1526:
100:
648:
energy state to a slightly different one. Thus, not only do they become delocalized, forming a sea of electrons permeating the structure, but they are also able to migrate through the structure when an external electrical field is applied, leading to electrical conductivity. Without the field, there are electrons moving equally in all directions. Within such a field, some electrons will adjust their state slightly, adopting a different
432:, in an attempt to explain why intermetallic alloys with certain compositions would form and others would not. Initially Hume-Rothery's attempts were quite successful. His idea was to add electrons to inflate the spherical Fermi-balloon inside the series of Brillouin-boxes and determine when a certain box would be full. This predicted a fairly large number of alloy compositions that were later observed. As soon as
293:
1520:
45:
1532:
1014:
the metallic bonding is confined to a tiny metallic particle, which prevents the oscillation wave of the plasmon from 'running away'. The momentum selection rule is therefore broken, and the plasmon resonance causes an extremely intense absorption in the green, with a resulting purple-red color. Such
930:
As these phenomena involve the movement of the atoms toward or away from each other, they can be interpreted as the coupling between the electronic and the vibrational states (i.e. the phonons) of the material. A different such electron-phonon interaction is thought to lead to a very different result
647:
elements and the communal sharing does not change that. There remain far more available energy states than there are shared electrons. Both requirements for conductivity are therefore fulfilled: strong delocalization and partly filled energy bands. Such electrons can therefore easily change from one
549:
is another example of delocalization, this time often in three-dimensional arrangements. Metals take the delocalization principle to its extreme, and one could say that a crystal of a metal represents a single molecule over which all conduction electrons are delocalized in all three dimensions. This
965:
consists of a combination of an electrical and a magnetic field. The electrical field is usually able to excite an elastic response from the electrons involved in the metallic bonding. The result is that photons cannot penetrate very far into the metal and are typically reflected, although some may
848:
Metals are insoluble in water or organic solvents, unless they undergo a reaction with them. Typically, this is an oxidation reaction that robs the metal atoms of their itinerant electrons, destroying the metallic bonding. However metals are often readily soluble in each other while retaining the
705:
When comparing periodic trends in the size of atoms it is often desirable to apply the so-called
Goldschmidt correction, which converts atomic radii to the values the atoms would have if they were 12-coordinated. Since metallic radii are largest for the highest coordination number, correction for
655:
The freedom of electrons to migrate also gives metal atoms, or layers of them, the capacity to slide past each other. Locally, bonds can easily be broken and replaced by new ones after a deformation. This process does not affect the communal metallic bonding very much, which gives rise to metals'
459:
showed that, in the case of a one-dimensional row of metallic atoms—say, hydrogen—an inevitable instability would break such a chain into individual molecules. This sparked an interest in the general question: when is collective metallic bonding stable, and when will a localized bonding take its
885:
The metallic bonding in complex compounds does not necessarily involve all constituent elements equally. It is quite possible to have one or more elements that do not partake at all. One could picture the conduction electrons flowing around them like a river around an island or a big rock. It is
463:
As powerful as the band structure model proved to be in describing metallic bonding, it remains a one-electron approximation of a many-body problem: the energy states of an individual electron are described as if all the other electrons form a homogeneous background. Researchers such as Mott and
813:
The strong bonding of metals in liquid form demonstrates that the energy of a metallic bond is not highly dependent on the direction of the bond; this lack of bond directionality is a direct consequence of electron delocalization, and is best understood in contrast to the directional bonding of
448:. These models either depart from the atomic orbitals of neutral atoms that share their electrons, or (in the case of density functional theory) departs from the total electron density. The free-electron picture has, nevertheless, remained a dominant one in introductory courses on metallurgy.
562:
participating in the bonding interaction (and, in pure elemental metals, none at all). Thus, metallic bonding is an extremely delocalized communal form of covalent bonding. In a sense, metallic bonding is not a 'new' type of bonding at all. It describes the bonding only as present in a
365:, it became clear that metals generally go into solution as positively charged ions, and the oxidation reactions of the metals became well understood in their electrochemical series. A picture emerged of metals as positive ions held together by an ocean of negative electrons.
910:
could be said to be held together by a combination of metallic bonding and high pressure induced by gravity. At lower pressures, however, the bonding becomes entirely localized into a regular covalent bond. The localization is so complete that the (more familiar)
598:
is very close to accurate (though not perfectly so). For other elements the electrons are less free, in that they still experience the potential of the metal atoms, sometimes quite strongly. They require a more intricate quantum mechanical treatment (e.g.,
443:
The nearly-free electron debacle compelled researchers to modify the assumpition that ions flowed in a sea of free electrons. A number of quantum mechanical models were developed, such as band structure calculations based on molecular orbitals, and the
849:
metallic character of their bonding. Gold, for example, dissolves easily in mercury, even at room temperature. Even in solid metals, the solubility can be extensive. If the structures of the two metals are the same, there can even be complete solid
897:
reminiscent of molecules; and these compounds are more a topic of chemistry than of metallurgy. The formation of the clusters could be seen as a way to 'condense out' (localize) the electron-deficient bonding into bonds of a more localized nature.
550:
means that inside the metal one can generally not distinguish molecules, so that the metallic bonding is neither intra- nor inter-molecular. 'Nonmolecular' would perhaps be a better term. Metallic bonding is mostly non-polar, because even in
810:. Even though gallium will melt from the heat of one's hand just above room temperature, its boiling point is not far from that of copper. Molten gallium is, therefore, a very nonvolatile liquid, thanks to its strong metallic bonding.
483:-electrons, the interaction with nearby individual electrons (and atomic displacements) may become stronger than the delocalized interaction that leads to broad bands. This gave a better explanation for the transition from localized
664:. This is particularly true for pure elements. In the presence of dissolved impurities, the normally easily formed cleavages may be blocked and the material become harder. Gold, for example, is very soft in pure form (24-
593:
is so strong that the electrons are virtually freed from the caesium atoms to form a gas constrained only by the surface of the metal. For caesium, therefore, the picture of Cs ions held together by a negatively charged
697:
The metallic radius is defined as one-half of the distance between the two adjacent metal ions in the metallic structure. This radius depends on the nature of the atom as well as its environment—specifically, on the
970:. The balance between reflection and absorption determines how white or how gray a metal is, although surface tarnish can obscure the lustre. Silver, a metal with high conductivity, is one of the whitest.
1001:
the limiting frequency is in the far ultraviolet, but for copper and gold it is closer to the visible. This explains the colors of these two metals. At the surface of a metal, resonance effects known as
981:
goes from negative (reflecting) to positive (transmitting); higher frequency photons are not reflected at the surface, and do not contribute to the color of the metal. There are some materials, such as
915:
gas results. A similar argument holds for an element such as boron. Though it is electron-deficient compared to carbon, it does not form a metal. Instead it has a number of complex structures in which
973:
Notable exceptions are reddish copper and yellowish gold. The reason for their color is that there is an upper limit to the frequency of the light that metallic electrons can readily respond to: the
1006:
can result. They are collective oscillations of the conduction electrons, like a ripple in the electronic ocean. However, even if photons have enough energy, they usually do not have enough
966:
also be absorbed. This holds equally for all photons in the visible spectrum, which is why metals are often silvery white or grayish with the characteristic specular reflection of metallic
671:
Metals are typically also good conductors of heat, but the conduction electrons only contribute partly to this phenomenon. Collective (i.e., delocalized) vibrations of the atoms, known as
682:, which conducts heat quite well, is not an electrical conductor. This is not a consequence of delocalization being absent in diamond, but simply that carbon is not electron deficient.
376:. In both models, the electrons are seen as a gas traveling through the structure of the solid with an energy that is essentially isotropic, in that it depends on the square of the
1010:
to set the ripple in motion. Therefore, plasmons are hard to excite on a bulk metal. This is why gold and copper look like lustrous metals albeit with a dash of color. However, in
1300:
Brewer, Scott H.; Franzen, Stefan (2002). "Indium Tin Oxide Plasma
Frequency Dependence on Sheet Resistance and Surface Adlayers Determined by Reflectance FTIR Spectroscopy".
436:
became available and the shape of the balloon could be determined, it was found that the balloon was not spherical as the Hume-Rothery believed, except perhaps in the case of
865:
is not valid; and often a range of stoichiometric ratios can be achieved. It is better to abandon such concepts as 'pure substance' or 'solute' in such cases and speak of
1619:
857:, an alloy of silver and gold. At times, however, two metals will form alloys with different structures than either of the two parents. One could call these materials
779:
The atoms in metals have a strong attractive force between them. Much energy is required to overcome it. Therefore, metals often have high boiling points, with
1015:
colors are orders of magnitude more intense than ordinary absorptions seen in dyes and the like, which involve individual electrons and their energy states.
814:
covalent bonds. The energy of a metallic bond is thus mostly a function of the number of electrons which surround the metallic atom, as exemplified by the
685:
Electron deficiency is important in distinguishing metallic from more conventional covalent bonding. Thus, we should amend the expression given above to:
421:
largely remained a mystery and their study was often merely empirical. Chemists generally steered away from anything that did not seem to follow Dalton's
1737:
1664:
1345:
1024:
233:
169:
1240:
1166:
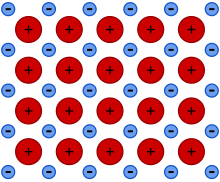
55:
66:
340:
314:
84:
499:
and the availability of a far larger number of delocalized energy states than of delocalized electrons. The latter could be called
440:. This revealed how a model can sometimes give a whole series of correct predictions, yet still be wrong in its basic assumptions.
1599:
1584:
887:
837:
1408:
1435:
832:
Much biochemistry is mediated by the weak interaction of metal ions and biomolecules. Such interactions, and their associated
1396:
1386:
318:
1391:
611:-electrons the delocalization is not strong at all and this explains why these electrons are able to continue behaving as
578:, held together by a more conventional covalent bond. This is why it is not correct to speak of a single 'metallic bond'.
398:
are added to k-space by the periodic potential experienced from the (ionic) structure, thus mildly breaking the isotropy.
1338:
862:
422:
162:
567:
of condensed matter: be it crystalline solid, liquid, or even glass. Metallic vapors, in contrast, are often atomic (
303:
595:
373:
1659:
1654:
357:
of the elements, and great progress was made in the description of the salts that can be formed in reactions with
322:
307:
1763:
1644:
1634:
1609:
1579:
1003:
445:
31:
1425:
987:
746:
738:
465:
368:
With the advent of quantum mechanics, this picture was given a more formal interpretation in the form of the
1686:
1589:
1561:
1331:
959:, rather than considering the states of individual electrons involved in more conventional covalent bonds.
758:
155:
1094:
Electron deficiency is a relative term: it means fewer than half of the electrons needed to complete the
1730:
1691:
833:
819:
221:
59:
that states a
Knowledge (XXG) editor's personal feelings or presents an original argument about a topic.
1725:
1260:
825:
Given high enough cooling rates and appropriate alloy composition, metallic bonding can occur even in
409:
made it possible to study the structure of crystalline solids, including metals and their alloys; and
1649:
1540:
1403:
1362:
1188:
761:
is observed—there is very little increase of the radius down the group due to the presence of poorly
496:
429:
229:
194:
190:
142:
1551:
1415:
1381:
1082:
978:
967:
924:
815:
699:
644:
500:
433:
369:
241:
1715:
795:, more and more when going down the periodic table, because the energy differential to the empty n
464:
Hubbard realized that the one-electron treatment was perhaps appropriate for strongly delocalized
451:
The electronic band structure model became a major focus for the study of metals and even more of
1470:
1212:
1204:
952:
762:
727:
687:
Metallic bonding is an extremely delocalized communal form of electron-deficient covalent bonding
377:
1154:
428:
The nearly-free electron model was eagerly taken up by some researchers in metallurgy, notably
1701:
1490:
1450:
1440:
1236:
1035:
1029:
974:
932:
903:
800:
742:
652:. Consequently, there will be more moving one way than another and a net current will result.
612:
568:
555:
542:
484:
402:
353:
As chemistry developed into a science, it became clear that metals formed the majority of the
260:
217:
209:
1230:
455:. Together with the electronic states, the vibrational states were also shown to form bands.
1742:
1525:
1455:
1309:
1280:
1196:
983:
799:
orbitals becomes larger. These metals are therefore relatively volatile, and are avoided in
784:
406:
362:
237:
1768:
1594:
1465:
1142:
765:
734:
263:
with metallic bonding between them. Another example of a metal–metal covalent bond is the
137:
132:
99:
1720:
1130:
994:, which is why they are transparent in the visible, but good reflectors in the infrared.
886:
possible to observe which elements do partake: e.g., by looking at the core levels in an
1192:
1629:
1430:
1264:
1067:
1011:
866:
858:
826:
665:
636:
603:) in which the atoms are viewed as neutral, much like the carbon atoms in benzene. For
456:
395:
354:
1757:
1678:
1638:
1571:
1500:
1373:
1354:
1285:
1256:
1216:
1078:
936:
894:
861:. But, because materials with metallic bonding are typically not molecular, Dalton's
600:
546:
514:
is an example of two-dimensional metallic bonding. Its metallic bonds are similar to
452:
414:
410:
391:
264:
256:
248:
186:
122:
1098:
noble gas configuration. For example, lithium is electron deficient with respect to
390:. In three-dimensional k-space, the set of points of the highest filled levels (the
1624:
940:
657:
640:
127:
783:(5828 K) being extremely high. A remarkable exception is the elements of the
1710:
1460:
1060:
916:
649:
523:
515:
425:; and the problem was considered the domain of a different science, metallurgy.
386:
292:
1519:
977:. At the plasmon frequency, the frequency-dependent dielectric function of the
951:
The presence of an ocean of mobile charge carriers has profound effects on the
806:
Otherwise, metallic bonding can be very strong, even in molten metals, such as
17:
870:
850:
527:
1445:
1420:
874:
869:
instead. The study of such phases has traditionally been more the domain of
661:
616:
225:
1200:
1179:
Okumura, K. & Templeton, I. M. (1965). "The Fermi
Surface of Caesium".
955:
of metals, which can only be understood by considering the electrons as a
1007:
991:
899:
854:
780:
632:
511:
1208:
943:
are formed that no longer experience any resistance to their mobility.
907:
807:
679:
590:
531:
519:
460:
place? Much research went into the study of clustering of metal atoms.
437:
252:
1313:
935:. Rather than blocking the mobility of the charge carriers by forming
902:
is an extreme example of this form of condensation. At high pressures
251:
a metal can exhibit, even as a pure substance. For example, elemental
1107:
1074:
998:
890:(XPS) spectrum. If an element partakes, its peaks tend to be skewed.
792:
702:(CN), which in turn depends on the temperature and applied pressure.
672:
572:
551:
213:
104:
1268:
1531:
394:) should therefore be a sphere. In the nearly-free model, box-like
962:
745:; but the radii increase down the group due to an increase in the
675:
that travel through the solid as a wave, are bigger contributors.
628:
418:
358:
198:
98:
1323:
495:
The combination of two phenomena gives rise to metallic bonding:
259:
pairs of atoms in both liquid and solid-state—these pairs form a
1099:
559:
1327:
1269:"The embedded-atom method: a review of theory and applications"
818:. This typically results in metals assuming relatively simple,
286:
201:
38:
737:: they decrease across the period due to the increase in the
189:
that arises from the electrostatic attractive force between
1059:, their energy would only depend on the magnitude of their
787:: Zn, Cd, and Hg. Their electron configurations end in ...n
56:
personal reflection, personal essay, or argumentative essay
791:, which resembles a noble gas configuration, like that of
413:
were developed. Despite all this progress, the nature of
1167:"Physics 133 Lecture Notes" Spring, 2004. Marion Campus
62:
1700:
1677:
1608:
1570:
1550:
1539:
1499:
1481:
1372:
1361:
881:Localization and clustering: from bonding to bonds
103:An example showing metallic bonding. + represents
1038: – Concept of aromaticity extended to metals
741:, which is not offset by the increased number of
706:less dense coordinations involves multiplying by
668:), which is why alloys are preferred in jewelry.
487:to itinerant ones partaking in metallic bonding.
877:, although the two fields overlap considerably.
730:who obtained the numerical values quoted above.
1235:. Oxford University Press. 2010. pp. 74–.
893:Some intermetallic materials, e.g., do exhibit
822:crystal structures, such as FCC, BCC, and HCP.
986:(ITO), that are metallic conductors (actually
1339:
163:
8:
1181:Proceedings of the Royal Society of London A
1085:and is never a sphere, not even for caesium.
615:that retain their spin, adding interesting
321:. Unsourced material may be challenged and
107:, - represents the free floating electrons.
1547:
1369:
1346:
1332:
1324:
170:
156:
110:
1284:
571:) or at times contain molecules, such as
341:Learn how and when to remove this message
247:Metallic bonding is not the only type of
85:Learn how and when to remove this message
1738:Polyhedral skeletal electron pair theory
1106:with respect to the previous noble gas,
1073:, the Fermi level should form a perfect
1025:Atomic radii of the elements (data page)
204:. It may be described as the sharing of
1232:Shriver and Atkins' Inorganic Chemistry
1123:
1048:
234:electrical resistivity and conductivity
113:
726:= 0.97. The correction is named after
581:Delocalization is most pronounced for
216:). Metallic bonding accounts for many
990:) for which this threshold is in the
554:there is little difference among the
384:the direction of the momentum vector
193:(in the form of an electron cloud of
7:
319:adding citations to reliable sources
1302:The Journal of Physical Chemistry B
829:, which have amorphous structures.
1032: – Classification of bondings
714:< 1. Specifically, for CN = 4,
25:
1066:, not its direction. That is, in
844:Solubility and compound formation
1530:
1524:
1518:
888:X-ray photoelectron spectroscopy
838:dual polarisation interferometry
623:Electron deficiency and mobility
291:
43:
372:and its further extension, the
27:Type of chemical bond in metals
589:-electrons. Delocalization in
491:The nature of metallic bonding
479:-electrons, and even more for
1:
931:at low temperatures, that of
678:However, a substance such as
639:relative to their periods or
1286:10.1016/0920-2307(93)90001-U
1055:If the electrons were truly
423:laws of multiple proportions
212:of positively charged ions (
863:law of integral proportions
836:, have been measured using
497:delocalization of electrons
1785:
1436:Metal–ligand multiple bond
927:are a related phenomenon.
374:nearly free electron model
29:
1516:
1273:Materials Science Reports
988:degenerate semiconductors
906:. The core of the planet
733:The radii follow general
446:density functional theory
197:) and positively charged
32:Metallophilic interaction
1275:(Submitted manuscript).
1169:. physics.ohio-state.edu
1079:shape of the Fermi level
747:principal quantum number
739:effective nuclear charge
722:= 0.96, and for CN = 8,
30:Not to be confused with
415:intermetallic compounds
1201:10.1098/rspa.1965.0170
834:conformational changes
759:lanthanide contraction
108:
65:by rewriting it in an
361:. With the advent of
195:delocalized electrons
102:
1426:Coordinate (dipolar)
939:in localized bonds,
925:Charge density waves
853:, as in the case of
775:Strength of the bond
718:= 0.88; for CN = 6,
315:improve this section
191:conduction electrons
143:Van der Waals radius
1600:C–H···O interaction
1382:Electron deficiency
1308:(50): 12986–12992.
1193:1965RSPSA.287...89O
1083:cyclotron resonance
1081:can be measured by
923:clusters dominate.
816:embedded atom model
700:coordination number
617:magnetic properties
556:electronegativities
501:electron deficiency
434:cyclotron resonance
370:free electron model
220:of metals, such as
218:physical properties
1585:Resonance-assisted
1265:Baskes, Michael I.
1261:Foiles, Stephen M.
953:optical properties
947:Optical properties
728:Victor Goldschmidt
645:electron-deficient
613:unpaired electrons
485:unpaired electrons
208:electrons among a
109:
67:encyclopedic style
54:is written like a
1751:
1750:
1702:Electron counting
1673:
1672:
1562:London dispersion
1514:
1513:
1491:Metal aromaticity
1314:10.1021/jp026600x
1242:978-0-19-923617-6
1157:. chemguide.co.uk
1145:. chemguide.co.uk
1133:. chemguide.co.uk
1036:Metal aromaticity
1030:Bonding in solids
979:free electron gas
975:plasmon frequency
933:superconductivity
801:ultra-high vacuum
743:valence electrons
619:to these metals.
607:- and especially
543:Metal aromaticity
403:X-ray diffraction
351:
350:
343:
261:crystal structure
180:
179:
95:
94:
87:
16:(Redirected from
1776:
1764:Chemical bonding
1743:Jemmis mno rules
1595:Dihydrogen bonds
1548:
1534:
1528:
1522:
1456:Hyperconjugation
1370:
1348:
1341:
1334:
1325:
1318:
1317:
1297:
1291:
1290:
1288:
1279:(7–8): 251–310.
1253:
1247:
1246:
1227:
1221:
1220:
1187:(1408): 89–104.
1176:
1170:
1164:
1158:
1152:
1146:
1143:Metal structures
1140:
1134:
1131:Metallic bonding
1128:
1111:
1092:
1086:
1053:
1004:surface plasmons
984:indium tin oxide
725:
721:
717:
713:
709:
516:aromatic bonding
407:thermal analysis
363:electrochemistry
346:
339:
335:
332:
326:
295:
287:
278:
277:
276:
257:covalently-bound
249:chemical bonding
187:chemical bonding
183:Metallic bonding
172:
165:
158:
111:
90:
83:
79:
76:
70:
47:
46:
39:
21:
1784:
1783:
1779:
1778:
1777:
1775:
1774:
1773:
1754:
1753:
1752:
1747:
1696:
1669:
1612:
1604:
1566:
1553:
1543:
1535:
1529:
1523:
1510:
1495:
1477:
1365:
1357:
1352:
1322:
1321:
1299:
1298:
1294:
1255:
1254:
1250:
1243:
1229:
1228:
1224:
1178:
1177:
1173:
1165:
1161:
1153:
1149:
1141:
1137:
1129:
1125:
1120:
1115:
1114:
1102:, but electron-
1093:
1089:
1054:
1050:
1045:
1021:
949:
922:
914:
883:
859:metal compounds
846:
777:
749:. Between the 4
735:periodic trends
723:
719:
715:
711:
710:, where 0 <
707:
695:
693:Metallic radius
656:characteristic
625:
576:
540:
509:
493:
396:Brillouin zones
347:
336:
330:
327:
312:
296:
285:
275:
272:
271:
270:
268:
176:
147:
138:Metallic radius
133:Covalent radius
91:
80:
74:
71:
63:help improve it
60:
48:
44:
35:
28:
23:
22:
18:Metallic radius
15:
12:
11:
5:
1782:
1780:
1772:
1771:
1766:
1756:
1755:
1749:
1748:
1746:
1745:
1740:
1735:
1734:
1733:
1728:
1723:
1718:
1707:
1705:
1698:
1697:
1695:
1694:
1689:
1683:
1681:
1675:
1674:
1671:
1670:
1668:
1667:
1662:
1657:
1652:
1647:
1642:
1632:
1627:
1622:
1616:
1614:
1606:
1605:
1603:
1602:
1597:
1592:
1587:
1582:
1576:
1574:
1568:
1567:
1565:
1564:
1558:
1556:
1545:
1541:Intermolecular
1537:
1536:
1517:
1515:
1512:
1511:
1509:
1508:
1505:
1503:
1497:
1496:
1494:
1493:
1487:
1485:
1479:
1478:
1476:
1475:
1474:
1473:
1468:
1458:
1453:
1448:
1443:
1438:
1433:
1428:
1423:
1418:
1413:
1412:
1411:
1401:
1400:
1399:
1394:
1389:
1378:
1376:
1367:
1363:Intramolecular
1359:
1358:
1355:Chemical bonds
1353:
1351:
1350:
1343:
1336:
1328:
1320:
1319:
1292:
1257:Daw, Murray S.
1248:
1241:
1222:
1171:
1159:
1155:Chemical Bonds
1147:
1135:
1122:
1121:
1119:
1116:
1113:
1112:
1087:
1047:
1046:
1044:
1041:
1040:
1039:
1033:
1027:
1020:
1017:
1012:colloidal gold
948:
945:
937:electron pairs
920:
912:
895:metal clusters
882:
879:
845:
842:
776:
773:
757:elements, the
694:
691:
637:valence shells
624:
621:
574:
547:metal clusters
539:
536:
508:
505:
492:
489:
457:Rudolf Peierls
453:semiconductors
411:phase diagrams
401:The advent of
355:periodic table
349:
348:
299:
297:
290:
284:
281:
273:
178:
177:
175:
174:
167:
160:
152:
149:
148:
146:
145:
140:
135:
130:
125:
119:
116:
115:
114:Types of radii
93:
92:
51:
49:
42:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1781:
1770:
1767:
1765:
1762:
1761:
1759:
1744:
1741:
1739:
1736:
1732:
1729:
1727:
1724:
1722:
1719:
1717:
1716:Hückel's rule
1714:
1713:
1712:
1709:
1708:
1706:
1703:
1699:
1693:
1690:
1688:
1685:
1684:
1682:
1680:
1679:Bond cleavage
1676:
1666:
1663:
1661:
1658:
1656:
1653:
1651:
1648:
1646:
1645:Intercalation
1643:
1640:
1636:
1635:Metallophilic
1633:
1631:
1628:
1626:
1623:
1621:
1618:
1617:
1615:
1611:
1607:
1601:
1598:
1596:
1593:
1591:
1588:
1586:
1583:
1581:
1578:
1577:
1575:
1573:
1569:
1563:
1560:
1559:
1557:
1555:
1552:Van der Waals
1549:
1546:
1542:
1538:
1533:
1527:
1521:
1507:
1506:
1504:
1502:
1498:
1492:
1489:
1488:
1486:
1484:
1480:
1472:
1469:
1467:
1464:
1463:
1462:
1459:
1457:
1454:
1452:
1449:
1447:
1444:
1442:
1439:
1437:
1434:
1432:
1429:
1427:
1424:
1422:
1419:
1417:
1414:
1410:
1407:
1406:
1405:
1402:
1398:
1395:
1393:
1390:
1388:
1385:
1384:
1383:
1380:
1379:
1377:
1375:
1371:
1368:
1364:
1360:
1356:
1349:
1344:
1342:
1337:
1335:
1330:
1329:
1326:
1315:
1311:
1307:
1303:
1296:
1293:
1287:
1282:
1278:
1274:
1270:
1266:
1262:
1258:
1252:
1249:
1244:
1238:
1234:
1233:
1226:
1223:
1218:
1214:
1210:
1206:
1202:
1198:
1194:
1190:
1186:
1182:
1175:
1172:
1168:
1163:
1160:
1156:
1151:
1148:
1144:
1139:
1136:
1132:
1127:
1124:
1117:
1109:
1105:
1101:
1097:
1091:
1088:
1084:
1080:
1076:
1072:
1070:
1065:
1062:
1058:
1052:
1049:
1042:
1037:
1034:
1031:
1028:
1026:
1023:
1022:
1018:
1016:
1013:
1009:
1005:
1000:
995:
993:
989:
985:
980:
976:
971:
969:
964:
960:
958:
954:
946:
944:
942:
938:
934:
928:
926:
918:
909:
905:
904:it is a metal
901:
896:
891:
889:
880:
878:
876:
872:
868:
864:
860:
856:
852:
843:
841:
839:
835:
830:
828:
823:
821:
817:
811:
809:
804:
802:
798:
794:
790:
786:
782:
774:
772:
770:
768:
764:
760:
756:
752:
748:
744:
740:
736:
731:
729:
703:
701:
692:
690:
688:
683:
681:
676:
674:
669:
667:
663:
659:
653:
651:
646:
642:
641:energy levels
638:
634:
630:
622:
620:
618:
614:
610:
606:
602:
601:tight binding
597:
592:
588:
584:
579:
577:
570:
566:
561:
557:
553:
548:
544:
537:
535:
533:
529:
525:
521:
517:
513:
506:
504:
502:
498:
490:
488:
486:
482:
478:
474:
472:
468:
461:
458:
454:
449:
447:
441:
439:
435:
431:
426:
424:
420:
416:
412:
408:
404:
399:
397:
393:
392:Fermi surface
389:
388:
383:
379:
375:
371:
366:
364:
360:
356:
345:
342:
334:
324:
320:
316:
310:
309:
305:
300:This section
298:
294:
289:
288:
282:
280:
266:
265:mercurous ion
262:
258:
254:
250:
245:
243:
239:
235:
231:
227:
223:
219:
215:
211:
207:
203:
200:
196:
192:
188:
185:is a type of
184:
173:
168:
166:
161:
159:
154:
153:
151:
150:
144:
141:
139:
136:
134:
131:
129:
126:
124:
123:Atomic radius
121:
120:
118:
117:
112:
106:
101:
97:
89:
86:
78:
75:February 2021
68:
64:
58:
57:
52:This article
50:
41:
40:
37:
33:
19:
1721:Baird's rule
1482:
1441:Charge-shift
1404:Hypervalence
1305:
1301:
1295:
1276:
1272:
1251:
1231:
1225:
1184:
1180:
1174:
1162:
1150:
1138:
1126:
1103:
1095:
1090:
1068:
1063:
1056:
1051:
996:
972:
961:
956:
950:
941:Cooper pairs
929:
892:
884:
847:
831:
824:
820:close-packed
812:
805:
796:
788:
778:
766:
754:
750:
732:
704:
696:
686:
684:
677:
670:
658:malleability
654:
631:contain few
626:
608:
604:
596:electron gas
586:
582:
580:
564:
541:
510:
494:
480:
476:
470:
466:
462:
450:
442:
430:Hume-Rothery
427:
400:
385:
381:
367:
352:
337:
331:October 2009
328:
313:Please help
301:
255:consists of
246:
205:
182:
181:
128:Ionic radius
96:
81:
72:
53:
36:
1711:Aromaticity
1687:Heterolysis
1665:Salt bridge
1610:Noncovalent
1580:Low-barrier
1461:Aromaticity
1451:Conjugation
1431:Pi backbond
1061:wave vector
917:icosahedral
650:wave vector
643:. They are
524:naphthalene
1758:Categories
1639:aurophilic
1620:Mechanical
1118:References
957:collective
871:metallurgy
851:solubility
785:zinc group
528:anthracene
475:; but for
473:-electrons
1731:spherical
1692:Homolysis
1655:Cation–pi
1630:Chalcogen
1590:Symmetric
1446:Hapticity
1217:123127614
875:chemistry
803:systems.
763:shielding
662:ductility
635:in their
633:electrons
378:magnitude
302:does not
226:ductility
210:structure
1660:Anion–pi
1650:Stacking
1572:Hydrogen
1483:Metallic
1374:Covalent
1366:(strong)
1267:(1993).
1019:See also
1008:momentum
992:infrared
900:Hydrogen
873:than of
855:electrum
781:tungsten
769:orbitals
512:Graphene
222:strength
1625:Halogen
1471:bicyclo
1416:Agostic
1209:2415064
1189:Bibcode
908:Jupiter
827:glasses
808:gallium
680:diamond
673:phonons
591:caesium
558:of the
534:, etc.
532:ovalene
520:benzene
438:caesium
323:removed
308:sources
283:History
253:gallium
238:opacity
230:thermal
214:cations
105:cations
61:Please
1769:Metals
1726:Möbius
1554:forces
1544:(weak)
1239:
1215:
1207:
1108:helium
1077:. The
1075:sphere
1071:-space
999:silver
968:lustre
867:phases
793:helium
627:Metal
585:- and
552:alloys
469:- and
419:alloys
242:lustre
240:, and
1704:rules
1613:other
1501:Ionic
1409:3c–4e
1397:8c–2e
1392:4c–2e
1387:3c–2e
1213:S2CID
1205:JSTOR
1043:Notes
963:Light
753:and 5
666:karat
629:atoms
565:chunk
560:atoms
538:In 3D
507:In 2D
359:acids
199:metal
1466:homo
1421:Bent
1237:ISBN
1104:rich
1100:neon
1096:next
1057:free
997:For
660:and
417:and
405:and
306:any
304:cite
232:and
206:free
202:ions
1310:doi
1306:106
1281:doi
1197:doi
1185:287
545:in
518:in
382:not
317:by
279:).
1760::
1304:.
1271:.
1263:;
1259:;
1211:.
1203:.
1195:.
1183:.
921:12
840:.
771:.
689:.
573:Na
569:Hg
530:,
526:,
522:,
503:.
380:,
269:Hg
244:.
236:,
228:,
224:,
1641:)
1637:(
1347:e
1340:t
1333:v
1316:.
1312::
1289:.
1283::
1277:9
1245:.
1219:.
1199::
1191::
1110:.
1069:k
1064:k
919:B
913:2
911:H
797:p
789:s
767:f
755:d
751:d
724:x
720:x
716:x
712:x
708:x
609:f
605:d
587:p
583:s
575:2
481:f
477:d
471:p
467:s
387:k
344:)
338:(
333:)
329:(
325:.
311:.
274:2
267:(
171:e
164:t
157:v
88:)
82:(
77:)
73:(
69:.
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.