596:doi
2556:doi
2481:116
2379:PMC
2371:doi
2160:doi
1996:PMC
1988:doi
1927:doi
1898:doi
1846:doi
1811:doi
1720:doi
1657:doi
1653:133
1606:doi
1559:doi
1518:PMC
1500:doi
1440:doi
1436:355
1388:doi
1306:hdl
1296:doi
1248:doi
1196:doi
1192:101
1147:doi
1143:118
607:in
439:PhD
306:of
84:VAD
4505::
3764:.
3738:.
3695:.
3687:.
3675:.
3649:.
3639:.
3627:.
3621:.
3598:.
3588:.
3578:22
3576:.
3572:.
3546:.
3536:75
3534:.
3509:.
3497:.
3472:.
3462:.
3454:.
3444:64
3442:.
3438:.
3424:^
3410:.
3400:.
3392:.
3382:19
3380:.
3376:.
3353:.
3345:.
3333:79
3331:.
3327:.
3304:.
3290:.
3286:.
3263:.
3255:.
3243:.
3239:.
3216:.
3208:.
3198:28
3196:.
3192:.
3169:.
3159:75
3157:.
3143:^
3129:.
3117:91
3115:.
3109:.
3097:^
3083:.
3073:85
3071:.
3046:.
3038:.
3024:.
3020:.
2997:.
2989:.
2977:79
2975:.
2971:.
2959:^
2945:.
2935:50
2933:.
2908:.
2896:67
2894:.
2888:.
2862:.
2837:15
2835:.
2811:.
2799:54
2797:.
2791:.
2764:.
2723:.
2709:.
2703:.
2680:.
2612:.
2602:.
2592:34
2590:.
2586:.
2562:.
2552:93
2550:.
2527:}}
2523:{{
2479:.
2473:.
2436:^
2419:^
2409:.
2387:.
2377:.
2367:65
2365:.
2359:.
2322:.
2304:.
2284:.
2264:.
2237:.
2213:.
2190:.
2168:.
2156:54
2154:.
2148:.
2127:.
2004:.
1994:.
1982:.
1976:.
1955:.
1933:.
1923:30
1921:.
1896:.
1884:.
1880:.
1842:14
1840:.
1817:.
1807:29
1805:.
1779:.
1751:.
1726:.
1716:25
1714:.
1687:.
1665:.
1651:.
1645:.
1622:.
1612:.
1581:.
1573:.
1565:.
1555:63
1553:.
1549:.
1526:.
1516:.
1508:.
1494:.
1490:.
1472:.
1462:^
1448:.
1434:.
1428:.
1416:^
1402:.
1394:.
1384:17
1382:.
1378:.
1352:.
1330:.
1322:.
1314:.
1304:.
1292:77
1290:.
1286:.
1262:.
1254:.
1244:44
1242:.
1218:.
1210:.
1202:.
1190:.
1186:.
1163:.
1155:.
1141:.
1137:.
1003:)
711:.
662:,
647:,
619:.
326:,
277:.
106:,
102:,
78:A
3827:e
3820:t
3813:v
3777:.
3749:.
3703:.
3683::
3677:7
3657:.
3635::
3629:7
3606:.
3592::
3584::
3554:.
3542::
3517:.
3505::
3499:6
3480:.
3450::
3418:.
3388::
3361:.
3339::
3312:.
3298::
3271:.
3251::
3224:.
3204::
3177:.
3165::
3137:.
3123::
3091:.
3079::
3054:.
3032::
3005:.
2983::
2953:.
2941::
2916:.
2902::
2873:.
2847:.
2843::
2819:.
2805::
2776:.
2731:.
2717::
2646:.
2620:.
2598::
2570:.
2558::
2533:)
2519:.
2458:.
2413:.
2395:.
2373::
2270:.
2249:.
2176:.
2162::
2133:.
2112:.
2012:.
1990::
1984:1
1941:.
1929::
1906:.
1900::
1892::
1886:2
1852:.
1848::
1825:.
1813::
1790:.
1762:.
1734:.
1722::
1698:.
1673:.
1659::
1630:.
1608::
1589:.
1561::
1534:.
1502::
1496:9
1456:.
1442::
1410:.
1390::
1363:.
1338:.
1308::
1298::
1270:.
1250::
1226:.
1198::
1171:.
1149::
750:)
746:(
740:.
653:)
82:(
71:]
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.