148:
232:
254:
Selectivity can be a challenge in nitrations because as a rule more than one compound may result but only one is desired, so alternative products act as contaminants or are simply wasted. Accordingly, it is desirable to design syntheses with suitable selectivity; for example, by controlling the
565:
Esteves, P. M.; Carneiro, J. W. M.; Cardoso, S. P.; Barbosa, A. G. H.; Laali, K. K.; Rasul, G.; Prakash, G. K. S.; e Olah, G. A. (2003). "Unified
Mechanism Concept of Electrophilic Aromatic Nitration Revisited: Convergence of Computational Results and Experimental Data".
365:
followed by the actual nitration. Because the amide is a regular activating group the products formed are the para and ortho isomers. Heating the reaction mixture is sufficient to hydrolyze the amide back to the nitrated aniline.
4057:
602:
Queiroz, J. F.; Carneiro, J. W. M.; Sabino A. A.; Sparapan, R.; Eberlin, M. N.; Esteves, P. M. (2006). "Electrophilic
Aromatic Nitration: Understanding Its Mechanism and Substituent Effects".
135:
or another nitrogen atom), whereas in nitrate esters (also called organic nitrates), the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom (nitrito group).
3173:
638:
Bordwell, F. G.; Garbisch, Edgar W. (July 1960). "Nitrations with Acetyl
Nitrate. I. The Nature of the Nitrating Agent and the Mechanism of Reaction with Simple Alkenes".
3118:
3886:
3228:
138:
There are many major industrial applications of nitration in the strict sense; the most important by volume are for the production of nitroaromatic compounds such as
3378:
2012:
4107:
3881:
2983:
1707:
2753:
904:
3553:
1497:
747:
357:), which may explain this reaction product distribution. According to another source, a more controlled nitration of aniline starts with the formation of
3648:
1752:
3628:
3123:
2290:
2171:
1727:
3718:
421:
3473:
940:
2305:
278:
groups have an electron-withdrawing effect. Such groups deactivate (slow) the reaction and directs the electrophilic nitronium ion to attack the
3951:
3408:
3901:
3513:
3493:
3453:
2260:
810:
Perrin, C. L.; Skinner, G. A. (1971). "Directive effects in electrophilic aromatic substitution ("ipso factors"). Nitration of haloanisoles".
4047:
3972:
3856:
2468:
1872:
1203:
4042:
3871:
3528:
3383:
3013:
2858:
2095:
3218:
2708:
2383:
4122:
3906:
2928:
370:
267:
219:
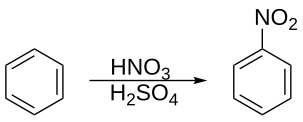
210:, also effects nitration without the need for the mixed acid. In mixed-acid syntheses sulfuric acid is not consumed and hence acts as a
3483:
4117:
3831:
3693:
3448:
495:
4007:
3478:
3393:
3363:
3343:
3208:
3203:
2578:
2503:
2146:
2100:
1967:
1228:
3946:
4112:
4072:
4022:
3498:
3248:
3178:
1667:
3708:
3313:
2206:
1927:
3698:
1238:
231:
3866:
3623:
3573:
2363:
2295:
2186:
1762:
1517:
1442:
1223:
4206:
3578:
3388:
2863:
2773:
897:
4152:
3936:
3876:
3278:
3253:
3163:
2743:
2623:
1657:
1153:
4037:
3523:
3318:
1587:
4142:
3728:
3238:
2748:
2693:
2538:
2498:
2330:
2085:
1802:
1652:
4102:
3663:
3618:
3108:
2963:
405:
was first used by Perrin and
Skinner in 1971, in an investigation into chloroanisole nitration. In one protocol, 4-chloro-
4137:
4052:
3911:
3826:
3723:
2798:
2453:
2121:
1532:
1093:
4201:
4027:
4002:
3987:
3683:
3548:
3503:
3268:
2813:
2663:
1877:
1557:
1502:
4032:
3977:
3508:
2923:
2638:
2633:
2126:
1942:
1932:
1647:
1507:
1457:
1452:
1427:
1333:
4087:
3688:
3608:
3223:
3188:
3033:
2458:
2418:
2315:
2090:
1842:
1787:
1387:
1098:
1088:
1063:
207:
4062:
3763:
3568:
3003:
2568:
2543:
2483:
2075:
1782:
1432:
1617:
3353:
2888:
2340:
1562:
1527:
1123:
1058:
890:
398:
3921:
3543:
2603:
2528:
2052:
1887:
1572:
1348:
1308:
1053:
4162:
4067:
3801:
3773:
3743:
3658:
3588:
3518:
3438:
3338:
3298:
2993:
2613:
1912:
1907:
1369:
1233:
458:
4127:
3997:
3861:
3703:
3563:
3083:
2057:
1607:
1567:
1318:
4017:
3613:
3583:
3458:
3413:
3243:
3153:
2968:
2958:
2788:
2345:
2285:
2250:
2037:
1997:
1772:
1642:
1158:
1148:
1078:
744:
239:
3593:
2573:
1597:
1143:
1023:
3806:
4097:
3956:
3748:
3673:
3653:
3373:
3323:
3183:
3148:
3088:
3018:
2320:
2300:
2032:
1952:
1847:
1807:
1777:
1712:
1582:
1492:
1482:
1358:
1068:
432:
218:, the reaction is conducted at a warm temperature, not exceeding 50 °C. The process is one example of
3836:
3558:
3308:
3288:
3263:
3213:
3128:
3103:
3058:
3028:
3008:
2978:
2943:
2898:
2873:
2848:
2733:
2658:
2438:
2131:
2067:
1867:
1592:
1512:
1198:
1173:
950:
945:
279:
4172:
2918:
1542:
3758:
3713:
3428:
3398:
3368:
3303:
3283:
3198:
3193:
3158:
3113:
3098:
3093:
3073:
3063:
2998:
2988:
2868:
2388:
2191:
1767:
1722:
1552:
1288:
1008:
970:
175:
171:
147:
1218:
1213:
3811:
3941:
3891:
3841:
3821:
3668:
3643:
3358:
3348:
3233:
3048:
3043:
2973:
2758:
2558:
2518:
2448:
2413:
2368:
2335:
2201:
2176:
2156:
1977:
1937:
1897:
1862:
1792:
1547:
1417:
1392:
930:
154:
Nitration reactions are notably used for the production of explosives, for example the conversion of
4157:
4147:
4132:
3778:
3753:
3738:
3733:
3463:
3418:
3403:
3293:
3273:
3168:
3053:
3038:
2883:
2828:
2818:
2808:
2783:
2548:
2423:
2398:
2310:
2166:
2151:
2136:
1992:
1957:
1902:
1672:
1522:
1467:
1338:
1253:
1113:
1038:
378:
100:
84:
1183:
353:) exists in equilibrium with the more abundant but less reactive (deactivated) anilinium ion (ArNH
3896:
3846:
3816:
3678:
3468:
3258:
3143:
3078:
3068:
2833:
2763:
2728:
2723:
2703:
2698:
2643:
2553:
2403:
2265:
2255:
2161:
1947:
1892:
1822:
1742:
1637:
1537:
1472:
1397:
1243:
1108:
1043:
551:
271:
1028:
3633:
2953:
2838:
2803:
2768:
2713:
2668:
2628:
2583:
2563:
2513:
2508:
2478:
2463:
2373:
2280:
2216:
2181:
2007:
1882:
1757:
1682:
1662:
1577:
1412:
1407:
1353:
1263:
1168:
1128:
1083:
965:
960:
925:
868:
792:
722:
694:
620:
584:
568:
529:
491:
92:
46:
4167:
4012:
3982:
3926:
3851:
3783:
3538:
3488:
3333:
3138:
2913:
2908:
2853:
2843:
2618:
2428:
2408:
2378:
2275:
2211:
2196:
2027:
1982:
1972:
1962:
1857:
1837:
1832:
1817:
1812:
1692:
1687:
1627:
1612:
1602:
1447:
1437:
1303:
1293:
1283:
1193:
1188:
1163:
1103:
955:
914:
858:
850:
819:
784:
670:
647:
612:
576:
483:
453:
362:
303:
69:
54:
4077:
3768:
3603:
3598:
2893:
2878:
2823:
2778:
2738:
2688:
2653:
2648:
2593:
2588:
2523:
2473:
2393:
2221:
2105:
2080:
2042:
2017:
2002:
1987:
1922:
1797:
1747:
1737:
1717:
1677:
1487:
1477:
1462:
1258:
1178:
1003:
998:
751:
448:
167:
1048:
1018:
839:"Pd-Catalyzed Conversion of Aryl Chlorides, Triflates, and Nonaflates to Nitroaromatics"
4082:
3992:
3931:
3023:
2933:
2903:
2678:
2533:
2270:
2047:
1917:
1732:
1702:
1402:
1298:
1073:
935:
863:
838:
520:
410:
275:
243:
159:
349:-nitroaniline isomers. In this reaction the fast-reacting and activating aniline (ArNH
4195:
4092:
3793:
3638:
3533:
3328:
2718:
2683:
2673:
2608:
2598:
2488:
2325:
2141:
1852:
1827:
1697:
1343:
1328:
1313:
1208:
1138:
1118:
1033:
604:
338:
263:
195:
191:
124:
96:
73:
31:
186:
Typical nitration syntheses apply so-called "mixed acid", a mixture of concentrated
3133:
2493:
2245:
2022:
1622:
1422:
1273:
1268:
1133:
988:
414:
139:
1632:
1278:
1248:
1013:
717:
689:
524:
382:
358:
334:
299:
187:
88:
58:
35:
3916:
3443:
2793:
882:
256:
674:
487:
155:
39:
872:
796:
788:
624:
588:
72:. The term also is applied incorrectly to the different process of forming
1323:
993:
765:
Peter Taylor, Royal
Society of Chemistry (Great Britain), Open University
394:
311:
283:
211:
120:
17:
823:
651:
238:
Alternative mechanisms have also been proposed, including one involving
983:
374:
330:
223:
215:
163:
104:
854:
616:
580:
315:
291:
132:
128:
323:
319:
307:
295:
287:
27:
Chemical reaction which adds a nitro (–NO₂) group onto a molecule
214:
as well as an absorbent for water. In the case of nitration of
1367:
886:
206:. This active ingredient, which can be isolated in the case of
775:
Prakash, G.; Mathew, T. (2010). "Ipso-Nitration of Arenes".
178:. Millions of tons of nitroaromatics are produced annually.
341:, according to one source, results in a 50/50 mixture of
4058:
Erlenmeyer–Plöchl azlactone and amino-acid synthesis
3965:
3792:
3427:
2942:
2437:
2354:
2234:
2114:
2066:
1376:
282:. Deactivating meta-directing substituents include
777:Angewandte Chemie International Edition in English
478:Gerald Booth (2007). "Nitro Compounds, Aromatic".
3119:Divinylcyclopropane-cycloheptadiene rearrangement
259:can be selectively trinitrated or tetranitrated.
222:, which involves the attack by the electron-rich
665:Louw, Robert (15 April 2001). "Acetyl Nitrate".
170:(TNT). However, they are of wide importance as
3379:Thermal rearrangement of aromatic hydrocarbons
2013:Thermal rearrangement of aromatic hydrocarbons
667:Encyclopedia of Reagents for Organic Synthesis
480:Ullmann's Encyclopedia of Industrial Chemistry
262:The substituents on aromatic rings affect the
4108:Lectka enantioselective beta-lactam synthesis
898:
8:
3887:Inverse electron-demand Diels–Alder reaction
1708:Heterogeneous metal catalyzed cross-coupling
3229:Lobry de Bruyn–Van Ekenstein transformation
3789:
2063:
1364:
905:
891:
883:
401:nitration may also take place. The phrase
146:
3719:Petrenko-Kritschenko piperidone synthesis
3174:Fritsch–Buttenberg–Wiechell rearrangement
862:
246:had also been used as a nitration agent.
3882:Intramolecular Diels–Alder cycloaddition
843:Journal of the American Chemical Society
812:Journal of the American Chemical Society
640:Journal of the American Chemical Society
552:"Nitration of benzene and methylbenzene"
99:). The difference between the resulting
79:
64:
470:
3902:Metal-centered cycloaddition reactions
3554:Debus–Radziszewski imidazole synthesis
1498:Bodroux–Chichibabin aldehyde synthesis
510:John McMurry Organic Chemistry 2nd Ed.
4048:Diazoalkane 1,3-dipolar cycloaddition
3952:Vinylcyclopropane (5+2) cycloaddition
3857:Diazoalkane 1,3-dipolar cycloaddition
3629:Hurd–Mori 1,2,3-thiadiazole synthesis
3124:Dowd–Beckwith ring-expansion reaction
2291:Hurd–Mori 1,2,3-thiadiazole synthesis
1204:LFER solvent coefficients (data page)
525:"Benzonitrile, 2-methyl-3,5-dinitro-"
326:resulting in para and ortho isomers.
7:
2859:Sharpless asymmetric dihydroxylation
2096:Methoxymethylenetriphenylphosphorane
123:atom in nitro compounds is directly
2984:Allen–Millar–Trippett rearrangement
268:electrophilic aromatic substitution
220:electrophilic aromatic substitution
4123:Nitrone-olefin (3+2) cycloaddition
4118:Niementowski quinazoline synthesis
3907:Nitrone-olefin (3+2) cycloaddition
3832:Azide-alkyne Huisgen cycloaddition
3694:Niementowski quinazoline synthesis
3449:Azide-alkyne Huisgen cycloaddition
2754:Meerwein–Ponndorf–Verley reduction
2306:Leimgruber–Batcho indole synthesis
688:E. O. Woolfolk and Milton Orchin.
420:in the presence of 0.5 mol%
302:. Nitration can be accelerated by
202:), which is the active species in
25:
3947:Trimethylenemethane cycloaddition
3649:Johnson–Corey–Chaykovsky reaction
3514:Cadogan–Sundberg indole synthesis
3494:Bohlmann–Rahtz pyridine synthesis
3454:Baeyer–Emmerling indole synthesis
2261:Cadogan–Sundberg indole synthesis
1753:Johnson–Corey–Chaykovsky reaction
431:, a biarylphosphine ligand and a
4043:Cook–Heilbron thiazole synthesis
3872:Hexadehydro Diels–Alder reaction
3699:Niementowski quinoline synthesis
3529:Cook–Heilbron thiazole synthesis
3474:Bischler–Möhlau indole synthesis
3384:Tiffeneau–Demjanov rearrangement
3014:Baker–Venkataraman rearrangement
2172:Horner–Wadsworth–Emmons reaction
1843:Mizoroki-Heck vs. Reductive Heck
1728:Horner–Wadsworth–Emmons reaction
1239:Neighbouring group participation
230:
3579:Fiesselmann thiophene synthesis
3409:Westphalen–Lettré rearrangement
3389:Vinylcyclopropane rearrangement
3219:Kornblum–DeLaMare rearrangement
2864:Epoxidation of allylic alcohols
2774:Noyori asymmetric hydrogenation
2709:Kornblum–DeLaMare rearrangement
2384:Gallagher–Hollander degradation
837:Fors, B.; Buchwald, S. (2009).
716:Melvin S. Newman and H. Boden.
4038:Chichibabin pyridine synthesis
3524:Chichibabin pyridine synthesis
3484:Blum–Ittah aziridine synthesis
3319:Ring expansion and contraction
1588:Cross dehydrogenative coupling
718:"2,4,5,7-Tetranitrofluorenone"
409:-butylbenzene is reacted with
1:
4008:Bischler–Napieralski reaction
3966:Heterocycle forming reactions
3619:Hemetsberger indole synthesis
3479:Bischler–Napieralski reaction
3394:Wagner–Meerwein rearrangement
3364:Sommelet–Hauser rearrangement
3344:Seyferth–Gilbert homologation
3209:Ireland–Claisen rearrangement
3204:Hofmann–Martius rearrangement
2964:2,3-sigmatropic rearrangement
2579:Corey–Winter olefin synthesis
2504:Barton–McCombie deoxygenation
2147:Corey–Winter olefin synthesis
2101:Seyferth–Gilbert homologation
1968:Seyferth–Gilbert homologation
4113:Lehmstedt–Tanasescu reaction
4073:Gabriel–Colman rearrangement
4028:Bucherer carbazole synthesis
4023:Borsche–Drechsel cyclization
4003:Bernthsen acridine synthesis
3988:Bamberger triazine synthesis
3973:Algar–Flynn–Oyamada reaction
3684:Nazarov cyclization reaction
3549:De Kimpe aziridine synthesis
3504:Bucherer carbazole synthesis
3499:Borsche–Drechsel cyclization
3269:Nazarov cyclization reaction
3249:Meyer–Schuster rearrangement
3179:Gabriel–Colman rearrangement
2929:Wolffenstein–Böters reaction
2814:Reduction of nitro compounds
2664:Grundmann aldehyde synthesis
2469:Algar–Flynn–Oyamada reaction
1878:Olefin conversion technology
1873:Nozaki–Hiyama–Kishi reaction
1668:Gabriel–Colman rearrangement
1558:Claisen-Schmidt condensation
1503:Bouveault aldehyde synthesis
377:reacts with nitric acid and
371:Wolffenstein–Böters reaction
194:. This mixture produces the
4088:Hantzsch pyridine synthesis
3867:Enone–alkene cycloadditions
3689:Nenitzescu indole synthesis
3609:Hantzsch pyridine synthesis
3574:Ferrario–Ackermann reaction
3224:Kowalski ester homologation
3189:Halogen dance rearrangement
3034:Benzilic acid rearrangement
2459:Akabori amino-acid reaction
2419:Von Braun amide degradation
2364:Barbier–Wieland degradation
2316:Nenitzescu indole synthesis
2296:Kharasch–Sosnovsky reaction
2187:Julia–Kocienski olefination
2091:Kowalski ester homologation
1788:Kowalski ester homologation
1763:Julia–Kocienski olefination
1518:Cadiot–Chodkiewicz coupling
1443:Aza-Baylis–Hillman reaction
1388:Acetoacetic ester synthesis
1099:Dynamic binding (chemistry)
1089:Conrotatory and disrotatory
1064:Charge remote fragmentation
732:, vol. 5, p. 1029
208:nitronium tetrafluoroborate
4223:
4153:Robinson–Gabriel synthesis
4103:Kröhnke pyridine synthesis
3937:Retro-Diels–Alder reaction
3877:Imine Diels–Alder reaction
3664:Kröhnke pyridine synthesis
3279:Newman–Kwart rearrangement
3254:Mislow–Evans rearrangement
3164:Fischer–Hepp rearrangement
3109:Di-π-methane rearrangement
2889:Stephen aldehyde synthesis
2624:Eschweiler–Clarke reaction
2341:Williamson ether synthesis
1658:Fujiwara–Moritani reaction
1563:Combes quinoline synthesis
1528:Carbonyl olefin metathesis
1229:More O'Ferrall–Jencks plot
1154:Grunwald–Winstein equation
1124:Electron-withdrawing group
1059:Catalytic resonance theory
704:, vol. 3, p. 837
690:"2,4,7-Trinitrofluorenone"
539:, vol. 5, p. 480
57:for the introduction of a
29:
4163:Urech hydantoin synthesis
4143:Pomeranz–Fritsch reaction
4068:Fischer oxazole synthesis
3802:1,3-Dipolar cycloaddition
3774:Urech hydantoin synthesis
3744:Reissert indole synthesis
3729:Pomeranz–Fritsch reaction
3659:Knorr quinoline synthesis
3589:Fischer oxazole synthesis
3519:Camps quinoline synthesis
3439:1,3-Dipolar cycloaddition
3339:Semipinacol rearrangement
3314:Ramberg–Bäcklund reaction
3299:Piancatelli rearrangement
3239:McFadyen–Stevens reaction
2994:Alpha-ketol rearrangement
2749:McFadyen–Stevens reaction
2694:Kiliani–Fischer synthesis
2614:Elbs persulfate oxidation
2539:Bouveault–Blanc reduction
2499:Baeyer–Villiger oxidation
2331:Schotten–Baumann reaction
2207:Ramberg–Bäcklund reaction
2086:Kiliani–Fischer synthesis
1928:Ramberg–Bäcklund reaction
1913:Pinacol coupling reaction
1908:Piancatelli rearrangement
1803:Liebeskind–Srogl coupling
1653:Fujimoto–Belleau reaction
1370:List of organic reactions
1234:Negative hyperconjugation
979:
921:
459:Reactive nitrogen species
4138:Pictet–Spengler reaction
4053:Einhorn–Brunner reaction
4018:Boger pyridine synthesis
3912:Oxo-Diels–Alder reaction
3827:Aza-Diels–Alder reaction
3724:Pictet–Spengler reaction
3624:Hofmann–Löffler reaction
3614:Hegedus indole synthesis
3584:Fischer indole synthesis
3459:Bartoli indole synthesis
3414:Willgerodt rearrangement
3244:McLafferty rearrangement
3154:Ferrier carbocyclization
2969:2,3-Wittig rearrangement
2959:1,2-Wittig rearrangement
2799:Parikh–Doering oxidation
2789:Oxygen rebound mechanism
2454:Adkins–Peterson reaction
2346:Yamaguchi esterification
2286:Hegedus indole synthesis
2251:Bartoli indole synthesis
2122:Bamford–Stevens reaction
2038:Weinreb ketone synthesis
1998:Stork enamine alkylation
1773:Knoevenagel condensation
1643:Ferrier carbocyclization
1533:Castro–Stephens coupling
1159:Hammett acidity function
1149:Free-energy relationship
1094:Curtin–Hammett principle
1079:Conformational isomerism
675:10.1002/047084289X.ra032
488:10.1002/14356007.a17_411
329:The direct nitration of
240:single electron transfer
30:Not to be confused with
4098:Knorr pyrrole synthesis
4033:Bucherer–Bergs reaction
3978:Allan–Robinson reaction
3957:Wagner-Jauregg reaction
3749:Ring-closing metathesis
3674:Larock indole synthesis
3654:Knorr pyrrole synthesis
3509:Bucherer–Bergs reaction
3374:Stieglitz rearrangement
3354:Skattebøl rearrangement
3324:Ring-closing metathesis
3184:Group transfer reaction
3149:Favorskii rearrangement
3089:Cornforth rearrangement
3019:Bamberger rearrangement
2924:Wolff–Kishner reduction
2744:Markó–Lam deoxygenation
2639:Fleming–Tamao oxidation
2634:Fischer–Tropsch process
2321:Oxymercuration reaction
2301:Knorr pyrrole synthesis
2127:Barton–Kellogg reaction
2033:Wagner-Jauregg reaction
1953:Ring-closing metathesis
1943:Reimer–Tiemann reaction
1933:Rauhut–Currier reaction
1848:Nef isocyanide reaction
1808:Malonic ester synthesis
1778:Knorr pyrrole synthesis
1713:High dilution principle
1648:Friedel–Crafts reaction
1583:Cross-coupling reaction
1508:Bucherer–Bergs reaction
1493:Blanc chloromethylation
1483:Blaise ketone synthesis
1458:Baylis–Hillman reaction
1453:Barton–Kellogg reaction
1428:Allan–Robinson reaction
1334:Woodward–Hoffmann rules
1069:Charge-transfer complex
763:Mechanism and synthesis
482:. Weinheim: Wiley-VCH.
433:phase-transfer catalyst
103:of nitro compounds and
4207:Substitution reactions
4063:Feist–Benary synthesis
3837:Bradsher cycloaddition
3807:4+4 Photocycloaddition
3764:Simmons–Smith reaction
3709:Paternò–Büchi reaction
3569:Feist–Benary synthesis
3559:Dieckmann condensation
3309:Pummerer rearrangement
3289:Oxy-Cope rearrangement
3264:Myers allene synthesis
3214:Jacobsen rearrangement
3129:Electrocyclic reaction
3104:Demjanov rearrangement
3059:Buchner ring expansion
3029:Beckmann rearrangement
3009:Aza-Cope rearrangement
3004:Arndt–Eistert reaction
2979:Alkyne zipper reaction
2899:Transfer hydrogenation
2874:Sharpless oxyamination
2849:Selenoxide elimination
2734:Lombardo methylenation
2659:Griesbaum coozonolysis
2569:Corey–Itsuno reduction
2544:Boyland–Sims oxidation
2484:Angeli–Rimini reaction
2132:Boord olefin synthesis
2076:Arndt–Eistert reaction
2068:Homologation reactions
1868:Nitro-Mannich reaction
1783:Kolbe–Schmitt reaction
1593:Cross-coupling partner
1513:Buchner ring expansion
1433:Arndt–Eistert reaction
1199:Kinetic isotope effect
946:Rearrangement reaction
789:10.1002/anie.200906940
280:aromatic meta position
172:chemical intermediates
162:and the conversion of
53:is a general class of
3922:Pauson–Khand reaction
3759:Sharpless epoxidation
3714:Pechmann condensation
3594:Friedländer synthesis
3544:Davis–Beirut reaction
3399:Wallach rearrangement
3369:Stevens rearrangement
3304:Pinacol rearrangement
3284:Overman rearrangement
3199:Hofmann rearrangement
3194:Hayashi rearrangement
3159:Ferrier rearrangement
3114:Dimroth rearrangement
3099:Curtius rearrangement
3094:Criegee rearrangement
3074:Claisen rearrangement
3064:Carroll rearrangement
2999:Amadori rearrangement
2989:Allylic rearrangement
2869:Sharpless epoxidation
2604:Dess–Martin oxidation
2529:Bohn–Schmidt reaction
2389:Hofmann rearrangement
2192:Kauffmann olefination
2115:Olefination reactions
2053:Wurtz–Fittig reaction
1888:Palladium–NHC complex
1768:Kauffmann olefination
1723:Homologation reaction
1573:Corey–House synthesis
1553:Claisen rearrangement
1349:Yukawa–Tsuno equation
1309:Swain–Lupton equation
1289:Spherical aromaticity
1224:Möbius–Hückel concept
1009:Aromatic ring current
971:Substitution reaction
523:and Stephen J. Kuhn.
393:With aryl chlorides,
255:reaction conditions,
4128:Paal–Knorr synthesis
3998:Barton–Zard reaction
3942:Staudinger synthesis
3892:Ketene cycloaddition
3862:Diels–Alder reaction
3842:Cheletropic reaction
3822:Alkyne trimerisation
3704:Paal–Knorr synthesis
3669:Kulinkovich reaction
3644:Jacobsen epoxidation
3564:Diels–Alder reaction
3359:Smiles rearrangement
3349:Sigmatropic reaction
3234:Lossen rearrangement
3084:Corey–Fuchs reaction
3049:Boekelheide reaction
3044:Bergmann degradation
2974:Achmatowicz reaction
2759:Methionine sulfoxide
2559:Clemmensen reduction
2519:Bergmann degradation
2449:Acyloin condensation
2414:Strecker degradation
2369:Bergmann degradation
2336:Ullmann condensation
2202:Peterson olefination
2177:Hydrazone iodination
2157:Elimination reaction
2058:Zincke–Suhl reaction
1978:Sonogashira coupling
1938:Reformatsky reaction
1898:Peterson olefination
1863:Nierenstein reaction
1793:Kulinkovich reaction
1608:Diels–Alder reaction
1568:Corey–Fuchs reaction
1548:Claisen condensation
1418:Alkyne trimerisation
1393:Acyloin condensation
1359:Σ-bishomoaromaticity
1319:Thorpe–Ingold effect
931:Elimination reaction
101:molecular structures
4202:Nitration reactions
4148:Prilezhaev reaction
4133:Pellizzari reaction
3812:(4+3) cycloaddition
3779:Van Leusen reaction
3754:Robinson annulation
3739:Pschorr cyclization
3734:Prilezhaev reaction
3464:Bergman cyclization
3419:Wolff rearrangement
3404:Weerman degradation
3294:Pericyclic reaction
3274:Neber rearrangement
3169:Fries rearrangement
3054:Brook rearrangement
3039:Bergman cyclization
2884:Staudinger reaction
2829:Rosenmund reduction
2819:Reductive amination
2784:Oppenauer oxidation
2574:Corey–Kim oxidation
2549:Cannizzaro reaction
2424:Weerman degradation
2399:Isosaccharinic acid
2311:Mukaiyama hydration
2167:Hofmann elimination
2152:Dehydrohalogenation
2137:Chugaev elimination
1958:Robinson annulation
1903:Pfitzinger reaction
1673:Gattermann reaction
1618:Wulff–Dötz reaction
1598:Dakin–West reaction
1523:Carbonyl allylation
1468:Bergman cyclization
1254:Kennedy J. P. Orton
1174:Hammond's postulate
1144:Flippin–Lodge angle
1114:Electromeric effect
1039:Beta-silicon effect
1024:Baker–Nathan effect
849:(36): 12898–12899.
824:10.1021/ja00743a015
652:10.1021/ja01499a029
435:to provide 4-nitro-
379:mercury(II) nitrate
272:Deactivating groups
3897:McCormack reaction
3847:Conia-ene reaction
3679:Madelung synthesis
3469:Biginelli reaction
3259:Mumm rearrangement
3144:Favorskii reaction
3079:Cope rearrangement
3069:Chan rearrangement
2834:Rubottom oxidation
2764:Miyaura borylation
2729:Lipid peroxidation
2724:Lindgren oxidation
2704:Kornblum oxidation
2699:Kolbe electrolysis
2644:Fukuyama reduction
2554:Carbonyl reduction
2404:Marker degradation
2266:Diazonium compound
2256:Boudouard reaction
2235:Carbon-heteroatom
2162:Grieco elimination
1948:Rieche formylation
1893:Passerini reaction
1823:Meerwein arylation
1743:Hydroxymethylation
1638:Favorskii reaction
1538:Chan rearrangement
1473:Biginelli reaction
1398:Aldol condensation
1244:2-Norbornyl cation
1219:Möbius aromaticity
1214:Markovnikov's rule
1109:Effective molarity
1054:Bürgi–Dunitz angle
1044:Bicycloaromaticity
750:2012-03-20 at the
204:aromatic nitration
182:Aromatic nitration
91:(as occurs in the
55:chemical processes
4189:
4188:
4185:
4184:
4181:
4180:
4173:Wohl–Aue reaction
3817:6+4 Cycloaddition
3634:Iodolactonization
2954:1,2-rearrangement
2919:Wohl–Aue reaction
2839:Sabatier reaction
2804:Pinnick oxidation
2769:Mozingo reduction
2714:Leuckart reaction
2669:Haloform reaction
2584:Criegee oxidation
2564:Collins oxidation
2514:Benkeser reaction
2509:Bechamp reduction
2479:Andrussow process
2464:Alcohol oxidation
2374:Edman degradation
2281:Haloform reaction
2230:
2229:
2217:Takai olefination
2182:Julia olefination
2008:Takai olefination
1883:Olefin metathesis
1758:Julia olefination
1683:Grignard reaction
1663:Fukuyama coupling
1578:Coupling reaction
1543:Chan–Lam coupling
1413:Alkyne metathesis
1408:Alkane metathesis
1264:Phosphaethynolate
1169:George S. Hammond
1129:Electronic effect
1084:Conjugated system
966:Stereospecificity
961:Stereoselectivity
926:Addition reaction
915:organic reactions
855:10.1021/ja905768k
783:(10): 1726–1728.
745:warren-wilson.edu
730:Collected Volumes
723:Organic Syntheses
702:Collected Volumes
695:Organic Syntheses
646:(14): 3588–3598.
617:10.1021/jo0609475
581:10.1021/ja021307w
569:J. Am. Chem. Soc.
537:Collected Volumes
530:Organic Syntheses
361:by reaction with
304:activating groups
47:organic chemistry
16:(Redirected from
4214:
4168:Wenker synthesis
4158:Stollé synthesis
4013:Bobbitt reaction
3983:Auwers synthesis
3927:Povarov reaction
3852:Cyclopropanation
3790:
3784:Wenker synthesis
3539:Darzens reaction
3489:Bobbitt reaction
3334:Schmidt reaction
3139:Enyne metathesis
2914:Whiting reaction
2909:Wharton reaction
2854:Shapiro reaction
2844:Sarett oxidation
2809:Prévost reaction
2619:Emde degradation
2429:Wohl degradation
2409:Ruff degradation
2379:Emde degradation
2276:Grignard reagent
2212:Shapiro reaction
2197:McMurry reaction
2064:
2028:Ullmann reaction
1993:Stollé synthesis
1983:Stetter reaction
1973:Shapiro reaction
1963:Sakurai reaction
1858:Negishi coupling
1838:Minisci reaction
1833:Michael reaction
1818:McMurry reaction
1813:Mannich reaction
1693:Hammick reaction
1688:Grignard reagent
1628:Enyne metathesis
1613:Doebner reaction
1603:Darzens reaction
1448:Barbier reaction
1438:Auwers synthesis
1365:
1339:Woodward's rules
1304:Superaromaticity
1294:Spiroaromaticity
1194:Inductive effect
1189:Hyperconjugation
1164:Hammett equation
1104:Edwards equation
956:Regioselectivity
907:
900:
893:
884:
877:
876:
866:
834:
828:
827:
807:
801:
800:
772:
766:
760:
754:
741:
735:
733:
726:
713:
707:
705:
698:
685:
679:
678:
662:
656:
655:
635:
629:
628:
611:(16): 6192–203.
599:
593:
592:
562:
556:
555:
548:
542:
540:
533:
517:
511:
508:
502:
501:
475:
454:Zincke nitration
397:and nonaflates,
363:acetic anhydride
234:
150:
131:atom (typically
118:
117:
116:
113:
82:
70:organic compound
67:
21:
4222:
4221:
4217:
4216:
4215:
4213:
4212:
4211:
4192:
4191:
4190:
4177:
4078:Gewald reaction
3961:
3788:
3769:Skraup reaction
3604:Graham reaction
3599:Gewald reaction
3430:
3423:
2945:
2938:
2894:Swern oxidation
2879:Stahl oxidation
2824:Riley oxidation
2779:Omega oxidation
2739:Luche reduction
2689:Jones oxidation
2654:Glycol cleavage
2649:Ganem oxidation
2594:Davis oxidation
2589:Dakin oxidation
2524:Birch reduction
2474:Amide reduction
2440:
2433:
2394:Hooker reaction
2356:
2350:
2238:
2236:
2226:
2222:Wittig reaction
2110:
2106:Wittig reaction
2081:Hooker reaction
2062:
2043:Wittig reaction
2018:Thorpe reaction
2003:Suzuki reaction
1988:Stille reaction
1923:Quelet reaction
1798:Kumada coupling
1748:Ivanov reaction
1738:Hydrovinylation
1718:Hiyama coupling
1678:Glaser coupling
1488:Blaise reaction
1478:Bingel reaction
1463:Benary reaction
1380:
1378:
1372:
1363:
1259:Passive binding
1179:Homoaromaticity
1029:Baldwin's rules
1004:Antiaromaticity
999:Anomeric effect
975:
917:
911:
881:
880:
836:
835:
831:
809:
808:
804:
774:
773:
769:
761:
757:
752:Wayback Machine
742:
738:
728:
715:
714:
710:
700:
687:
686:
682:
664:
663:
659:
637:
636:
632:
601:
600:
596:
575:(16): 4836–49.
564:
563:
559:
550:
549:
545:
535:
519:
518:
514:
509:
505:
498:
477:
476:
472:
467:
449:Menke nitration
445:
439:-butylbenzene.
429:
425:
391:
356:
352:
252:
201:
184:
168:trinitrotoluene
114:
111:
110:
108:
81:
77:
66:
62:
43:
28:
23:
22:
15:
12:
11:
5:
4220:
4218:
4210:
4209:
4204:
4194:
4193:
4187:
4186:
4183:
4182:
4179:
4178:
4176:
4175:
4170:
4165:
4160:
4155:
4150:
4145:
4140:
4135:
4130:
4125:
4120:
4115:
4110:
4105:
4100:
4095:
4090:
4085:
4083:Hantzsch ester
4080:
4075:
4070:
4065:
4060:
4055:
4050:
4045:
4040:
4035:
4030:
4025:
4020:
4015:
4010:
4005:
4000:
3995:
3993:Banert cascade
3990:
3985:
3980:
3975:
3969:
3967:
3963:
3962:
3960:
3959:
3954:
3949:
3944:
3939:
3934:
3932:Prato reaction
3929:
3924:
3919:
3914:
3909:
3904:
3899:
3894:
3889:
3884:
3879:
3874:
3869:
3864:
3859:
3854:
3849:
3844:
3839:
3834:
3829:
3824:
3819:
3814:
3809:
3804:
3798:
3796:
3787:
3786:
3781:
3776:
3771:
3766:
3761:
3756:
3751:
3746:
3741:
3736:
3731:
3726:
3721:
3716:
3711:
3706:
3701:
3696:
3691:
3686:
3681:
3676:
3671:
3666:
3661:
3656:
3651:
3646:
3641:
3636:
3631:
3626:
3621:
3616:
3611:
3606:
3601:
3596:
3591:
3586:
3581:
3576:
3571:
3566:
3561:
3556:
3551:
3546:
3541:
3536:
3531:
3526:
3521:
3516:
3511:
3506:
3501:
3496:
3491:
3486:
3481:
3476:
3471:
3466:
3461:
3456:
3451:
3446:
3441:
3435:
3433:
3425:
3424:
3422:
3421:
3416:
3411:
3406:
3401:
3396:
3391:
3386:
3381:
3376:
3371:
3366:
3361:
3356:
3351:
3346:
3341:
3336:
3331:
3326:
3321:
3316:
3311:
3306:
3301:
3296:
3291:
3286:
3281:
3276:
3271:
3266:
3261:
3256:
3251:
3246:
3241:
3236:
3231:
3226:
3221:
3216:
3211:
3206:
3201:
3196:
3191:
3186:
3181:
3176:
3171:
3166:
3161:
3156:
3151:
3146:
3141:
3136:
3131:
3126:
3121:
3116:
3111:
3106:
3101:
3096:
3091:
3086:
3081:
3076:
3071:
3066:
3061:
3056:
3051:
3046:
3041:
3036:
3031:
3026:
3024:Banert cascade
3021:
3016:
3011:
3006:
3001:
2996:
2991:
2986:
2981:
2976:
2971:
2966:
2961:
2956:
2950:
2948:
2944:Rearrangement
2940:
2939:
2937:
2936:
2934:Zinin reaction
2931:
2926:
2921:
2916:
2911:
2906:
2904:Wacker process
2901:
2896:
2891:
2886:
2881:
2876:
2871:
2866:
2861:
2856:
2851:
2846:
2841:
2836:
2831:
2826:
2821:
2816:
2811:
2806:
2801:
2796:
2791:
2786:
2781:
2776:
2771:
2766:
2761:
2756:
2751:
2746:
2741:
2736:
2731:
2726:
2721:
2716:
2711:
2706:
2701:
2696:
2691:
2686:
2681:
2679:Hydrogenolysis
2676:
2671:
2666:
2661:
2656:
2651:
2646:
2641:
2636:
2631:
2629:Étard reaction
2626:
2621:
2616:
2611:
2606:
2601:
2596:
2591:
2586:
2581:
2576:
2571:
2566:
2561:
2556:
2551:
2546:
2541:
2536:
2534:Bosch reaction
2531:
2526:
2521:
2516:
2511:
2506:
2501:
2496:
2491:
2486:
2481:
2476:
2471:
2466:
2461:
2456:
2451:
2445:
2443:
2439:Organic redox
2435:
2434:
2432:
2431:
2426:
2421:
2416:
2411:
2406:
2401:
2396:
2391:
2386:
2381:
2376:
2371:
2366:
2360:
2358:
2352:
2351:
2349:
2348:
2343:
2338:
2333:
2328:
2323:
2318:
2313:
2308:
2303:
2298:
2293:
2288:
2283:
2278:
2273:
2271:Esterification
2268:
2263:
2258:
2253:
2248:
2242:
2240:
2232:
2231:
2228:
2227:
2225:
2224:
2219:
2214:
2209:
2204:
2199:
2194:
2189:
2184:
2179:
2174:
2169:
2164:
2159:
2154:
2149:
2144:
2139:
2134:
2129:
2124:
2118:
2116:
2112:
2111:
2109:
2108:
2103:
2098:
2093:
2088:
2083:
2078:
2072:
2070:
2061:
2060:
2055:
2050:
2048:Wurtz reaction
2045:
2040:
2035:
2030:
2025:
2020:
2015:
2010:
2005:
2000:
1995:
1990:
1985:
1980:
1975:
1970:
1965:
1960:
1955:
1950:
1945:
1940:
1935:
1930:
1925:
1920:
1918:Prins reaction
1915:
1910:
1905:
1900:
1895:
1890:
1885:
1880:
1875:
1870:
1865:
1860:
1855:
1850:
1845:
1840:
1835:
1830:
1825:
1820:
1815:
1810:
1805:
1800:
1795:
1790:
1785:
1780:
1775:
1770:
1765:
1760:
1755:
1750:
1745:
1740:
1735:
1733:Hydrocyanation
1730:
1725:
1720:
1715:
1710:
1705:
1703:Henry reaction
1700:
1695:
1690:
1685:
1680:
1675:
1670:
1665:
1660:
1655:
1650:
1645:
1640:
1635:
1630:
1625:
1620:
1615:
1610:
1605:
1600:
1595:
1590:
1585:
1580:
1575:
1570:
1565:
1560:
1555:
1550:
1545:
1540:
1535:
1530:
1525:
1520:
1515:
1510:
1505:
1500:
1495:
1490:
1485:
1480:
1475:
1470:
1465:
1460:
1455:
1450:
1445:
1440:
1435:
1430:
1425:
1420:
1415:
1410:
1405:
1403:Aldol reaction
1400:
1395:
1390:
1384:
1382:
1377:Carbon-carbon
1374:
1373:
1368:
1362:
1361:
1356:
1354:Zaitsev's rule
1351:
1346:
1341:
1336:
1331:
1326:
1321:
1316:
1311:
1306:
1301:
1299:Steric effects
1296:
1291:
1286:
1281:
1276:
1271:
1266:
1261:
1256:
1251:
1246:
1241:
1236:
1231:
1226:
1221:
1216:
1211:
1206:
1201:
1196:
1191:
1186:
1181:
1176:
1171:
1166:
1161:
1156:
1151:
1146:
1141:
1136:
1131:
1126:
1121:
1116:
1111:
1106:
1101:
1096:
1091:
1086:
1081:
1076:
1071:
1066:
1061:
1056:
1051:
1046:
1041:
1036:
1031:
1026:
1021:
1016:
1011:
1006:
1001:
996:
991:
986:
980:
977:
976:
974:
973:
968:
963:
958:
953:
951:Redox reaction
948:
943:
938:
936:Polymerization
933:
928:
922:
919:
918:
912:
910:
909:
902:
895:
887:
879:
878:
829:
802:
767:
755:
743:Web resource:
736:
708:
680:
657:
630:
594:
557:
543:
521:George A. Olah
512:
503:
497:978-3527306732
496:
469:
468:
466:
463:
462:
461:
456:
451:
444:
441:
427:
423:
411:sodium nitrite
403:ipso nitration
390:
389:Ipso nitration
387:
354:
350:
274:such as other
251:
248:
244:Acetyl nitrate
236:
235:
199:
192:sulfuric acids
183:
180:
160:nitroguanidine
152:
151:
119:) is that the
74:nitrate esters
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
4219:
4208:
4205:
4203:
4200:
4199:
4197:
4174:
4171:
4169:
4166:
4164:
4161:
4159:
4156:
4154:
4151:
4149:
4146:
4144:
4141:
4139:
4136:
4134:
4131:
4129:
4126:
4124:
4121:
4119:
4116:
4114:
4111:
4109:
4106:
4104:
4101:
4099:
4096:
4094:
4093:Herz reaction
4091:
4089:
4086:
4084:
4081:
4079:
4076:
4074:
4071:
4069:
4066:
4064:
4061:
4059:
4056:
4054:
4051:
4049:
4046:
4044:
4041:
4039:
4036:
4034:
4031:
4029:
4026:
4024:
4021:
4019:
4016:
4014:
4011:
4009:
4006:
4004:
4001:
3999:
3996:
3994:
3991:
3989:
3986:
3984:
3981:
3979:
3976:
3974:
3971:
3970:
3968:
3964:
3958:
3955:
3953:
3950:
3948:
3945:
3943:
3940:
3938:
3935:
3933:
3930:
3928:
3925:
3923:
3920:
3918:
3915:
3913:
3910:
3908:
3905:
3903:
3900:
3898:
3895:
3893:
3890:
3888:
3885:
3883:
3880:
3878:
3875:
3873:
3870:
3868:
3865:
3863:
3860:
3858:
3855:
3853:
3850:
3848:
3845:
3843:
3840:
3838:
3835:
3833:
3830:
3828:
3825:
3823:
3820:
3818:
3815:
3813:
3810:
3808:
3805:
3803:
3800:
3799:
3797:
3795:
3794:Cycloaddition
3791:
3785:
3782:
3780:
3777:
3775:
3772:
3770:
3767:
3765:
3762:
3760:
3757:
3755:
3752:
3750:
3747:
3745:
3742:
3740:
3737:
3735:
3732:
3730:
3727:
3725:
3722:
3720:
3717:
3715:
3712:
3710:
3707:
3705:
3702:
3700:
3697:
3695:
3692:
3690:
3687:
3685:
3682:
3680:
3677:
3675:
3672:
3670:
3667:
3665:
3662:
3660:
3657:
3655:
3652:
3650:
3647:
3645:
3642:
3640:
3639:Isay reaction
3637:
3635:
3632:
3630:
3627:
3625:
3622:
3620:
3617:
3615:
3612:
3610:
3607:
3605:
3602:
3600:
3597:
3595:
3592:
3590:
3587:
3585:
3582:
3580:
3577:
3575:
3572:
3570:
3567:
3565:
3562:
3560:
3557:
3555:
3552:
3550:
3547:
3545:
3542:
3540:
3537:
3535:
3534:Cycloaddition
3532:
3530:
3527:
3525:
3522:
3520:
3517:
3515:
3512:
3510:
3507:
3505:
3502:
3500:
3497:
3495:
3492:
3490:
3487:
3485:
3482:
3480:
3477:
3475:
3472:
3470:
3467:
3465:
3462:
3460:
3457:
3455:
3452:
3450:
3447:
3445:
3442:
3440:
3437:
3436:
3434:
3432:
3429:Ring forming
3426:
3420:
3417:
3415:
3412:
3410:
3407:
3405:
3402:
3400:
3397:
3395:
3392:
3390:
3387:
3385:
3382:
3380:
3377:
3375:
3372:
3370:
3367:
3365:
3362:
3360:
3357:
3355:
3352:
3350:
3347:
3345:
3342:
3340:
3337:
3335:
3332:
3330:
3329:Rupe reaction
3327:
3325:
3322:
3320:
3317:
3315:
3312:
3310:
3307:
3305:
3302:
3300:
3297:
3295:
3292:
3290:
3287:
3285:
3282:
3280:
3277:
3275:
3272:
3270:
3267:
3265:
3262:
3260:
3257:
3255:
3252:
3250:
3247:
3245:
3242:
3240:
3237:
3235:
3232:
3230:
3227:
3225:
3222:
3220:
3217:
3215:
3212:
3210:
3207:
3205:
3202:
3200:
3197:
3195:
3192:
3190:
3187:
3185:
3182:
3180:
3177:
3175:
3172:
3170:
3167:
3165:
3162:
3160:
3157:
3155:
3152:
3150:
3147:
3145:
3142:
3140:
3137:
3135:
3132:
3130:
3127:
3125:
3122:
3120:
3117:
3115:
3112:
3110:
3107:
3105:
3102:
3100:
3097:
3095:
3092:
3090:
3087:
3085:
3082:
3080:
3077:
3075:
3072:
3070:
3067:
3065:
3062:
3060:
3057:
3055:
3052:
3050:
3047:
3045:
3042:
3040:
3037:
3035:
3032:
3030:
3027:
3025:
3022:
3020:
3017:
3015:
3012:
3010:
3007:
3005:
3002:
3000:
2997:
2995:
2992:
2990:
2987:
2985:
2982:
2980:
2977:
2975:
2972:
2970:
2967:
2965:
2962:
2960:
2957:
2955:
2952:
2951:
2949:
2947:
2941:
2935:
2932:
2930:
2927:
2925:
2922:
2920:
2917:
2915:
2912:
2910:
2907:
2905:
2902:
2900:
2897:
2895:
2892:
2890:
2887:
2885:
2882:
2880:
2877:
2875:
2872:
2870:
2867:
2865:
2862:
2860:
2857:
2855:
2852:
2850:
2847:
2845:
2842:
2840:
2837:
2835:
2832:
2830:
2827:
2825:
2822:
2820:
2817:
2815:
2812:
2810:
2807:
2805:
2802:
2800:
2797:
2795:
2792:
2790:
2787:
2785:
2782:
2780:
2777:
2775:
2772:
2770:
2767:
2765:
2762:
2760:
2757:
2755:
2752:
2750:
2747:
2745:
2742:
2740:
2737:
2735:
2732:
2730:
2727:
2725:
2722:
2720:
2719:Ley oxidation
2717:
2715:
2712:
2710:
2707:
2705:
2702:
2700:
2697:
2695:
2692:
2690:
2687:
2685:
2684:Hydroxylation
2682:
2680:
2677:
2675:
2674:Hydrogenation
2672:
2670:
2667:
2665:
2662:
2660:
2657:
2655:
2652:
2650:
2647:
2645:
2642:
2640:
2637:
2635:
2632:
2630:
2627:
2625:
2622:
2620:
2617:
2615:
2612:
2610:
2609:DNA oxidation
2607:
2605:
2602:
2600:
2599:Deoxygenation
2597:
2595:
2592:
2590:
2587:
2585:
2582:
2580:
2577:
2575:
2572:
2570:
2567:
2565:
2562:
2560:
2557:
2555:
2552:
2550:
2547:
2545:
2542:
2540:
2537:
2535:
2532:
2530:
2527:
2525:
2522:
2520:
2517:
2515:
2512:
2510:
2507:
2505:
2502:
2500:
2497:
2495:
2492:
2490:
2489:Aromatization
2487:
2485:
2482:
2480:
2477:
2475:
2472:
2470:
2467:
2465:
2462:
2460:
2457:
2455:
2452:
2450:
2447:
2446:
2444:
2442:
2436:
2430:
2427:
2425:
2422:
2420:
2417:
2415:
2412:
2410:
2407:
2405:
2402:
2400:
2397:
2395:
2392:
2390:
2387:
2385:
2382:
2380:
2377:
2375:
2372:
2370:
2367:
2365:
2362:
2361:
2359:
2353:
2347:
2344:
2342:
2339:
2337:
2334:
2332:
2329:
2327:
2326:Reed reaction
2324:
2322:
2319:
2317:
2314:
2312:
2309:
2307:
2304:
2302:
2299:
2297:
2294:
2292:
2289:
2287:
2284:
2282:
2279:
2277:
2274:
2272:
2269:
2267:
2264:
2262:
2259:
2257:
2254:
2252:
2249:
2247:
2244:
2243:
2241:
2237:bond forming
2233:
2223:
2220:
2218:
2215:
2213:
2210:
2208:
2205:
2203:
2200:
2198:
2195:
2193:
2190:
2188:
2185:
2183:
2180:
2178:
2175:
2173:
2170:
2168:
2165:
2163:
2160:
2158:
2155:
2153:
2150:
2148:
2145:
2143:
2142:Cope reaction
2140:
2138:
2135:
2133:
2130:
2128:
2125:
2123:
2120:
2119:
2117:
2113:
2107:
2104:
2102:
2099:
2097:
2094:
2092:
2089:
2087:
2084:
2082:
2079:
2077:
2074:
2073:
2071:
2069:
2065:
2059:
2056:
2054:
2051:
2049:
2046:
2044:
2041:
2039:
2036:
2034:
2031:
2029:
2026:
2024:
2021:
2019:
2016:
2014:
2011:
2009:
2006:
2004:
2001:
1999:
1996:
1994:
1991:
1989:
1986:
1984:
1981:
1979:
1976:
1974:
1971:
1969:
1966:
1964:
1961:
1959:
1956:
1954:
1951:
1949:
1946:
1944:
1941:
1939:
1936:
1934:
1931:
1929:
1926:
1924:
1921:
1919:
1916:
1914:
1911:
1909:
1906:
1904:
1901:
1899:
1896:
1894:
1891:
1889:
1886:
1884:
1881:
1879:
1876:
1874:
1871:
1869:
1866:
1864:
1861:
1859:
1856:
1854:
1853:Nef synthesis
1851:
1849:
1846:
1844:
1841:
1839:
1836:
1834:
1831:
1829:
1828:Methylenation
1826:
1824:
1821:
1819:
1816:
1814:
1811:
1809:
1806:
1804:
1801:
1799:
1796:
1794:
1791:
1789:
1786:
1784:
1781:
1779:
1776:
1774:
1771:
1769:
1766:
1764:
1761:
1759:
1756:
1754:
1751:
1749:
1746:
1744:
1741:
1739:
1736:
1734:
1731:
1729:
1726:
1724:
1721:
1719:
1716:
1714:
1711:
1709:
1706:
1704:
1701:
1699:
1698:Heck reaction
1696:
1694:
1691:
1689:
1686:
1684:
1681:
1679:
1676:
1674:
1671:
1669:
1666:
1664:
1661:
1659:
1656:
1654:
1651:
1649:
1646:
1644:
1641:
1639:
1636:
1634:
1631:
1629:
1626:
1624:
1621:
1619:
1616:
1614:
1611:
1609:
1606:
1604:
1601:
1599:
1596:
1594:
1591:
1589:
1586:
1584:
1581:
1579:
1576:
1574:
1571:
1569:
1566:
1564:
1561:
1559:
1556:
1554:
1551:
1549:
1546:
1544:
1541:
1539:
1536:
1534:
1531:
1529:
1526:
1524:
1521:
1519:
1516:
1514:
1511:
1509:
1506:
1504:
1501:
1499:
1496:
1494:
1491:
1489:
1486:
1484:
1481:
1479:
1476:
1474:
1471:
1469:
1466:
1464:
1461:
1459:
1456:
1454:
1451:
1449:
1446:
1444:
1441:
1439:
1436:
1434:
1431:
1429:
1426:
1424:
1421:
1419:
1416:
1414:
1411:
1409:
1406:
1404:
1401:
1399:
1396:
1394:
1391:
1389:
1386:
1385:
1383:
1379:bond forming
1375:
1371:
1366:
1360:
1357:
1355:
1352:
1350:
1347:
1345:
1344:Y-aromaticity
1342:
1340:
1337:
1335:
1332:
1330:
1329:Walsh diagram
1327:
1325:
1322:
1320:
1317:
1315:
1314:Taft equation
1312:
1310:
1307:
1305:
1302:
1300:
1297:
1295:
1292:
1290:
1287:
1285:
1284:Σ-aromaticity
1282:
1280:
1277:
1275:
1272:
1270:
1267:
1265:
1262:
1260:
1257:
1255:
1252:
1250:
1247:
1245:
1242:
1240:
1237:
1235:
1232:
1230:
1227:
1225:
1222:
1220:
1217:
1215:
1212:
1210:
1209:Marcus theory
1207:
1205:
1202:
1200:
1197:
1195:
1192:
1190:
1187:
1185:
1184:Hückel's rule
1182:
1180:
1177:
1175:
1172:
1170:
1167:
1165:
1162:
1160:
1157:
1155:
1152:
1150:
1147:
1145:
1142:
1140:
1139:Evelyn effect
1137:
1135:
1132:
1130:
1127:
1125:
1122:
1120:
1119:Electron-rich
1117:
1115:
1112:
1110:
1107:
1105:
1102:
1100:
1097:
1095:
1092:
1090:
1087:
1085:
1082:
1080:
1077:
1075:
1072:
1070:
1067:
1065:
1062:
1060:
1057:
1055:
1052:
1050:
1047:
1045:
1042:
1040:
1037:
1035:
1034:Bema Hapothle
1032:
1030:
1027:
1025:
1022:
1020:
1017:
1015:
1012:
1010:
1007:
1005:
1002:
1000:
997:
995:
992:
990:
987:
985:
982:
981:
978:
972:
969:
967:
964:
962:
959:
957:
954:
952:
949:
947:
944:
942:
939:
937:
934:
932:
929:
927:
924:
923:
920:
916:
908:
903:
901:
896:
894:
889:
888:
885:
874:
870:
865:
860:
856:
852:
848:
844:
840:
833:
830:
825:
821:
817:
813:
806:
803:
798:
794:
790:
786:
782:
778:
771:
768:
764:
759:
756:
753:
749:
746:
740:
737:
731:
725:
724:
719:
712:
709:
703:
697:
696:
691:
684:
681:
676:
672:
668:
661:
658:
653:
649:
645:
641:
634:
631:
626:
622:
618:
614:
610:
607:
606:
605:J. Org. Chem.
598:
595:
590:
586:
582:
578:
574:
571:
570:
561:
558:
553:
547:
544:
538:
532:
531:
526:
522:
516:
513:
507:
504:
499:
493:
489:
485:
481:
474:
471:
464:
460:
457:
455:
452:
450:
447:
446:
442:
440:
438:
434:
430:
419:
417:
412:
408:
404:
400:
396:
388:
386:
384:
380:
376:
372:
367:
364:
360:
348:
344:
340:
339:sulfuric acid
336:
332:
327:
325:
321:
317:
313:
309:
305:
301:
297:
293:
289:
285:
281:
277:
273:
269:
265:
260:
258:
249:
247:
245:
241:
233:
229:
228:
227:
225:
221:
217:
213:
209:
205:
197:
196:nitronium ion
193:
189:
181:
179:
177:
173:
169:
165:
161:
157:
149:
145:
144:
143:
141:
136:
134:
130:
126:
122:
106:
102:
98:
97:nitroglycerin
94:
90:
86:
75:
71:
60:
56:
52:
48:
41:
37:
33:
32:Nitrification
19:
3134:Ene reaction
2494:Autoxidation
2355:Degradation
2246:Azo coupling
2023:Ugi reaction
1623:Ene reaction
1423:Alkynylation
1274:Polyfluorene
1269:Polar effect
1134:Electrophile
1049:Bredt's rule
1019:Baird's rule
989:Alpha effect
846:
842:
832:
818:(14): 3389.
815:
811:
805:
780:
776:
770:
762:
758:
739:
729:
721:
711:
701:
693:
683:
666:
660:
643:
639:
633:
608:
603:
597:
572:
567:
560:
546:
536:
528:
515:
506:
479:
473:
436:
415:
406:
402:
392:
368:
346:
342:
328:
318:groups also
300:carboxylates
261:
253:
237:
203:
185:
153:
140:nitrobenzene
137:
50:
44:
1633:Ethenolysis
1279:Ring strain
1249:Nucleophile
1074:Clar's rule
1014:Aromaticity
383:picric acid
359:acetanilide
335:nitric acid
188:nitric acid
89:nitric acid
59:nitro group
36:Nitrosation
4196:Categories
3917:Ozonolysis
3444:Annulation
2794:Ozonolysis
913:Topics in
465:References
257:fluorenone
176:precursors
83:) between
68:) into an
3431:reactions
2946:reactions
2441:reactions
2357:reactions
2239:reactions
1381:reactions
669:: ra032.
395:triflates
156:guanidine
127:to a non-
93:synthesis
51:nitration
40:Nitriding
18:Nitrating
1324:Vinylogy
994:Annulene
941:Reagents
873:19737014
797:20146295
748:Archived
625:16872205
589:12696903
443:See also
418:-butanol
381:to give
306:such as
290:groups,
284:sulfonyl
266:of this
212:catalyst
121:nitrogen
105:nitrates
85:alcohols
984:A value
864:2773681
375:benzene
369:In the
331:aniline
312:hydroxy
242:(SET).
224:benzene
216:benzene
164:toluene
871:
861:
795:
623:
587:
494:
345:- and
324:ethers
320:amides
316:methyl
298:, and
296:esters
226:ring:
133:carbon
129:oxygen
125:bonded
426:(dba)
333:with
308:amino
288:cyano
276:nitro
250:Scope
38:, or
869:PMID
793:PMID
621:PMID
585:PMID
492:ISBN
399:ipso
347:meta
343:para
337:and
322:and
314:and
292:keto
264:rate
190:and
174:and
87:and
78:−ONO
859:PMC
851:doi
847:131
820:doi
785:doi
671:doi
648:doi
613:doi
577:doi
573:125
484:doi
413:in
198:(NO
166:to
158:to
142:.
95:of
63:−NO
45:In
4198::
867:.
857:.
845:.
841:.
816:93
814:.
791:.
781:49
779:.
727:;
720:.
699:;
692:.
644:82
642:.
619:.
609:71
583:.
534:;
527:.
490:.
422:Pd
385:.
373:,
310:,
294:,
286:,
270:.
109:NO
49:,
34:,
906:e
899:t
892:v
875:.
853::
826:.
822::
799:.
787::
734:.
706:.
677:.
673::
654:.
650::
627:.
615::
591:.
579::
554:.
541:.
500:.
486::
437:n
428:3
424:2
416:t
407:n
355:3
351:2
200:2
115:3
112:−
107:(
80:2
76:(
65:2
61:(
42:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.