185:
166:. The sliding contact or wiper of the potentiometer is adjusted and the galvanometer briefly connected between the sliding contact and the unknown voltage. The deflection of the galvanometer is observed and the sliding tap adjusted until the galvanometer no longer deflects from zero. At that point the galvanometer draws no current from the unknown source, and the magnitude of voltage can be calculated from the position of the sliding contact.
43:
193:
form of potentiometer is a uniform high-resistance wire attached to an insulating support, marked with a linear measuring scale. In use, an adjustable regulated voltage source E, of greater magnitude than the potential to be measured, is connected across the wire so as to pass a steady current through it.
196:
Between the end of the wire and any point along it will be a potential proportional to the length of wire to that point. By comparing the potential at points along the wire with an unknown potential, the magnitude of the unknown potential can be determined. The instrument used for comparison must be
192:
The principle of a potentiometer is that the potential dropped across a segment of a wire of uniform cross-section carrying a constant current is directly proportional to its length. The potentiometer is a simple device used to measure the electrical potentials (or compare the e.m.f of a cell). One
560:
To make a potentiometric determination of an analyte in a solution, the potential of the cell is measured. This measurement must be corrected for the reference and junction potentials. It can also be used in standardisation methods. The concentration of the analyte can then be calculated from the
355:
The galvanometer does not need to be calibrated, as its only function is to read zero or not zero. When measuring an unknown voltage and the galvanometer reads zero, no current is drawn from the unknown voltage and so the reading is independent of the source's internal resistance, as if by a
367:
Because the resistance wire can be made very uniform in cross-section and resistivity, and the position of the wiper can be measured easily, this method can be used to measure unknown DC voltages greater than or less than a calibration voltage produced by a standard cell without drawing any
552:. Potentiometers for use with thermocouples also measure the temperature at which the thermocouple wires are connected, so that cold-junction compensation may be applied to correct the apparent measured EMF to the standard cold-junction temperature of 0 degrees C.
394:
portion of the resistance wire when the galvanometer gives a zero reading for an unknown voltage, the distance AX is measured or read from a pre-printed scale next to the resistance wire. The unknown voltage can then be calculated:
573:
A metre bridge is a simple type of potentiometer which may be used in school science laboratories to demonstrate the principle of resistance measurement by potentiometric means. A resistance wire is laid along the length of a
312:
539:
This is a form of the constant resistance potentiometer described above but designed to minimize the effects of contact resistance and thermal emf. This equipment is satisfactorily used down to readings of 1000 nV or so.
582:
by a slider. When the galvanometer reads zero, the ratio between the lengths of wire to the left and right of the slider is equal to the ratio between the values of a known and an unknown resistor in a parallel circuit.
346:
of the resistance wire. The wiper is moved until no current flows into or out of the source of unknown voltage, as indicated by the galvanometer in series with the unknown voltage. The voltage across the selected
530:
The constant resistance potentiometer is a variation of the basic idea in which a variable current is fed through a fixed resistor. These are used primarily for measurements in the millivolt and microvolt range.
184:
521:
351:
section of wire is then equal to the unknown voltage. The final step is to calculate the unknown voltage from the fraction of the length of the resistance wire that was connected to the unknown voltage.
151:. If a sensitive indicating instrument is used, very little current is drawn from the source of the unknown voltage. Since the reference voltage can be produced from an accurately calibrated
60:
652:
736:
250:
668:
709:
107:
79:
86:
197:
sensitive, but need not be particularly well-calibrated or accurate so long as its deflection from zero position can be easily detected.
649:
206:
398:
704:
637:
617:
126:
93:
1011:
548:
Another development of the standard types was the 'thermocouple potentiometer' especially adapted for temperature measurement with
342:
An unknown DC voltage, in series with the galvanometer, is then connected to the sliding wiper, across a variable-length section R
759:
390:
resistance wire is AB, where A is the (-) end and B is the (+) end, and the movable wiper is at point X at a distance AX on the R
162:
In this arrangement, a fraction of a known voltage from a resistive slide wire is compared with an unknown voltage by means of a
729:
75:
1068:
895:
799:
779:
379:, then a second variable resistor (not shown) can be used to calibrate the potentiometer by varying the current through the R
64:
834:
1001:
930:
769:
156:
950:
940:
986:
1063:
875:
722:
945:
100:
1006:
819:
814:
745:
925:
900:
53:
839:
809:
764:
376:
844:
224:
1038:
981:
321:
317:
1058:
955:
920:
870:
905:
794:
996:
965:
885:
804:
784:
774:
597:
369:
1026:
562:
152:
155:, a potentiometer can provide high precision in measurement. The method was described by
1021:
232:
215:
is the resistance of the entire resistance wire. The arrow head represents the moving
1052:
1016:
991:
890:
829:
656:
592:
147:
or 'potential difference' by comparison of an unknown voltage with a known reference
31:
915:
824:
579:
549:
332:
163:
307:{\displaystyle {R_{2} \over R_{1}}={{\mbox{cell voltage}} \over V_{\mathrm {S} }}}
30:
This article is about the measuring instrument. For the electrical component, see
935:
865:
849:
681:
240:
42:
960:
789:
575:
565:. Many varieties of this basic principle exist for quantitative measurements.
205:
710:
Electrical calibration equipment including various measurement potentiometers
17:
880:
714:
357:
176:
Measurement potentiometers are divided into four main classes listed below.
170:
375:
If the potentiometer is attached to a constant voltage DC supply such as a
188:
Dial potentiometer, with built-in galvanometer and reference voltage source
910:
361:
209:
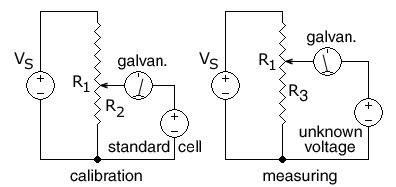
A potentiometer being calibrated and then measuring an unknown voltage.
148:
144:
169:
This null balance measuring method is still important in electrical
173:
and standards work and is also used in other areas of electronics.
204:
183:
159:
around 1841 and became a standard laboratory measuring technique.
247:
wire that corresponds to the voltage of a standard cell so that
718:
516:{\displaystyle V_{U}=(Calibration\ Cell\ Voltage){AX \over AB}}
36:
671:
Dept of
Physics. Thermodynamics: Thermocouple Potentiometer.
239:
for use as a voltage divider. The potentiometer is first
284:
401:
253:
243:
by positioning the wiper (arrow) at the spot on the R
320:
is used whose emf is known (e.g. 1.0183 volts for a
974:
858:
752:
67:. Unsourced material may be challenged and removed.
515:
306:
730:
8:
578:and contact with the wire is made through a
737:
723:
715:
493:
406:
400:
295:
294:
283:
281:
270:
260:
254:
252:
127:Learn how and when to remove this message
76:"Potentiometer" measuring instrument
682:"Ian Hickson's Metre Bridge Experiment"
608:
339:is equal to the standard cell voltage.
335:shows zero, indicating the voltage on R
223:In this circuit, the ends of a uniform
7:
705:Pictures of measuring potentiometers
65:adding citations to reliable sources
296:
25:
526:Constant resistance potentiometer
760:Adsorptive stripping voltammetry
41:
593:Potentiometer (voltage divider)
143:is an instrument for measuring
52:needs additional citations for
1012:Faraday's laws of electrolysis
896:Hanging mercury drop electrode
800:Differential pulse voltammetry
780:Cathodic stripping voltammetry
490:
415:
201:Constant current potentiometer
1:
835:Rotated electrode voltammetry
231:are connected to a regulated
931:Rotating ring-disk electrode
770:Anodic stripping voltammetry
157:Johann Christian Poggendorff
951:Standard hydrogen electrode
941:Saturated calomel electrode
331:is then adjusted until the
1085:
876:Dropping mercury electrode
616:Thomas B. Greenslade, Jr.
544:Thermocouple potentiometer
29:
1035:
946:Silver chloride electrode
746:Electroanalytical methods
820:Normal pulse voltammetry
815:Linear sweep voltammetry
372:from the standard cell.
926:Rotating disk electrode
901:Ion selective electrode
535:Microvolt potentiometer
987:Butler–Volmer equation
840:Squarewave voltammetry
810:Hydrodynamic technique
765:Amperometric titration
517:
386:If the length of the R
308:
220:
189:
180:Principle of operation
1069:Measuring instruments
1002:Debye–Hückel equation
845:Staircase voltammetry
518:
309:
208:
187:
1039:Analytical Chemistry
982:Activity coefficient
620:. Physics.kenyon.edu
556:Analytical chemistry
399:
327:The supply voltage V
322:Weston standard cell
318:electrochemical cell
251:
61:improve this article
956:Ultramicroelectrode
921:Reference electrode
871:Auxiliary electrode
684:. Academia.hixie.ch
618:"The Potentiometer"
906:Mercury coulometer
795:Cyclic voltammetry
513:
304:
288:
221:
190:
1064:Electrical meters
1046:
1045:
997:Cottrell equation
966:Working electrode
886:Electrolytic cell
805:Electrogravimetry
785:Chronoamperometry
775:Bulk electrolysis
598:Wheatstone bridge
511:
468:
453:
383:resistance wire.
377:lead–acid battery
302:
287:
276:
137:
136:
129:
111:
16:(Redirected from
1076:
739:
732:
725:
716:
693:
692:
690:
689:
678:
672:
666:
660:
647:
641:
640:Dept of Physics.
635:
629:
628:
626:
625:
613:
522:
520:
519:
514:
512:
510:
502:
494:
466:
451:
411:
410:
313:
311:
310:
305:
303:
301:
300:
299:
289:
285:
282:
277:
275:
274:
265:
264:
255:
132:
125:
121:
118:
112:
110:
69:
45:
37:
21:
1084:
1083:
1079:
1078:
1077:
1075:
1074:
1073:
1049:
1048:
1047:
1042:
1031:
1027:Nernst equation
970:
859:Instrumentation
854:
748:
743:
701:
696:
687:
685:
680:
679:
675:
667:
663:
648:
644:
636:
632:
623:
621:
615:
614:
610:
606:
589:
571:
563:Nernst Equation
558:
546:
537:
528:
503:
495:
402:
397:
396:
393:
389:
382:
350:
345:
338:
330:
290:
266:
256:
249:
248:
246:
238:
230:
214:
210:
203:
182:
153:voltage divider
133:
122:
116:
113:
70:
68:
58:
46:
35:
28:
23:
22:
15:
12:
11:
5:
1082:
1080:
1072:
1071:
1066:
1061:
1051:
1050:
1044:
1043:
1036:
1033:
1032:
1030:
1029:
1024:
1022:Ionic strength
1019:
1014:
1009:
1004:
999:
994:
989:
984:
978:
976:
972:
971:
969:
968:
963:
958:
953:
948:
943:
938:
933:
928:
923:
918:
913:
908:
903:
898:
893:
888:
883:
878:
873:
868:
862:
860:
856:
855:
853:
852:
847:
842:
837:
832:
827:
822:
817:
812:
807:
802:
797:
792:
787:
782:
777:
772:
767:
762:
756:
754:
750:
749:
744:
742:
741:
734:
727:
719:
713:
712:
707:
700:
699:External links
697:
695:
694:
673:
661:
655:2012-09-11 at
642:
630:
607:
605:
602:
601:
600:
595:
588:
585:
570:
567:
557:
554:
545:
542:
536:
533:
527:
524:
509:
506:
501:
498:
492:
489:
486:
483:
480:
477:
474:
471:
465:
462:
459:
456:
450:
447:
444:
441:
438:
435:
432:
429:
426:
423:
420:
417:
414:
409:
405:
391:
387:
380:
348:
343:
336:
328:
298:
293:
280:
273:
269:
263:
259:
244:
236:
228:
212:
202:
199:
181:
178:
135:
134:
49:
47:
40:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1081:
1070:
1067:
1065:
1062:
1060:
1057:
1056:
1054:
1041:
1040:
1034:
1028:
1025:
1023:
1020:
1018:
1017:Half-reaction
1015:
1013:
1010:
1008:
1005:
1003:
1000:
998:
995:
993:
992:Cell notation
990:
988:
985:
983:
980:
979:
977:
973:
967:
964:
962:
959:
957:
954:
952:
949:
947:
944:
942:
939:
937:
934:
932:
929:
927:
924:
922:
919:
917:
914:
912:
909:
907:
904:
902:
899:
897:
894:
892:
891:Galvanic cell
889:
887:
884:
882:
879:
877:
874:
872:
869:
867:
864:
863:
861:
857:
851:
848:
846:
843:
841:
838:
836:
833:
831:
830:Potentiometry
828:
826:
823:
821:
818:
816:
813:
811:
808:
806:
803:
801:
798:
796:
793:
791:
788:
786:
783:
781:
778:
776:
773:
771:
768:
766:
763:
761:
758:
757:
755:
751:
747:
740:
735:
733:
728:
726:
721:
720:
717:
711:
708:
706:
703:
702:
698:
683:
677:
674:
670:
665:
662:
658:
657:archive.today
654:
651:
646:
643:
639:
634:
631:
619:
612:
609:
603:
599:
596:
594:
591:
590:
586:
584:
581:
577:
568:
566:
564:
555:
553:
551:
550:thermocouples
543:
541:
534:
532:
525:
523:
507:
504:
499:
496:
487:
484:
481:
478:
475:
472:
469:
463:
460:
457:
454:
448:
445:
442:
439:
436:
433:
430:
427:
424:
421:
418:
412:
407:
403:
384:
378:
373:
371:
365:
363:
359:
353:
340:
334:
325:
323:
319:
314:
291:
278:
271:
267:
261:
257:
242:
234:
226:
218:
207:
200:
198:
194:
186:
179:
177:
174:
172:
167:
165:
160:
158:
154:
150:
146:
142:
141:potentiometer
131:
128:
120:
117:December 2014
109:
106:
102:
99:
95:
92:
88:
85:
81:
78: –
77:
73:
72:Find sources:
66:
62:
56:
55:
50:This article
48:
44:
39:
38:
33:
32:Potentiometer
19:
18:Potentiometry
1037:
1007:Double layer
916:Potentiostat
825:Polarography
686:. Retrieved
676:
664:
650:scenta.co.uk
645:
633:
622:. Retrieved
611:
580:galvanometer
572:
569:Metre bridge
559:
547:
538:
529:
385:
374:
366:
364:resistance.
354:
341:
333:galvanometer
326:
315:
286:cell voltage
222:
216:
195:
191:
175:
168:
164:galvanometer
161:
140:
138:
123:
114:
104:
97:
90:
83:
71:
59:Please help
54:verification
51:
936:Salt bridge
850:Voltammetry
316:A standard
1059:Voltmeters
1053:Categories
961:Voltameter
866:Amperostat
790:Coulometry
753:Techniques
688:2013-06-01
669:Kenyon.edu
638:Kenyon.edu
624:2013-06-01
604:References
576:metre rule
241:calibrated
225:resistance
87:newspapers
881:Electrode
358:voltmeter
171:metrology
27:Voltmeter
911:pH meter
653:Archived
587:See also
362:infinite
235:supply V
659:Scenta.
370:current
149:voltage
145:voltage
101:scholar
975:Theory
467:
452:
227:wire R
103:
96:
89:
82:
74:
217:wiper
108:JSTOR
94:books
80:news
360:of
324:).
63:by
1055::
233:DC
139:A
738:e
731:t
724:v
691:.
627:.
508:B
505:A
500:X
497:A
491:)
488:e
485:g
482:a
479:t
476:l
473:o
470:V
464:l
461:l
458:e
455:C
449:n
446:o
443:i
440:t
437:a
434:r
431:b
428:i
425:l
422:a
419:C
416:(
413:=
408:U
404:V
392:3
388:1
381:1
349:3
347:R
344:3
337:2
329:S
297:S
292:V
279:=
272:1
268:R
262:2
258:R
245:1
237:S
229:1
219:.
213:1
211:R
130:)
124:(
119:)
115:(
105:·
98:·
91:·
84:·
57:.
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.